Abstract
To study telomere length dynamics in hematopoietic cells with age, we analyzed the average length of telomere repeat sequences in diverse populations of nucleated blood cells. More than 500 individuals ranging in age from 0 to 90 yr, including 36 pairs of monozygous and dizygotic twins, were analyzed using quantitative fluorescence in situ hybridization and flow cytometry. Granulocytes and naive T cells showed a parallel biphasic decline in telomere length with age that most likely reflected accumulated cell divisions in the common precursors of both cell types: hematopoietic stem cells. Telomere loss was very rapid in the first year, and continued for more than eight decades at a 30-fold lower rate. Memory T cells also showed an initial rapid decline in telomere length with age. However, in contrast to naive T cells, this decline continued for several years, and in older individuals lymphocytes typically had shorter telomeres than did granulocytes. Our findings point to a dramatic decline in stem cell turnover in early childhood and support the notion that cell divisions in hematopoietic stem cells and T cells result in loss of telomeric DNA.
Keywords: telomere length dynamics, telomere length inheritance, cellular turnover, immune senescence, stem cell self-renewal
Most mature blood cells have a limited life span. The maintenance of adequate numbers of such cells requires their continuous production, which in adults may exceed 1012 cells per day. This impressive production of cells (∼4.1015 cells over a lifetime) is achieved by the proliferation and differentiation of committed progenitor cells which themselves are derived from a population of pluripotent stem cells. It is generally believed that hematopoietic stem cells have “self-renewal” properties to sustain life-long hematopoiesis. However, many questions about the actual number, phenotype, turnover, and regenerative potential of hematopoietic stem cells remain unanswered. One possibility is that stem cells have a limited replicative potential like most other somatic cells. This hypothesis is supported by the consideration that, in theory, only 52 divisions are enough to yield 4.1015 cells and observations in three areas 1. First, it was shown that “candidate” stem cells purified from fetal, neonatal, and adult tissue display dramatic differences in their ability to produce CD34+ progenitor cells 2. Second, these differences in “expansion potential” were found to correlate with a measurable loss of telomere repeats at the ends of chromosomes 3. Third, it was found that telomeres in blood cells of bone marrow transplant recipients are shorter than those in cells from the marrow donor 4 5 6, suggesting that the additional cell divisions in the stem cell compartment (required for marrow regeneration) result in a measurable decline in telomere length.
Questions about the number of times cells can and do divide are also pertinent in studies of the immune system. Effective immune responses may require repeated expansion of a limited number of antigen-specific T lymphocytes, and limitations in the replicative potential of such cells could eventually compromise immune function. All T cells are derived from hematopoietic stem cells via selection and expansion in the thymus 7. Upon antigen encounter, naive CD4+ and CD8+ T cells are activated to proliferate and generate effector as well as memory T cells that can be distinguished from naive T cells by their membrane marker phenotype and other functional characteristics 8 9. Although it is well accepted that memory or primed T cells arise from naive or unprimed precursor cells, the interrelationship and relative life span of these types of T lymphocytes is controversial. The life span of naive T cells seems to exceed that of memory T cells 10 11, raising questions about how immunological memory is maintained. Recently, it was found that although thymic function decreases with age, substantial output is maintained into late adulthood 12.
Several previous studies have shown that the length of telomeres in blood cells decreases with age 13 14 15 16 17 18 19. In normal diploid fibroblasts 13 20, T lymphocytes 16, and hematopoietic cells 3, the average telomere length decreases with each cell division in vitro. These observations suggest that telomere length could possibly be used as an indicator of cell divisions in vivo. However, most of these studies have been focused on the decline that begins in early adult life and progresses gradually with advancing age, and very little information is available on telomere length dynamics in early childhood. Furthermore, none of these previous studies have adequately addressed questions about the length of telomeres in subpopulations of leukocytes from individual donors, (e.g., granulocytes and T lymphocyte subpopulations). Different individuals of a given age may show marked genetic variation in telomere length 21. Questions about the dynamics of telomere length in relation to age should therefore ideally be addressed in longitudinal studies of the same individuals. Time constraints limit this approach. Alternatively, to compensate for the variation at any given age, the telomere length dynamics in cells could be studied in a large number of individuals. This approach has been hampered by the laborious nature of conventional telomere length analysis by Southern blot analysis. We recently described the development of fluorescence in situ hybridization (FISH)1 techniques to measure the average telomere length in cells by flow cytometry 22. Through this approach, a large number of samples can be processed in a single day with the additional advantage that different subpopulations of blood cells can be analyzed separately.
