Abstract
Pneumocystis carinii causes severe pneumonia in immunocompromised hosts. The binding of P. carinii to alveolar epithelial cells and extracellular matrix constituents such as fibronectin and vitronectin is a central feature of infection, which initiates proliferation of the organism. Herein, we demonstrate that P. carinii binding to lung cells specifically alters the gene expression of the organism, regulating fungal growth. Subtractive hybridization was performed to isolate P. carinii genes expressed following binding to mammalian extracellular matrix constituents. P. carinii STE20 (PCSTE20), a gene participating in mating and pseudohyphal growth of other fungi, was identified following adherence to the extracellular matrix constituents fibronectin, vitronectin, collagen, and lung epithelial cells. The expression of PCSTE20 and a related P. carinii mitogen-activated protein kinase (MAPK) kinase gene, also implicated in signaling of mating, were both specifically upregulated by binding to matrix protein. The expression of general cyclin-dependent kinases and other MAPKs not involved in mating pathways were not altered by organism binding. PCSTE20 expression was also strongly enhanced following organism attachment to A549 lung epithelial cells. When expressed in a Saccharomyces cerevisiae ste20Δ mutant, PCSTE20 suppressed defects in both mating and pseudohyphal growth. These findings are consistent with the observed proliferation and filopodial extension of Pneumocystis organisms adherent to the epithelium in the lungs of immunocompromised hosts. PCSTE20 expression appears to represent a significant component in the regulation of the life cycle of this intractable opportunistic pathogen.
Pneumocystis pneumonia continues to inflict severe morbidity and mortality in immunocompromised patients, including those with AIDS and malignancies (3, 19). Even with effective treatment, the mortality of an individual episode of Pneumocystis carinii pneumonia continues to range within an unacceptable 15 to 40% (3, 19). The binding of P. carinii trophic forms to alveolar epithelial cells and extracellular matrix components of the host such as fibronectin and vitronectin is an important component of infection (28, 30). The attachment of the organism to host cells is associated with the extension of filopodia, which interdigitate with the membranes of host epithelial cells to mediate firm adherence (2, 18, 30, 31). In addition, the adherence of P. carinii to lung epithelial cells has been shown to induce proliferation of the organism (5, 30).
The continuous in vitro cultivation of P. carinii has been extremely problematic (30, 39). Our group and others have observed limited short-term proliferation of P. carinii when the organisms are cultured on feeder A549 lung epithelial cells. We demonstrated a 5.8 (± 2.2)-fold increase in P. carinii numbers after 10 days of incubation on A549 cells (a P value of <0.05 was obtained by comparing the initial inoculum at day 0 to the organism number at day 10) (30). Longer culture times showed declining organism numbers, and serial passage was not possible. In those studies, organism numbers were assessed by direct enumeration of P. carinii nuclei. In addition, maneuvers that prevented the adherence of P. carinii to the A549 lung epithelial cell substrate, such as separation of the organisms from the lung cells with uncoated Transwell membranes, fully inhibited this limited short-term proliferation of P. carinii. Thus, adherence of P. carinii to lung cells appears to facilitate proliferative growth of the organism.
The extracellular matrix (ECM) is a complex mixture of molecules containing, among other components, fibronectin, vitronectin, collagens, and proteoglycans. The composition of the ECM varies in different tissues and during states of injury, inflammation, and repair. Host ECM components such as fibronectin and vitronectin strongly promote the attachment of P. carinii to lung cells (26, 29, 36, 37, 44). Other mammalian ECM components, such as collagen, can also serve as adhesive substrates for the organism (32). The major surface glycoprotein of P. carinii (termed msg or gpA), cell wall β-glucans, and other fungal matrix receptors have been shown to interact with ECM proteins (11, 16, 29, 34-36). Cognate integrin receptors on lung epithelial cells have been implicated in the mediation of interactions of matrix proteins coating the surface of the organism (37).
Recent studies have further demonstrated multiple signaling pathways that regulate the P. carinii cell cycle (8, 9, 13, 41, 42). However, despite evidence that P. carinii organisms substantially alter their morphology and initiate proliferation following contact with the host epithelium, surprisingly little has been learned about signal transduction mechanisms activated upon organism binding to mammalian surfaces. Such investigations may yield important clues to better understand the life cycle of Pneumocystis organisms and may provide insights for developing new agents to prevent and treat this devastating infection of immunocompromised patients.
MATERIALS AND METHODS
Strains and materials.
P. carinii (American Type Culture Collection, Rockville, Md.) pneumonia was induced in dexamethasone-treated rats (6, 27). The rat lungs were homogenized, and P. carinii was harvested by passage through 10-μm-pore-size filters (Millipore) (26). Preparations containing detectable bacteria or other fungi were discarded. Nitrocellulose membranes with separated P. carinii chromosomes were provided by Melanie T. Cushion, University of Cincinnati (4). Saccharomyces cerevisiae strains L5366 (STE20+ wild type, MATa/α ura3-52/ura3-52 in the Sigma 1278b strain background), L5624 (MATa/α ste20::TRP1/ste20::TRP1 ura3-52/ura3-52 trp1::hisG/trp1::hisG in the Sigma 1278b strain background), and JKY40 (MATa ste20::TRP1 ura3-52 leu2-3,112 his3Δ200 or his3-11,15 trp1Δ63 ade2 lys2::FUS1-lacZ in the S288c strain background) were used as described previously. The latter has a slightly leaky sterility phenotype (20). The mating tester strain was JBY311 (MATα kss1 ura3-52 lys9). The TJK1 strain of S. cerevisiae, employed in mating assays, was constructed by transformation of JKY40 with plasmid pYES2.1/PCSTE20, a vector with URA3 for selection (Invitrogen) and carrying P. carinii STE20 (PCSTE20) under control of the GAL1 promoter. The TJK2 strain of S. cerevisiae, used for studying pseudohyphal growth, was similarly derived from strain L5624 transformed with pYES2.1/PCSTE20.
Isolation of P. carinii transcripts induced by adherence to ECM collagens.
