Abstract
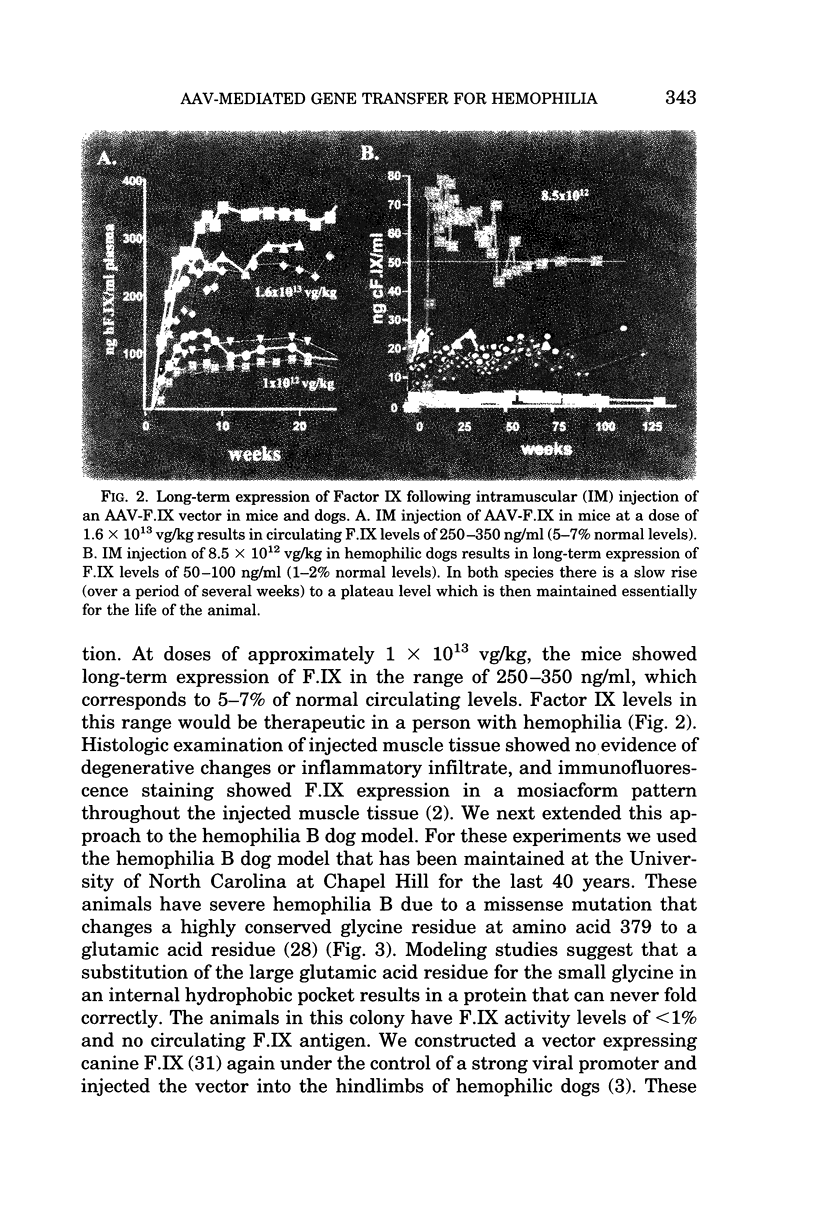
Our research efforts have been focussed on developing a gene transfer strategy for the treatment of the hemophilias. Hemophilia is an attractive target for studies in gene transfer because even small amounts of clotting factor can improve the clinical symptoms of the disease, the factor can be expressed in almost any tissue as long as it gains access to the circulation, and there are large and small animal models of the disease, so that promising approaches can be assessed for efficacy before moving into clinical studies (1). We have developed recombinant adeno-associated viral (AAV) vectors expressing blood coagulation Factor IX. AAV has a number of advantages as a gene transfer vector including: 1) the absence of viral coding sequences in the recombinant vector; 2) the ability to transduce a variety of non-dividing target cells, including liver, muscle and nervous system; 3) the ability to direct long-term expression of the transgene in immunocompetent animals. We have introduced AAV-F.IX vectors into skeletal muscle and liver, and shown long-term correction of the bleeding diatheses in both small and large animal models of hemophilia B (2-5). In the initial clinical trial, rAAV was introduced into skeletal muscle of subjects with severe hemophilia B. Results showed that the general characteristics of transduction were similar in mouse, canine and human muscle, and muscle biopsies of injected sites showed evidence of gene transfer and expression, but circulating levels of F.IX failed to reach the desired target of 3-10%. There were no serious adverse events associated with rAAV injection in skeletal muscle (6). Work has also proceeded on development of a liver-directed approach. Engineering of the expression cassette has resulted in better expression per particle, and circulating F.IX levels of 4-12% have now been achieved in hemophilia B dogs treated with vector doses lower than those already administered in the clinical study in skeletal muscle (5). After extensive safety studies in mice, rats, hemophilic dogs and non-human primates, a Phase I study of an AAV-mediated, liver-directed approach to treating hemophilia B has begun. There were no acute toxicities associated with administration of vector to the first two subjects, but subsequently a PCR assay on the subjects' semen was found to be positive for vector sequences. After a period of weeks, the positive signal disappeared. These findings were distinct from those seen in pre-clinical animal studies. To gain a clearer understanding of the biodistribution of vector to the gonads, we undertook additional studies in rabbits and mice. These showed that, following intravascular delivery of vector, there is hematogenous dissemination to the gonads and gradual washout of vector over time. Direct transduction of germ cells does not appear to occur (7). Based on these and other safety studies, the clinical trial has now resumed. A goal of this work will be to determine whether the therapeutic levels achieved in a large animal model of hemophilia can be realized in humans.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed M. M., Mutimer D. J., Elias E., Linin J., Garrido M., Hubscher S., Jarvis L., Simmonds P., Wilde J. T. A combined management protocol for patients with coagulation disorders infected with hepatitis C virus. Br J Haematol. 1996 Nov;95(2):383–388. doi: 10.1046/j.1365-2141.1996.d01-1899.x. [DOI] [PubMed] [Google Scholar]