Here we report results of flow FISH measurements in granulocytes and lymphocytes as well as T lymphocyte subsets from peripheral blood samples of >500 individuals ranging in age from 0 to 90 yr. Both granulocytes and total lymphocytes, as well as subpopulations of CD4+ and CD8+ T cells, showed a biphasic decline in telomere length that was rapid during early childhood and more gradual, although still highly significant, thereafter. These results point to an unexpected, age-related physiological change in the turnover of hematopoietic stem cells soon after birth. The data are compatible with a finite replicative life span of stem cells and rapid expansion of the number of stem cells early in life followed by a marked decrease in the rate of stem cell divisions in the years that follow.
Materials and Methods
Human Subjects.
For this study, leftover EDTA blood samples (0.5–5 ml) after coagulation tests and heparinized blood samples from 436 individuals (0-90 yr of age) were obtained. Patients with known malignant, immunological, and infectious diseases were excluded and all patient information except sex and date of birth were removed from the samples before shipment to the Terry Fox Laboratory. In addition, heparinized blood samples from 17 pairs of monozygous (MZ) and 19 pairs of dizygotic (DZ) elderly (mean age of 74 yr) twins from the Longitudinal Study of Aging Danish Twins (LSADT) were also included. The blood samples were given a numerical code and sent by overnight courier from Denmark to Vancouver. The code was broken after results of flow FISH analysis were sent back to Denmark.
Isolation of Leukocytes and T Lymphocyte Subpopulations.
Peripheral blood leukocytes were obtained after osmotic lysis of red cells using ammonium chloride. Cells were washed with PBS containing 0.1% BSA (Calbiochem Corp.). After hybridization, two populations were observed by light scatter. Cells with high light scatter signals were mainly granulocytes but also included monocytes. No attempt was made to distinguish between the two cell types, which are referred to in the text as granulocytes. For the preparation of lymphocyte subpopulations, PBMCs were obtained after density centrifugation using Ficoll-Hypaque (Ficoll-Hypaque; Amersham Pharmacia Biotech). Typically, 2 × 106 PBMCs were stained with the following antibodies: allophycocyanin-labeled antibodies to CD4 (CD4-allophycocyanin; Becton Dickinson), CD45RO-FITC (PharMingen), and CD45RA-PE 3, or CD8-APC (PharMingen), CD27-FITC (PharMingen), and CD45RA-PE. CD4+CD45RA+ CD45RO−, CD4+CD45RA−CD45RO+, CD8+CD45RA+ CD27+, CD8+CD45RA+CD27−, and CD8+CD45RA−CD27+ were then sorted by a FACS® (FACStarPLUS ™; Becton Dickinson) and stimulated in RPMI 1640 medium (GIBCO BRL) containing 10% (vol/vol) human serum supplemented with 1.0 μg/ml of phytohemagglutinin PHA (Murex Diagnostics), 100 U/ml of rIL-2 (Roche), and 106/ml irradiated allogeneic mononuclear cells. Typically, 1–5 × 105 cells were sorted. The sorted cells were cultured for 10–15 d as described above in order to obtain sufficient numbers of cells (∼106 cells) for the analysis of telomere fluorescence. The telomere fluorescence in T cells derived from cultures initiated with 2,000, 5,000, 10,000, 20,000, or 50,000 sorted CD4+CD45RA+ or CD4+CD45RO+ T cells was very similar and directly comparable to freshly isolated CD4+CD45RA+ or CD4+CD45RO+ T lymphocytes (data not shown).
Telomere Fluorescence In Situ Hybridization and Flow Cytometry (Flow FISH).