Freshly isolated P. carinii organisms (2 × 106) were suspended in Dulbecco modified Eagle medium with 10% fetal calf serum, containing penicillin (10 μg/ml), gentamicin (4 μg/ml), and amphotericin (0.5 μg/ml), applied either to Transwell inserts coated with an equimolar mixture of types I and III collagen or uncoated tissue culture inserts (Becton-Dickinson, Inc., Bedford, Mass.), and incubated at 30°C for 2.0 h, and total RNA was isolated. This time of incubation was selected because it was consistent with a period in which P. carinii is known to adhere to ECM components but before any observable changes in organism numbers were observed in prior studies (26, 28, 29). Subtractive hybridization was used to isolate genes induced by P. carinii binding to matrix-coated surfaces, as previously reported (14). To obtain separate commonly expressed genes from those of interest, a pool of driver mRNA (P. carinii incubated on uncoated surfaces) and target mRNA (P. carinii incubated on collagen) were bound to oligo(dT) Dynabeads (Dynal, Inc.) for 10 min at room temperature, the supernatant was removed, and the beads were washed three times. Over multiple rounds of subtractive hybridization, a gene with strong homology to Ste20 kinase genes was identified. The full-length PCSTE20 cDNA was obtained by probing a rat-derived P. carinii cDNA library in Uni-ZAP XR phage (National Institutes of Health AIDS Research Reagent Program, Bethesda, Md.). Hybridizing clones were plaque-purified to homogeneity, and both DNA strands were sequenced.
Southern and Northern hybridization.
A 371-bp PCSTE20 probe was generated between bp 507 and 878 of PCSTE20. Southern hybridization was performed with digested P. carinii genomic DNA or whole P. carinii chromosomes separated by contour-clamped homogeneous electric field electrophoresis as described previously (4, 21, 22). To verify that PCSTE20 expression was induced upon P. carinii binding to collagen, RNA was isolated from P. carinii incubated for 2.0 h under the exact conditions used for subtractive hybridization. Total RNA (5.0 μg) was separated by electrophoresis, and Northern hybridization was completed for PCSTE20 as reported previously (21). Northern hybridizations were similarly performed for other P. carinii target genes, including MAPK (involved in mating pathways), MKP1 (participating in cell wall integrity), and CDC2 and CDC13 (cell cycle regulatory genes) (8, 23, 41, 42).
Effect of matrix and A549 lung cells on PCSTE20 expression.
Transwell inserts were coated with fibronectin, vitronectin, or albumin (10 μg/cm2) in 0.1 mM NaHCO3 by overnight incubation, followed by washing. P. carinii organisms (2 × 106) were incubated on the inserts for 2.0 h, and Northern hybridization was performed for PCSTE20 in Dulbecco modified Eagle medium in the presence of 10% fetal calf serum exactly as described in the original subtractive hybridization protocol. PCSTE20 expression was also evaluated after culturing P. carinii organisms (2 × 106) on A549 cells (1 × 106 per well) or uncoated inserts suspended above the A549 cell layers for 2 h. The presence of serum in the culture medium was necessary in order to maintain the viability of the P. carinii organisms as measured by recovering intact RNA. While serum contains some fibronectin and vitronectin, these soluble matrix proteins themselves did not alter the expression of PCSTE20. In preliminary studies, no induction of PCSTE20 gene expression was noted when P. carinii was incubated on uncoated plastic or on bovine serum albumin (BSA)-coated surfaces in the presence of media containing serum.
Regulation of PCSTE20 expression in separated life cycle forms of P. carinii.
Whole P. carinii isolates were separated into cyst and trophic form populations by differential filtration (21, 41). In brief, P. carinii cysts are retained by 3-μm-pore-size Nuclepore filters and resuspended after exhaustive washing. P. carinii trophic forms pass through the device and are recovered by centrifugation. This separation procedure yielded trophic form populations containing 99.5% trophic forms and cyst preparations, which were 40-fold enriched (41). Isolated cysts or trophic forms (50 × 106) were cultured on either collagen-coated inserts or directly on A549 cells for 2.0 h. RNA from the P. carinii forms was analyzed for PCSTE20 expression by Northern hybridization.
Suppression of defects in pseudohyphal growth and mating in an S. cerevisiae ste20Δ strain by PCSTE20.
Studies of P. carinii gene function are hindered by the inability to culture and transform this organism (39). Thus, analysis of gene function has been addressed by heterologous expression of P. carinii proteins in other fungi (22, 41). To address the potential role of PCSTE20 in pseudohyphal morphology change, an S. cerevisiae ste20Δ mutant (L5624) was transformed with PCSTE20 (20). Transformants were selected by uracil auxotrophy and evaluated for pseudohyphal growth on synthetic low-ammonium medium with galactose (SLAG medium) containing yeast nitrogen base without amino acids (6.7 g/liter), galactose (20.0 g/liter), ammonium sulfate (7.0 mg/liter), and agar (40.0 g/liter) (12). S. cerevisiae L5624 transformed with vector alone and wild-type S. cerevisiae (L5366) were studied in parallel.
Finally, the ability of PCSTE20 to restore the mating ability of sterile S. cerevisiae ste20Δ mutant strains was also evaluated. The ste20Δ strain JKY40 (MATa) was transformed with PCSTE20 and assessed for mating with the MATα tester strain JKY311 by the replica patch method (20, 22). Specifically, the ste20Δ strain JKY40 had mutations in the biosynthesis pathways of leucine, histidine, adenine, lysine, and uracil. The defect in uracil biosynthesis was complemented by the vector for PCSTE20, but on minimal medium lacking the amino acids listed above, JKY40 could not grow. The tester strain, JBY311, was wild type for the biosynthesis genes, which were defective in JKY40. Conversely, the tester strain had a mutation in a different gene in the lysine biosynthesis pathway, which was wild type in JKY40. Thus, JBY311 was also unable to grow on minimal medium. If the two strains mated, each would bring to the resulting diploid the biosynthesis genes defective in its mating partner. The resulting diploid would therefore be able to grow on minimal medium. If the sterility of the ste20Δ strain JKY40 was complemented by PCSTE20, mating with the tester strain could occur and diploid cells would grow on the minimal medium. A multiplex PCR assay was further employed to demonstrate both the MATa locus (544-bp PCR product) and the MATα locus (404-bp product) following mating (17). Finally, the efficiency of diploid formation in the presence of PCSTE20 was additionally determined by quantitative mating assays, as described previously (43).