- Anderson W. French. The current status of clinical gene therapy. Hum Gene Ther. 2002 Jul 20;13(11):1261–1262. doi: 10.1089/104303402760128496. [DOI] [PubMed] [Google Scholar]
- Arruda V. R., Fields P. A., Milner R., Wainwright L., De Miguel M. P., Donovan P. J., Herzog R. W., Nichols T. C., Biegel J. A., Razavi M. Lack of germline transmission of vector sequences following systemic administration of recombinant AAV-2 vector in males. Mol Ther. 2001 Dec;4(6):586–592. doi: 10.1006/mthe.2001.0491. [DOI] [PubMed] [Google Scholar]
- Arruda V. R., Hagstrom J. N., Deitch J., Heiman-Patterson T., Camire R. M., Chu K., Fields P. A., Herzog R. W., Couto L. B., Larson P. J. Posttranslational modifications of recombinant myotube-synthesized human factor IX. Blood. 2001 Jan 1;97(1):130–138. doi: 10.1182/blood.v97.1.130. [DOI] [PubMed] [Google Scholar]
- Bedossa P., Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996 Aug;24(2):289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- Bi L., Lawler A. M., Antonarakis S. E., High K. A., Gearhart J. D., Kazazian H. H., Jr Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995 May;10(1):119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- Boyce N. Trial halted after gene shows up in semen. Nature. 2001 Dec 13;414(6865):677–677. doi: 10.1038/414677a. [DOI] [PubMed] [Google Scholar]
- Cameron C., Notley C., Hoyle S., McGlynn L., Hough C., Kamisue S., Giles A., Lillicrap D. The canine factor VIII cDNA and 5' flanking sequence. Thromb Haemost. 1998 Feb;79(2):317–322. [PubMed] [Google Scholar]
- Epstein S., Bauer S., Miller A., Pilaro A., Noguchi P. FDA comments on phase I clinical trials without vector biodistribution data. Nat Genet. 1999 Aug;22(4):326–326. doi: 10.1038/11895. [DOI] [PubMed] [Google Scholar]
- Evans J. P., Brinkhous K. M., Brayer G. D., Reisner H. M., High K. A. Canine hemophilia B resulting from a point mutation with unusual consequences. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10095–10099. doi: 10.1073/pnas.86.24.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. P., Watzke H. H., Ware J. L., Stafford D. W., High K. A. Molecular cloning of a cDNA encoding canine factor IX. Blood. 1989 Jul;74(1):207–212. [PubMed] [Google Scholar]
- Hanley J. P., Jarvis L. M., Andrews J., Dennis R., Lee R., Simmonds P., Piris J., Hayes P., Ludlam C. A. Investigation of chronic hepatitis C infection in individuals with haemophilia: assessment of invasive and non-invasive methods. Br J Haematol. 1996 Jul;94(1):159–165. doi: 10.1046/j.1365-2141.1996.6192064.x. [DOI] [PubMed] [Google Scholar]
- Herzog R. W., Hagstrom J. N., Kung S. H., Tai S. J., Wilson J. M., Fisher K. J., High K. A. Stable gene transfer and expression of human blood coagulation factor IX after intramuscular injection of recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997 May 27;94(11):5804–5809. doi: 10.1073/pnas.94.11.5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog R. W., Yang E. Y., Couto L. B., Hagstrom J. N., Elwell D., Fields P. A., Burton M., Bellinger D. A., Read M. S., Brinkhous K. M. Long-term correction of canine hemophilia B by gene transfer of blood coagulation factor IX mediated by adeno-associated viral vector. Nat Med. 1999 Jan;5(1):56–63. doi: 10.1038/4743. [DOI] [PubMed] [Google Scholar]
- High K. A. Gene therapy in haematology and oncology. Lancet. 2000 Dec;356 (Suppl):s8–s8. doi: 10.1016/s0140-6736(00)91994-9. [DOI] [PubMed] [Google Scholar]
- High K. A. Gene transfer as an approach to treating hemophilia. Circ Res. 2001 Feb 2;88(2):137–144. doi: 10.1161/01.res.88.2.137. [DOI] [PubMed] [Google Scholar]
- Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994 Jul;20(1 Pt 1):15–20. [PubMed] [Google Scholar]
- Kay M. A., Manno C. S., Ragni M. V., Larson P. J., Couto L. B., McClelland A., Glader B., Chew A. J., Tai S. J., Herzog R. W. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000 Mar;24(3):257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- Kay M. A., Rothenberg S., Landen C. N., Bellinger D. A., Leland F., Toman C., Finegold M., Thompson A. R., Read M. S., Brinkhous K. M. In vivo gene therapy of hemophilia B: sustained partial correction in factor IX-deficient dogs. Science. 1993 Oct 1;262(5130):117–119. doi: 10.1126/science.8211118. [DOI] [PubMed] [Google Scholar]
- Kessler P. D., Podsakoff G. M., Chen X., McQuiston S. A., Colosi P. C., Matelis L. A., Kurtzman G. J., Byrne B. J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu R. K., Sangiorgi F., Wu L. Y., Kurachi K., Anderson W. F., Maxson R., Gordon E. M. Targeted inactivation of the coagulation factor IX gene causes hemophilia B in mice. Blood. 1998 Jul 1;92(1):168–174. [PubMed] [Google Scholar]
- Lieber A., Vrancken Peeters M. J., Meuse L., Fausto N., Perkins J., Kay M. A. Adenovirus-mediated urokinase gene transfer induces liver regeneration and allows for efficient retrovirus transduction of hepatocytes in vivo. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6210–6214. doi: 10.1073/pnas.92.13.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H. F., Maeda N., Smithies O., Straight D. L., Stafford D. W. A coagulation factor IX-deficient mouse model for human hemophilia B. Blood. 1997 Nov 15;90(10):3962–3966. [PubMed] [Google Scholar]
- Ljung R. C. Can haemophilic arthropathy be prevented? Br J Haematol. 1998 May;101(2):215–219. doi: 10.1046/j.1365-2141.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- Lozier Jay N., Dutra Amalia, Pak Evgenia, Zhou Nan, Zheng Zhili, Nichols Timothy C., Bellinger Dwight A., Read Marjorie, Morgan Richard A. The Chapel Hill hemophilia A dog colony exhibits a factor VIII gene inversion. Proc Natl Acad Sci U S A. 2002 Sep 19;99(20):12991–12996. doi: 10.1073/pnas.192219599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löfqvist T., Nilsson I. M., Berntorp E., Pettersson H. Haemophilia prophylaxis in young patients--a long-term follow-up. J Intern Med. 1997 May;241(5):395–400. doi: 10.1046/j.1365-2796.1997.130135000.x. [DOI] [PubMed] [Google Scholar]
- Mannucci P. M. Modern treatment of hemophilia: from the shadows towards the light. Thromb Haemost. 1993 Jul 1;70(1):17–23. [PubMed] [Google Scholar]
- Mannucci P. M., Tuddenham E. G. The hemophilias--from royal genes to gene therapy. N Engl J Med. 2001 Jun 7;344(23):1773–1779. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- Mauser A. E., Whitlark J., Whitney K. M., Lothrop C. D., Jr A deletion mutation causes hemophilia B in Lhasa Apso dogs. Blood. 1996 Nov 1;88(9):3451–3455. [PubMed] [Google Scholar]
- Mount Jane D., Herzog Roland W., Tillson D. Michael, Goodman Susan A., Robinson Nancy, McCleland Mark L., Bellinger Dwight, Nichols Timothy C., Arruda Valder R., Lothrop Clinton D., Jr Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002 Apr 15;99(8):2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Mount Jane D., Herzog Roland W., Tillson D. Michael, Goodman Susan A., Robinson Nancy, McCleland Mark L., Bellinger Dwight, Nichols Timothy C., Arruda Valder R., Lothrop Clinton D., Jr Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002 Apr 15;99(8):2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- Nakai H., Herzog R. W., Hagstrom J. N., Walter J., Kung S. H., Yang E. Y., Tai S. J., Iwaki Y., Kurtzman G. J., Fisher K. J. Adeno-associated viral vector-mediated gene transfer of human blood coagulation factor IX into mouse liver. Blood. 1998 Jun 15;91(12):4600–4607. [PubMed] [Google Scholar]