The average length of telomere repeats at chromosome ends in individual cells was measured by flow FISH 22 using the following minor modifications. To correct for daily shifts in the linearity of the flow cytometer, fluctuations in the laser intensity, and alignment, and to allow expression of results in standard fluorescence units, FITC-labeled fluorescent calibration beads (Quantum™-24 Premixed; Flow Cytometry Standards Corp.) were used. At the beginning and the end of each experiment, the fluorescence signals from calibration beads suspended in PBS/0.1% BSA were acquired. The bead solution contains four populations ranging from 3,000 to 50,000 MESF (molecules of equivalent soluble fluorochrome) units 23 as well as nonfluorescent beads. Voltage and amplification of the FL1 parameter were set in such a way that blank, 5,579, 15,842 and 36,990 MESF units of microbeads gave 25, 162, 456, and 942 FL1 channels on a linear scale, respectively. The resulting calibration curve (y = 0.02604 x) was then used to convert telomere fluorescence data to MESF units (×10−3), allowing comparison of results among experiments. To estimate the telomere length (in bp) from telomere fluorescence in MESF units, the slope of the calibration curve previously described for lymphocyte subset 22 (y = 0.019 x) was used in the following equation (bp = MESF units × 0.02604 × 0.019 × 103). To verify whether the same slope could be used for the analysis of the telomere fluorescence values obtained in the granulocyte subset, 15 granulocyte and lymphocyte subpopulations were further isolated and characterized separately by flow FISH and Southern blot 13. The telomere fluorescence of granulocyte and lymphocyte subsets was proportional to the mean size of terminal restriction fragments of DNA from the same cells. Furthermore, the slopes of the calibration curve obtained with purified granulocytes and lymphocytes were very similar to each other as well as to the previously described calibration curve 22. To analyze the day to day variation in flow FISH results, aliquots of the same frozen lymphoma cells 22 were analyzed in each experiment over the 6-mo period of the studies described in this paper. In 38 experiments, the mean (± SD) fluorescent value was 12,150 ± 1,840 MESF units with a variation coefficient (CV) of 15%. The method-related variation in the telomere fluorescence that was measured in blood cells may exceed the variation in these control cells. Apart from variations in the time between sample collection and analysis (which may have affected the viability and fluorescence of granulocytes), it is possible (but unlikely) that in some of the samples abnormal cells were included in the analysis. In view of the large number of samples analyzed in our study, individual aberrant data points are not expected to impact significantly on results or conclusions.
Statistical Analysis.
Linear and nonlinear least squares regression techniques were applied to analyze the relationship between telomere fluorescence and age for the different cell groups. Goodness-of-fit tests were carried out to compare various models using the extra sum of squares principle 24. Analysis of covariance methods were used to study the effect of gender on the regressions on age. When pairs of dependent data sets were compared in terms of their relationship to age, bivariate regression techniques were applied as well as corresponding regressions on age of the pairwise differences in telomere fluorescence. Bisegmental linear fits to the data were calculated by placing a fine grid over the fluorescence-by-age data plane, and by computation of an optimal point and an optimal pair of lines passing through that point so as to minimize the total residual sums of squares.
Results
Telomere Fluorescence Analysis of Leukocyte Subpopulations.
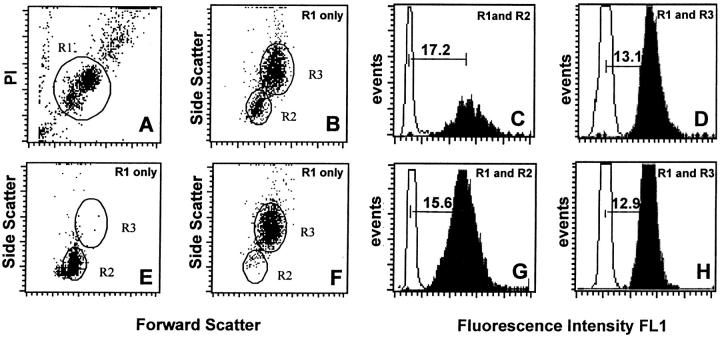
Peripheral blood leukocytes were analyzed using flow cytometry after fluorescence in situ hybridization with labeled telomere probes as previously described 22. Selected windows were used to allow analysis of granulocytes and lymphocytes as single cells from the same blood sample (Fig. 1A and Fig. B). For each cell subpopulation, the specific telomere fluorescence was calculated by subtracting the mean fluorescence of the background control (no probe) from the mean fluorescence obtained from cells hybridized with the telomere probe (Fig. 1C, Fig. D, Fig. G, and Fig. H, horizontal bars). The separation between lymphoid cells and granulocytes/monocytes on the basis of scatter properties was validated in experiments with purified cell suspensions (Fig. 1E, Fig. F, Fig. G, and Fig. H). The telomere fluorescence in both purified cell fractions was proportional to the mean size of terminal restriction fragments measured by Southern blot analysis (slopes and correlation coefficients similar to those previously reported in reference 22; results not shown).
Figure 1.