Nucleotide sequence accession number.
The PCSTE20 coding region that was isolated in this study has been submitted to GenBank under accession number AF332388.
RESULTS
PCSTE20 is expressed by P. carinii following interaction with ECM collagen.
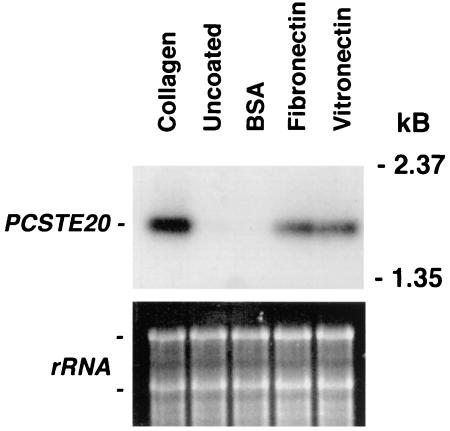
P. carinii binding to lung cells is facilitated through strong interactions with ECM proteins, including collagens (29, 32, 36). To characterize P. carinii gene activation resulting from interactions with mammalian surfaces, we implemented a PCR-based subtractive hybridization strategy to detect transcripts induced upon P. carinii binding to collagen-coated surfaces. Using this method, we isolated a 2.1-kb coding region that was homologous to the Ste20/p65PAK protein family and designated it PCSTE20 (GenBank accession no. AF332388). The subtractive hybridization screen was verified by Northern hybridization under conditions identical to those in the screen, confirming an enhanced amount of PCSTE20 mRNA transcript following binding of the organism to collagen-coated surfaces compared to that on uncoated plastic (Fig. 1).
FIG. 1.
Mammalian matrix proteins implicated in P. carinii adhesion to lung epithelium induce PCSTE20 expression in the organism. P. carinii organisms were cultured on tissue culture inserts coated either with collagen, vitronectin, fibronectin, or BSA, or inserts left uncoated (control). RNA was isolated and examined for PCSTE20 expression. Collagen-, vitronectin-, and fibronectin-coated surfaces induced expression of PCSTE20. In contrast, neither the BSA-coated nor the uncoated plastic surfaces induced expression of this gene. Equal loading of RNA was confirmed on the ethidium-stained gel prior to transfer.
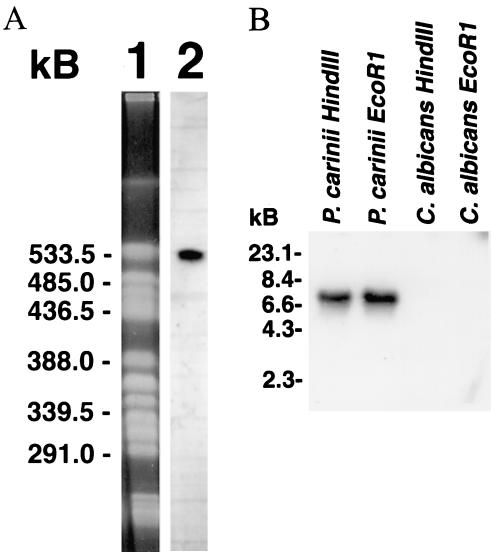
To verify that the isolated PCSTE20 was truly derived from Pneumocystis, we assessed its ability to hybridize specifically to separated P. carinii chromosomes and genomic DNA (Fig. 2). A 371-bp PCSTE20 probe hybridized to a single P. carinii chromosome (Fig. 2A), confirming its presence within the organism's genome, and to a single location on P. carinii genomic DNA digested with EcoRI or HindIII, suggesting it is represented as a single copy (Fig. 2B). A PCSTE20 gene has also been recently identified as part of a putative mating locus discovered through the P. carinii genome project (40).
FIG. 2.
(A) PCSTE20 hybridizes to a single P. carinii chromosome under high-stringency conditions. Lane 1, P. carinii chromosomes resolved by contour-clamped homogeneous electric field electrophoresis and stained with ethidium bromide. P. carinii chromosomes isolated from a single rat were separated. Lane 2, P. carinii chromosomes were transferred to nitrocellulose and hybridized to the 371-bp 32P-labeled PCSTE20 probe. The PCSTE20 probe hybridized specifically to one P. carinii chromosome. (B) PCSTE20 is specifically represented within Pneumocystis genomic DNA. PCSTE20 cDNA was radiolabeled and hybridized to P. carinii genomic DNA digested with EcoRI or HindIII as indicated. PCSTE20 was present in one location for each analysis. Parallel digestion and hybridization of Candida albicans DNA yielded no hybridization.
Ste20 protein (Ste20p) has been implicated in mating and morphological changes in fungi (10, 25, 38). Analysis of the predicted P. carinii Ste20p revealed it to be homologous to other fungal Ste20 proteins involved in growth regulation (Fig. 3). BLASTX analysis of the PCSTE20 product revealed the sequence to be most homologous to that of Cryptococcus neoformans Ste20 alpha kinase (51%). It also had significant homology to S. cerevisiae Ste20p (41%).
FIG. 3.
P. carinii Ste20p is homologous to other fungal Ste20 proteins involved in growth regulation. P.c., P. carinii; S.c., S. cerevisiae; C.n., C. neoformans. Dashes were used to maximize alignment. *, identical amino acid residues.
Fibronectin and vitronectin enhance expression of PCSTE20.
Since fibronectin and vitronectin are known to strongly participate in P. carinii attachment to lung cells, PCSTE20 expression was assessed following binding to these mammalian ECM components (Fig. 1). Enhanced PCSTE20 expression was detected when P. carinii organisms were adherent to both fibronectin- and vitronectin-coated surfaces in a fashion comparable to that to collagen surfaces. In comparison, both BSA-coated and uncoated plastic surfaces failed to alter PCSTE20 RNA levels. Thus, the adherence of P. carinii to a variety of mammalian matrix proteins present in the lower respiratory tract induces PCSTE20 expression by the organism.
Interaction of P. carinii with lung epithelial cells promotes expression of PCSTE20.