- Palmer T. D., Thompson A. R., Miller A. D. Production of human factor IX in animals by genetically modified skin fibroblasts: potential therapy for hemophilia B. Blood. 1989 Feb;73(2):438–445. [PubMed] [Google Scholar]
- Ragni M. V., Winkelstein A., Kingsley L., Spero J. A., Lewis J. H. 1986 update of HIV seroprevalence, seroconversion, AIDS incidence, and immunologic correlates of HIV infection in patients with hemophilia A and B. Blood. 1987 Sep;70(3):786–790. [PubMed] [Google Scholar]
- Russell D. W., Kay M. A. Adeno-associated virus vectors and hematology. Blood. 1999 Aug 1;94(3):864–874. [PMC free article] [PubMed] [Google Scholar]
- Snyder R. O., Miao C. H., Patijn G. A., Spratt S. K., Danos O., Nagy D., Gown A. M., Winther B., Meuse L., Cohen L. K. Persistent and therapeutic concentrations of human factor IX in mice after hepatic gene transfer of recombinant AAV vectors. Nat Genet. 1997 Jul;16(3):270–276. doi: 10.1038/ng0797-270. [DOI] [PubMed] [Google Scholar]
- Snyder R. O., Miao C., Meuse L., Tubb J., Donahue B. A., Lin H. F., Stafford D. W., Patel S., Thompson A. R., Nichols T. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999 Jan;5(1):64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- Soucie J. M., Nuss R., Evatt B., Abdelhak A., Cowan L., Hill H., Kolakoski M., Wilber N. Mortality among males with hemophilia: relations with source of medical care. The Hemophilia Surveillance System Project Investigators. Blood. 2000 Jul 15;96(2):437–442. [PubMed] [Google Scholar]
- Wagner J. A., Moran M. L., Messner A. H., Daifuku R., Conrad C. K., Reynolds T., Guggino W. B., Moss R. B., Carter B. J., Wine J. J. A phase I/II study of tgAAV-CF for the treatment of chronic sinusitis in patients with cystic fibrosis. Hum Gene Ther. 1998 Apr 10;9(6):889–909. doi: 10.1089/hum.1998.9.6-889. [DOI] [PubMed] [Google Scholar]
- Wagner J. A., Reynolds T., Moran M. L., Moss R. B., Wine J. J., Flotte T. R., Gardner P. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998 Jun 6;351(9117):1702–1703. doi: 10.1016/S0140-6736(05)77740-0. [DOI] [PubMed] [Google Scholar]
- Wang L., Zoppè M., Hackeng T. M., Griffin J. H., Lee K. F., Verma I. M. A factor IX-deficient mouse model for hemophilia B gene therapy. Proc Natl Acad Sci U S A. 1997 Oct 14;94(21):11563–11566. doi: 10.1073/pnas.94.21.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. M., Stafford D. W., Ware J. Deduced amino acid sequence of mouse blood-coagulation factor IX. Gene. 1990 Feb 14;86(2):275–278. doi: 10.1016/0378-1119(90)90290-8. [DOI] [PubMed] [Google Scholar]
- Xiao X., Li J., Samulski R. J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996 Nov;70(11):8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S. N., Kurachi K. Expression of human factor IX in mice after injection of genetically modified myoblasts. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3357–3361. doi: 10.1073/pnas.89.8.3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S. N., Wilson J. M., Nabel E. G., Kurachi S., Hachiya H. L., Kurachi K. Expression of human factor IX in rat capillary endothelial cells: toward somatic gene therapy for hemophilia B. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8101–8105. doi: 10.1073/pnas.88.18.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]