Flow FISH analysis of normal human peripheral blood cells. Nucleated cells after lysis of red blood cells from a healthy 32-yr-old donor were analyzed after hybridization with or without FITC-labeled (C3TA2)3 peptide nucleic acid (shaded and open histograms, respectively, in C, D, G, and H). The cells were gated on region 1 (R1) on the basis of propidium iodide (PI) fluorescence and forward light scatter (FSC) as is shown in A. Regions 2 and 3 (R2 and R3) were selected within R1 from forward versus side scatter (SCC) dot plot histograms as is shown in B, E, and F. Results with purified lymphocytes (E and G) and granulocytes (F and H) confirmed the validity of selected light scatter gates R2 and R3 for analysis of unseparated blood cells (B). All parameters were collected on a linear scale. Green fluorescence is expressed in MESF units using calibration beads analyzed at identical instrument settings at the beginning and end of each experiment (data not shown). Note that (i) the fluorescence of gated and purified cells is similar, (ii) granulocytes have a higher autofluorescence than lymphocytes, and (iii) the telomere fluorescence of lymphocytes (C and G) is more heterogeneous than that of granulocytes (D and H), reflecting a more diverse replicative history 22. The telomere fluorescence of cells was calculated by subtracting the mean background fluorescence from the mean fluorescence obtained with the telomere probe as is shown in C, D, G, and H.
Rate of Telomere Sequence Loss in Human Leukocyte Subpopulations Varies with Age.
The rate of telomere shortening in granulocytes and lymphocytes from 301 individuals ranging in age from 0 to 90 yr was analyzed by linear regression. As shown in Fig. 2, the mean telomere fluorescence declined in both granulocytes and total lymphocytes with age. The loss in telomere fluorescence in granulocytes corresponded to 39 bp per year (R = −0.52, P < 0.0001), and in lymphocytes to 59 bp per year (R = −0.74, P < 0.0001; Table , top). The average telomere fluorescence values in both cell populations were slightly higher in female than in male donors; however, this difference did not reach statistical significance (results not shown).
Figure 2.
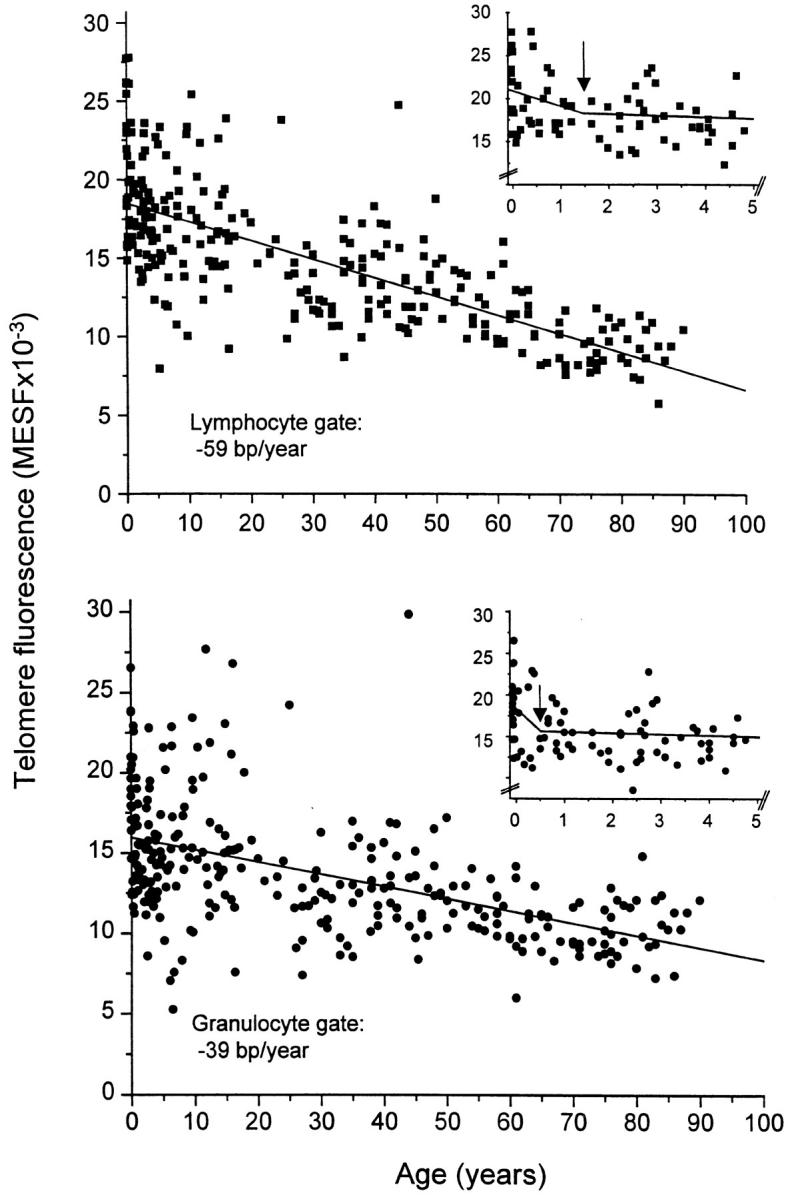
Loss of telomere fluorescence in lymphocytes and granulocytes from peripheral blood measured by flow FISH. The specific telomere fluorescence of lymphocytes and granulocytes was analyzed after gating on these cells as is shown in Fig. 1. Note the heterogeneity in telomere fluorescence values, the overall decline in telomere fluorescence in both cell types and the higher rate of telomere decline in lymphocytes. Insets show the results of bisegmented fit analysis for cells in the lymphocyte (top) and granulocyte (bottom) gate. Arrows indicate the optimal intersection of calculated regression lines (see also text and Table ).