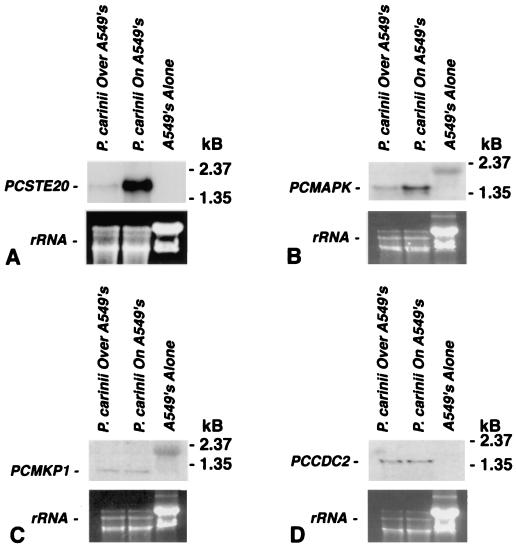
Adherence of P. carinii to lung epithelial cells is a central feature of infection, which promotes proliferation of the organism (5, 30). Thus, the expression of PCSTE20 was further evaluated when P. carinii organisms were cultured directly on A549 lung epithelial cells (Fig. 4A). Similar to the findings with surfaces coated with ECM constituents, P. carinii attachment to A549 cells also significantly stimulated PCSTE20 expression compared to that resulting when P. carinii was incubated on uncoated culture inserts cocultured above, but not in direct contact with, A549 cells. RNA from A549 cells alone exhibited no cross-hybridization to PCSTE20, confirming the specificity of the probe for Pneumocystis-derived STE20.
FIG. 4.
Adherence of P. carinii to A549 alveolar epithelial cells specifically induces expression of PCSTE20 and PCMAPK, which participate in fungal mating pathways. To assess whether lung epithelial cells also promote expression of PCSTE20, P. carinii was cultured either directly adherent to A549 lung cells (P. carinii on A549's) or separated from A549 cell monolayers by uncoated Transwell membranes (P. carinii over A549's) for 2 h. (A) Direct adherence of P. carinii to A549 lung cells enhanced PCSTE20 mRNA expression in the organism. In contrast, those organisms cocultured on uncoated tissue culture membranes separating them from the A549 cell layers did not exhibit enhanced expression of PCSTE20. RNA derived from A549 cells cultured without P. carinii exhibited no cross-reactivity to the PCSTE20 probe. Relative RNA loading was again verified by ethidium bromide staining of rRNA. (B) In a similar manner, PCMAPK, a kinase gene also implicated in fungal mating, showed enhanced expression following direct interaction with A549 lung cells. (C) In contrast, a distinct P. carinii kinase gene, PCMKP1, a gene involved in maintaining cell wall integrity, was expressed at a lower level and was not substantially altered by incubation of the organism on lung epithelial cells. (D and E) Furthermore, the P. carinii cyclin-dependent kinase gene PCCDC2 (D) and the cognate cyclin gene PCCDC13 (E) were also not induced following the binding of P. carinii to A549 lung cells.
It has previously been shown that the adherence of P. carinii to A549 lung epithelial cells induces limited proliferation of the organism (30). To additionally investigate whether expression of PCSTE20 represented a specific signaling response following organism binding to epithelial cells, or instead reflected generalized gene expression accompanying limited organism proliferation, we analyzed the expression of other P. carinii mitogen-activated protein kinases (MAPKs) and genes implicated in P. carinii life cycle regulation (Fig. 4). Accordingly, we assessed the effect of P. carinii binding to A549 cells on the expression of PCMAPK, a MAPK gene implicated in pheromone-initiated mating signaling (7, 42). We further analyzed expression of PCMKP1, a distinct MAPK gene participating in maintenance of cell wall integrity, and PCCDC2 and PCCDC13, cyclin-dependent kinase genes mediating cell cycle regulation of this organism (8, 23, 41). Notably, the expression of PCMAPK, another gene participating in the mating of other fungi, was similarly induced by the interaction of P. carinii with A549 epithelial cells (Fig. 4B). In contrast, expression of PCMKP1 and the cyclin-dependent kinase genes PCCDC2 and PCCDC13 were not altered by the organism binding to A549 lung cells (Fig. 4C to E). Thus, the enhanced expression of PCSTE20 and PCMAPK, involved in initiation of fungal mating, appears to be specifically expressed by the organism in response to interactions with lung epithelial cells.
There was absolutely no cross-reactivity for either the PCSTE20 or PCCDC2 probes with RNA derived from A549 cells, indicating that the mRNA of interest was derived from P. carinii. The probes for PCMAPK, PCMKP1, and PCCDC13 hybridized to P. carinii RNA at the appropriate molecular weight but also cross-hybridized slightly to mammalian RNA at a significantly higher molecular weight in the somewhat overloaded A549 cell RNA lanes. Nonetheless, the P. carinii-related hybridizations were easily discerned at the appropriate lower molecular weight, and thus contaminating RNA from the A549 cell substrates did not confound the interpretation of the relative changes of P. carinii gene expression following interaction with the lung cell substrates.
PCSTE20 expression is differentially regulated over the life cycle of P. carinii.
The life cycle of P. carinii alternates between diminutive trophic forms (1 to 2 μm in diameter) and larger cysts (8 μm) (2, 31). Studies have further shown that trophic forms, but not cysts, closely interact with the alveolar epithelium (2). To study PCSTE20 expression over the organism's life cycle, P. carinii trophic forms and cysts were separated and PCSTE20 expression was analyzed with and without binding to collagen (Fig. 5A). Basal PCSTE20 RNA levels in trophic and cyst forms were roughly similar. However, following binding to the collagen, PCSTE20 expression was stimulated only in the trophic form. Enhanced PCSTE20 expression was also observed following incubation of trophic forms, but not cysts, on A549 lung epithelial cells (Fig. 5B). Thus, the ability of P. carinii to regulate PCSTE20 expression in response to mammalian surfaces appears to be entirely restricted to trophic forms, the life cycle form which actively interacts with lung epithelial cells.
FIG. 5.
PCSTE20 is differentially expressed in P. carinii trophic forms and in P. carinii cysts in response to matrix proteins and epithelial cells. All of the P. carinii organisms were separated into trophic forms and cysts and then placed on uncoated or collagen-coated tissue culture inserts. (A) Comparable amounts of PCSTE20 gene expression were noted in cysts and trophic forms maintained on uncoated surfaces. However, only P. carinii trophic forms (not cysts) exhibited stimulation of PCSTE20 following interaction with collagen-coated surfaces. (B) Similarly, only trophic forms of P. carinii exhibited enhanced expression of PCSTE20 following incubation on A549 lung epithelial cells. Equal loading of RNA was verified on the ethidium bromide-stained gel.
PCSTE20 suppresses both the morphological and mating defects in an ste20 mutant yeast strain.
STE20 genes mediate morphological changes such as pseudohyphal growth in S. cerevisiae and other fungi (10, 20). Although P. carinii does not possess a true filamentous or pseudohyphal phase, it does exhibit a prominent extension of filopodia following interaction with lung cells (2, 28). To evaluate whether PCSTE20 could function in signaling morphology changes, we investigated the ability of PCSTE20 to induce pseudohyphal growth in an S. cerevisiae mutant strain deficient in endogenous STE20 kinase activity (20). To test this, an S. cerevisiae ste20Δ mutant (L5624) was transformed with either vector alone or PCSTE20 and cultured on SLAG medium, which induces pseudohyphal growth of the wild-type strain (L5366) (Fig. 6). PCSTE20, but not vector alone, fully restored pseudohyphal morphology in the ste20-deficient yeast strain.
FIG. 6.
PCSTE20 restores pseudohyphal growth in an S. cerevisiae ste20Δ strain. (A and B) Sterile diploid ste20Δ/ste20Δ S. cerevisiae (strain L5624) was transformed with either empty pYES2.1 plasmid (A) or plasmid containing PCSTE20 (B) and cultured on SLAG medium, which induces pseudohyphal growth. PCSTE20, but not vector alone, restored pseudohyphal growth. (C) As a positive control, wild-type S. cerevisiae (STE20+, strain 5366) containing the empty pYES2.1 vector demonstrated comparable growth characteristics on SLAG medium. (D) As another positive control, the diploid ste20Δ/ste20Δ strain L5624 was transformed with the wild-type S. cerevisiae STE20 gene on plasmid B2585 (S. cerevisiae STE20 CEN URA3 AmpR). (E) Organisms sampled from the leading edge of colonies containing sterile diploid ste20Δ/ste20Δ S. cerevisiae (strain L5624) transformed empty pYES2.1 plasmid (as in panel A) and demonstrate typical round yeast morphology when cultured on SLAG medium and viewed under a phase-contrast microscope. (F) However, Ste20 mutant yeast organisms sampled from the leading edges of colonies complemented with PCSTE20 (as in panel B) demonstrate typical pseudohyphal morphology when viewed under a phase-contrast microscope. Panels E and F are photomicrographic images of organisms sampled near the leading edge of these colonies, suspended in phosphate-buffered saline, spread onto glass slides, and imaged by phase-contrast microscopy. Magnification, ×100.
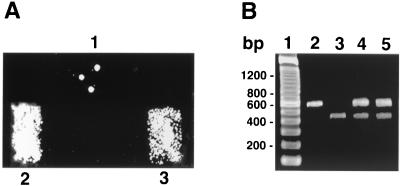
Recent studies demonstrating the presence of a putative mating locus and related genes in P. carinii have suggested the existence of a pheromone-driven mating pathway in this organism, though direct proof of mating remains elusive (40, 42). Ste20 kinases also have been implicated in, among other activities, mating signaling in fungi (15, 20, 43). To address the potential role of PCSTE20 in mating, we expressed PCSTE20 in the sterile S. cerevisiae ste20Δ strain. The ste20 mutant strain JKY40 (MATa) was transformed with either vector alone or PCSTE20 and mated with the MATα tester strain JBY311 by using the replica patch method (Fig. 7). Diploids were selected by using media lacking leucine, uracil, and tryptophan. Replica patch mating performed with vector alone yielded only rare colonies, due to the slight leakiness in the sterility phenotype of the parental S. cerevisiae ste20Δ strain (20, 24). In contrast, transfection with the Pneumocystis gene PCSTE20 conferred abundant mating in this previously sterile mutant yeast. Multiplex PCR of the MATa (or MATα) loci further confirmed successful mating in the replica patch mating assays (Fig. 7).
FIG. 7.
PCSTE20 suppresses mating defect in an S. cerevisiae ste20Δ strain. To address the potential role of PCSTE20 in mating, we employed heterologous expression in an S. cerevisiae ste20Δ (sterile) strain. (A) The ste20Δ strain JKY40 (MATa) was transformed with either vector alone (patch 1) or PCSTE20 in pYES2.1 vector (patch 3) and mated with the MATα tester strain JBY311, with diploids selected by using galactose medium lacking leucine, uracil, and tryptophan. Replica patch mating performed in the presence of vector alone yielded only rare colonies, representing slight sterility phenotype leakiness of the parental ste20Δ strain. In contrast, PCSTE20 strongly confers efficient mating in the S. cerevisiae ste20Δ strain (patch 3). As a positive control (patch 2), the sterile ste20Δ strain JKY40 was transformed with wild-type S. cerevisiae STE20 on plasmid B2585 (S. cerevisiae STE20 CEN URA3 AmpR), which also permitted efficient diploid formation. (B) Multiplex PCR of the MATa (or MATα) loci confirmed successful mating. Lane 1, molecular weight markers (100-bp ladder). Lane 2, JKY40 MATa strain yielding a 544-bp product. Lane 3, JBY311 MATα strain (MATα lys9 ura3-52) yielding a 404-bp product. Lane 4, diploid colonies from strain JKY84 mated with JBY311 revealing both the MATa and MATα products. Lane 5, diploid colonies from JKY40 transformed with PCSTE20 and mated with JBY311 also revealing both the MATa and MATα loci.
Quantitative mating assays were further performed to determine the efficiency of diploid formation in the presence of the Pneumocystis gene PCSTE20 (43). The frequency of diploid formation after 4-h matings of the JKY40 strain transformed with PCSTE20 was 46.7%. In contrast, matings of the JKY40 strain transformed with pYES2.1 vector alone yielded only 1.4% diploids. For comparison, the diploid formation frequency of the JKY48 strain (S. cerevisiae ste20Δ mutant) restored with wild-type S. cerevisiae STE20 was 53.1%. Thus, PCSTE20 permits efficient mating comparable to that of the wild-type S. cerevisiae STE20 gene when heterologously expressed in the otherwise sterile ste20Δ yeast strain.
DISCUSSION
P. carinii exhibits a profound tropism for the lower respiratory tract, representing the preferred biological niche for this important opportunistic pathogen. P. carinii organisms tightly attach to lung epithelial cells by closely apposing their filopodial membranes with those of host cells without fusion or phagocytosis (2, 28, 31). The functional consequences of these interactions have long been elusive. Consistent growth of the organism has never been demonstrated outside the lung, and continuous axenic culture of P. carinii in defined media has not been established (39). However, self-limited proliferation of P. carinii has been shown to occur when Pneumocystis organisms attach to cultured lung epithelial cells (5, 30). The present study advances our understanding of the host-organism relationship in this important infection. Using a global genetic strategy, we demonstrated that PCSTE20 and PCMAPK, signaling molecules that participate in fungal mating pathways and morphology changes, are strongly induced by adherence of P. carinii organisms either to alveolar epithelial cells or to mammalian ECM proteins including fibronectin, vitronectin, and collagen.
Pneumocystis species have evolved redundant mechanisms to ensure stable interactions with the lung epithelium. P. carinii binds to fibronectin through its mannose-rich major surface glycoprotein complex, termed gpA (36). In contrast, vitronectin interacts with high-molecular-weight components of the organism's surface through the glycosaminoglycan-binding region of this matrix molecule (29). Both fibronectin and vitronectin strongly facilitate P. carinii adherence to cultured epithelial cells and immobilized matrix (29, 36). Attachment of P. carinii to matrix in the absence of feeder cells does not promote proliferation in culture. Additional investigations suggest that αv, b1, and α3 integrin receptors, present on lung epithelial cells, facilitate P. carinii attachment (37).
The induction of PCSTE20 in response to mammalian ECM components and lung epithelial cells may be a unique response of this organism to its environment. Interestingly, culture of the nonpathogenic yeast S. cerevisiae on purified matrix components such as fibronectin or collagen or on lung epithelial cells failed to cause any STE20 induction in that species (data not shown). The reasons why P. carinii has evolved this response to express PCSTE20 and PCMAPK in response to mammalian ECM components and epithelial cells are not fully known. One may speculate that recognition of ECM molecules, such as fibronectin and vitronectin, and epithelial cells, such as those found in lung, may be one of several means that the organism uses to determine that it is within an environment conducive for a variety of processes cognate to its life cycle and the establishment of infection, including alterations in shape and mating. These signals at the epithelial surface may be integrated with other environmental information, including pH and perhaps oxygen tension, to determine that conditions are ideal for metabolism and proliferation of the organism (22).
Ste20 protein kinases are active in a variety of signaling pathways in yeast species. These include maintenance of cell wall structure, in addition to morphology alteration and mating pathways (10, 20, 25, 33, 38). We focused our studies on two of these important activities. Pseudohyphal growth was studied as a prototypical signaling pathway associated with changes in organism morphology. Although P. carinii exhibits neither a true hyphal nor a true pseudohyphal phase, it does exhibit profound shape alterations characterized by filopodial extensions following binding to the host (2, 30, 31). Heterologous expression of PCSTE20 in an S. cerevisiae ste20Δ strain conferred pseudohyphal growth. These heterologous expression studies support our postulate that the morphological changes of P. carinii following epithelial binding may represent such a homologous morphological change. Furthermore, we provide the first functional evidence that PCSTE20 can function in mating signaling pathways when heterologously expressed in S. cerevisiae. Direct assessment of these activities in P. carinii will await availability of methods to culture, transform, or disrupt gene function in this organism.
The formation of pseudohyphae by Saccharomyces and other fungi results in filamentous structures comprised of multiple cells divided by septa, a process requiring remodeling of the fungal cell wall (10-12). In contrast, P. carinii trophic forms extend filopodial projections to appose their cell membranes directly to the membranes of host epithelial cells. Cystic forms may also be associated with the alveolar epithelium, but they lack such filopodial extensions. The formation of filopodia by P. carinii is likely mediated by rearrangement of the actin cytoskeleton (28). While it is clear from our experiments that PCSTE20 can complement the functional defects in pseudohyphal growth in an ste20 mutant yeast strain, the exact role of PCSTE20 in mediating morphology changes within P. carinii cannot presently be determined.
Most available evidence indicates that adherence of P. carinii to the alveolar epithelium benefits the organisms through enhancement of growth. However, the interaction is not without deleterious consequences to the host (26, 28, 30). Epithelial cells with adherent P. carinii appear vacuolated and exhibit abnormal electrical potential gradients (1, 31). Furthermore, the adherence of P. carinii to cultured epithelial cells reduces the ability of these cells to replicate, thereby slowing repair of damaged alveolar units during P. carinii pneumonia (26, 28).
Although Pneumocystis organisms observed in animals and humans with pneumonia demonstrate characteristic interactions with alveolar epithelial cells, many organisms are found within alveolar spaces, unattached to epithelium but enmeshed in proteinaceous exudates. These exudates contain substantial quantities of fibronectin, vitronectin, and the collectin (collagen-lectin) surfactant proteins SP-A and SP-D. It is plausible that these exudates may therefore further stimulate PCSTE20 expression in P. carinii organisms that are not physically attached to alveolar epithelium. Additional studies will be needed to determine the regional expression of PCSTE20 in P. carinii organisms present in the alveolar spaces during pneumonia.
In summary, the present study demonstrates that PCSTE20 is strongly and specifically expressed along with the mating pathway gene MAPK following interactions of P. carinii trophic forms with alveolar epithelial cells and matrix proteins. Expression of PCSTE20 in ste20-deficient yeast strains indicates that this protein can function in signaling pathways mediating morphological changes and mating. The induced expression of PCSTE20 following contact of the organism with lung epithelial cells is consistent with the proliferation and filopodial extension of Pneumocystis organisms consistently observed in the lungs of immunosuppressed hosts. Additional characterization of Ste20 kinase activity in P. carinii should provide important functional correlates to enhance our evolving understanding of life cycle regulation in this intractable opportunistic fungal pathogen.
Acknowledgments
This work was supported by NIH grants R01-HL55934, R01-HL57125, and R01-HL62150 to A.H.L.
We thank Melanie T. Cushion, University of Cincinnati College of Medicine, for providing the contour-clamped homogeneous electric field nitrocellulose membrane and the corresponding ethidium bromide-stained gel photograph. We also thank Joseph Standing for assistance in generating P. carinii and Kathy Streich for assistance during the final preparation of the manuscript.
Editor: T. R. Kozel
REFERENCES
- 1.Beck, J. M., A. M. Preston, J. G. Wagner, S. E. Wilcoxen, P. Hossler, S. R. Meshnick, and R. Paine. 1998. Interaction of rat Pneumocystis carinii and rat alveolar epithelial cells. Am. J. Physiol. 275:L118-L125. [DOI] [PubMed] [Google Scholar]
- 2.Campbell, W. G. 1972. Ultrastructure of Pneumocystis in lung. Arch. Pathol. 93:312-330. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 1999. CDC surveillance summaries, surveillance for AIDS-defining opportunistic illnesses, 1992-1997. Morb. Mortal. Wkly. Rep. 48(SS2):1-22. [PubMed] [Google Scholar]
- 4.Cushion, M. T., S. Orr, S. P. Keely, and J. R. Stringer. 2001. Time between inoculations and karyotype forms of Pneumocystis carinii f. sp. carinii influence outcome of experimental coinfections in rats. Infect. Immun. 69:97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cushion, M. T., and P. D. Walzer. 1984. Growth and serial passage of Pneumocystis carinii in the A549 cell line. Infect. Immun. 44:245-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Durkin, M. E., M. M. Shaw, M. S. Bartlett, and J. W. Smith. 1991. Culture and filtration methods for obtaining Pneumocystis trophozoites and cysts. J. Protozool. 38:210-212. [PubMed] [Google Scholar]
- 7.Elion, E. A., P. L. Grisafi, and G. R. Fink. 1990. FUS3 encodes a cdc2+/CDC28-related kinase required for the transition from mitosis into conjugation. Cell 60:649-664. [DOI] [PubMed] [Google Scholar]
- 8.Fox, D., and A. G. Smulian. 1999. Mitogen-activated protein kinase Mkp1 of Pneumocystis carinii complements the slt2Delta defect in the cell integrity pathway of Saccharomyces cerevisiae. Mol. Microbiol. 34:451-462. [DOI] [PubMed] [Google Scholar]
- 9.Fox, D., and A. G. Smulian. 2000. Mkp1 of Pneumocystis carinii associates with the yeast transcription factor Rlm1 via a mechanism independent of the activation state. Cell. Signal. 12:381-390. [DOI] [PubMed] [Google Scholar]
- 10.Fujita, A., A. Tonouchi, T. Hiroko, F. Inose, T. Nagashima, R. Satoh, and S. Tanaka. 1999. Hsl7p, a negative regulator of Ste20p protein kinase in the Saccharomyces cerevisiae filamentous growth-signaling pathway. Proc. Natl. Acad. Sci. USA 96:8522-8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gale, C. A., C. M. Bendel, M. McClellan, M. Hauser, J. M. Becker, J. Berman, and M. K. Hostetter. 1998. Linkage of adhesion, filamentous growth, and virulence in Candida albicans to a single gene, INT1. Science 279:1355-1358. [DOI] [PubMed] [Google Scholar]
- 12.Gimeno, C. J., P. O. Ljungdahl, C. A. Styles, and G. R. Fink. 1992. Unipolar cell divisions in the yeast S. cerevisiae lead to filamentous growth: regulation by starvation and RAS. Cell 68:1077-1090. [DOI] [PubMed] [Google Scholar]
- 13.Gustafson, M. P., C. F. Thomas, F. Rusnak, A. H. Limper, and E. B. Leof. 2001. Differential regulation of growth and checkpoint control mediated by a Cdc25 mitotic phosphatase from Pneumocystis carinii. J. Biol. Chem. 276:835-843. [DOI] [PubMed] [Google Scholar]
- 14.Heinrich, T., S. Washer, J. Marshall, M. G. Jones, and R. H. Potter. 1997. Subtractive hybridization of cDNA from small amounts of plant tissue. Mol. Biotechnol. 8:7-12. [DOI] [PubMed] [Google Scholar]
- 15.Hilti, N., D. Baumann, A. M. Schweingruber, P. Bigler, and M. E. Schweingruber. 1999. Gene ste20 controls amiloride sensitivity and fertility in Schizosaccharomyces pombe. Curr. Genet. 35:585-592. [DOI] [PubMed] [Google Scholar]
- 16.Hostetter, M. K. 1999. Integrin-like proteins in Candida spp. and other microorganisms. Fungal Genet. Biol. 28:135-145. [DOI] [PubMed] [Google Scholar]
- 17.Huxley, C., E. D. Green, and I. Dunham. 1990. Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet. 6:236-237. [DOI] [PubMed] [Google Scholar]
- 18.Itatani, C. A. 1996. Ultrastructural morphology of intermediate forms and forms suggestive of conjugation in the life cycle of Pneumocystis carinii. J. Parasitol. 82:163-171. [PubMed] [Google Scholar]
- 19.Kaplan, J. E., D. L. Hanson, J. L. Jones, C. B. Beard, D. D. Juranek, and C. A. Dykewicz. 1998. Opportunistic infections (OIs) as emerging infectious diseases: challenges posed by OIs in the 1990s and beyond, p. 257-272. In W. M. Sheld, W. A. Craig, and J. M. Hughes (ed.), Emerging infections, vol. 2. ASM Press, Washington, D.C.
- 20.Köhler, J. R., and G. R. Fink. 1996. Candida albicans strains heterozygous and homozygous for mutations in mitogen-activated protein kinase signaling components have defects in hyphal development. Proc. Natl. Acad. Sci. USA 3:13223-13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kottom, T. J., and A. H. Limper. 2000. Cell wall assembly by Pneumocystis carinii: evidence for a unique Gsc-1 subunit mediating β-1,3-glucan deposition. J. Biol. Chem. 275:40628-40634. [DOI] [PubMed] [Google Scholar]
- 22.Kottom, T. J., C. F. Thomas, Jr., and A. H. Limper. 2001. Characterization of Pneumocystis carinii PHR1, a pH-regulated gene important for fungal cell wall integrity. J. Bacteriol. 183:6740-6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kottom, T. J., C. F. Thomas, Jr., K. K. Mubarak, E. B. Leof, and A. H. Limper. 2000. Pneumocystis carinii utilizes a functional cdc13 B-type cyclin complex during its life cycle. Am. J. Respir. Cell Mol. Biol. 22:722-731. [DOI] [PubMed] [Google Scholar]
- 24.Leberer, E., D. Dignard, D. Harcus, D. Y. Thomas, and M. Whiteway. 1992. The protein kinase homologue Ste20p is required to link the yeast pheromone response G-protein beta gamma subunits to downstream signalling components. EMBO J. 11:4815-4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lengeler, K. B., P. Wang, G. M. Cox, J. R. Perfect, and J. Heitman. 2000. Identification of the MATa mating-type locus of Cryptococcus neoformans reveals a serotype A MATa strain thought to have been extinct. Proc. Natl. Acad. Sci. USA 97:14455-14460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Limper, A. H., M. Edens, R. A. Anders, and E. B. Leof. 1998. Pneumocystis carinii inhibits cyclin-dependent kinase activity in lung epithelial cells. J. Clin. Investig. 101:1148-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limper, A. H., J. S. Hoyte, and J. E. Standing. 1997. Alveolar macrophages mediate Pneumocystis carinii degradation and organism clearance from the lung. J. Clin. Investig. 99:2110-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limper, A. H., and W. J. Martin II. 1990. Pneumocystis carinii inhibition of lung cell growth mediated by parasite attachment. J. Clin. Investig. 85:391-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Limper, A. H., J. E. Standing, O. A. Hoffman, M. Castro, and L. W. Neese. 1993. Vitronectin binds to Pneumocystis carinii and mediates organism attachment to cultured lung epithelial cells. Infect. Immun. 61:4302-4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Limper, A. H., C. F. Thomas, Jr., R. A. Anders, and E. B. Leof. 1997. Interactions of parasite and host epithelial cell cycle regulation during Pneumocystis carinii pneumonia. J. Lab. Clin. Med. 130:132-138. [DOI] [PubMed] [Google Scholar]
- 31.Long, E. G., J. S. Smith, and J. L. Meier. 1986. Attachment of Pneumocystis carinii to rat pneumocytes. Lab. Investig. 54:609-615. [PubMed] [Google Scholar]
- 32.Merali, S., U. Frevert, H. Williams, K. Chin, R. Bryan, and A. B. Clarkson, Jr. 1999. Continuous axenic cultivation of Pneumocystis carinii. Proc. Natl. Acad. Sci. USA 96:2402-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosch, H. U., and G. R. Fink. 1997. Dissection of filamentous growth by transposon mutagenesis in Saccharomyces cerevisiae. Genetics 145:671-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narasimhan, S., M. Y. Armstrong, K. Rhee, J. C. Edman, F. F. Richards, and E. Spicer. 1994. Gene for an extracellular matrix receptor protein from Pneumocystis carinii. Proc. Natl. Acad. Sci. USA 91:7440-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson, E. J., J. E. Standing, N. Griego-Harper, O. A. Hoffman, and A. H. Limper. 1996. Fungal β-glucan interacts with vitronectin and stimulates tumor necrosis factor alpha release from macrophages. Infect. Immun. 64:3548-3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pottratz, S. T., J. Paulsrud, J. S. Smith, and W. J. Martin II. 1991. Pneumocystis carinii attachment to cultured lung cells by Pneumocystis gp120, a fibronectin binding protein. J. Clin. Investig. 88:403-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pottratz, S. T., A. L. Weir, and P. E. Wisniowski. 1994. Pneumocystis carinii attachment increases expression of fibronectin-binding integrins on cultured lung cells. Infect. Immun. 62:5464-5469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raitt, D. C., F. Posas, and H. Saito. 2000. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 19:4623-4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sloand, E., B. Laughon, M. Armstrong, M. S. Bartlett, W. Blumenfeld, M. Cushion, A. Kalica, J. A. Kovacs, W. J. Martin, and E. Pitt. 1993. The challenge of Pneumocystis carinii culture. J. Eukaryot. Microbiol. 40:188-195. [DOI] [PubMed] [Google Scholar]
- 40.Smulian, A. G., T. Sesterhenn, R. Tanaka, and M. T. Cushion. 2001. The ste3 pheromone receptor gene of Pneumocystis carinii is surrounded by a cluster of signal transduction genes. Genetics 157:991-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas, C. F., R. A. Anders, M. P. Gustafson, E. B. Leof, and A. H. Limper. 1998. Pneumocystis carinii contains a functional cell-division-cycle Cdc2 homologue. Am. J. Respir. Cell Mol. Biol. 18:297-306. [DOI] [PubMed] [Google Scholar]
- 42.Thomas, C. F., Jr., T. J. Kottom, E. B. Leof, and A. H. Limper. 1998. Characterization of a mitogen activated protein kinase from Pneumocystis carinii. Am. J. Physiol. Lung Cell Mol. Physiol. 275:L193-L199. [DOI] [PubMed] [Google Scholar]
- 43.Trueheart, J., J. D. Boeke, and G. R. Fink. 1987. Two genes required for cell fusion during yeast conjugation: evidence for a pheromone-induced surface protein. Mol. Cell. Biol. 7:2316-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wisniowski, P., and W. J. Martin II. 1995. Interaction of vitronectin with Pneumocystis carinii: evidence for binding via the heparin-binding domain. J. Lab. Clin. Med. 125:38-45. [PubMed] [Google Scholar]