Table 1.
Loss of Telomere Fluorescence with Age in Blood Cells
| Subset | Age range | n | y intercept (MESF × 10−3) | Slope (MESF × 10−3/yr) | Slope(bp/yr) | R value |
|---|---|---|---|---|---|---|
| Linear regression analysis | ||||||
| yr | ||||||
| TL | 0–90 | 284 | 18.48 | −0.119 | −59 | −0.74 |
| G | 292 | 15.96 | −0.079 | −39 | −0.52 | |
| TL | 0–4 | 67 | 20.34 | −0.923 | −457 | −0.34 |
| G | 69 | 17.43 | −0.977 | −483 | −0.34 | |
| TL | 4–90 | 217 | 17.73 | −0.105 | −52 | −0.69 |
| G | 223 | 15.73 | −0.072 | −36 | −0.48 | |
| Bisegmented line analysis | ||||||
| TL | 0–1.5 | 38 | 20.96 | −2.2 | −1,088 | |
| ≥1.5 | 246 | 17.82 | −0.106 | −52 | ||
| G | 0–0.5 | 23 | 18.59 | −6.168 | −3,052 | |
| ≥0.5 | 269 | 15.54 | −0.068 | −34 | ||
A more detailed analysis of the data shown in Fig. 2 revealed that both granulocytes and lymphocytes showed a rapid and significant decline in telomere length during the first years of life (Table , top). This observation suggested that a linear distribution of telomere fluorescence values over the whole age range did not adequately describe the telomere length dynamics in either population of cells. Although various models such as polynomial and logarithmic curve fitting were tested, only bisegmented line analysis increased the statistical significance over linear regression analysis (Table , bottom, and Fig. 2 insets). The optimal cut-off point for granulocytes was at 0.5 yr and for lymphocytes at 1.5 yr. In these time intervals both granulocytes and lymphocytes showed a remarkably rapid decline in telomere length, corresponding to 3,052 and 1,088 bp/yr, respectively, with a more gradual but still significant telomere loss thereafter (Table , bottom). Of note, the shape of individual telomere fluorescence histograms also showed age- and cell type–specific patterns (results not shown). In early childhood the CV in granulocytes and lymphocytes was ∼18%, whereas at >60 yr, lymphocytes showed a markedly higher CV (∼25%), which was not observed in granulocytes.
Analysis of Telomere Length in MZ and DZ Twins.
Considerable variation in telomere fluorescence between individuals, especially for granulocytes, was observed, with some young children having apparently fewer telomere repeats per cell than individuals over the age of 50 (Fig. 2). To further investigate the nature of this variation, we analyzed the correlation between telomere fluorescence values in granulocytes and lymphocytes in 36 pairs of MZ 17 and DZ 19 twins (mean age of 74 yr) as well as 17 pairs of unrelated individuals within the same age range. As shown in Table , the MZ correlations were very high and statistically significant for both granulocytes and lymphocytes and so was the DZ correlation for granulocytes. Only a moderate (and statistically insignificant) correlation was found for DZ lymphocyte telomere fluorescence. No significant correlations were found in such comparisons between the cells from unrelated individuals.
Table 2.
Correlation of Telomere Fluorescence Values in Granulocytes and Lymphocytes of Elderly MZ and DZ Twins and Paired Unrelated Individuals
| TL | G | |
|---|---|---|
| MZ | 0.71 | 0.64 |
| DZ | 0.18 | 0.59 |
| UR | −0.22 | −0.34 |
The telomere fluorescence in peripheral blood granulocytes (G) and total lymphocytes (TL) from 17 pairs of MZ twins, 19 pairs of DZ twins (mean age of 74 yr), and 17 pairs of unrelated (UR) age-matched control individuals was analyzed as described (Fig. 1). The intrapair resemblances for MZ and DZ twin pairs as well as age-matched pairs of unrelated individuals were estimated by intraclass correlation coefficients [r = 2cov/(var1 + var2)].
Because age effects can bias analyses of twin resemblance, telomere fluorescence values were adjusted for the effects of age by subtracting the age-specific telomere fluorescence value obtained from the regression analyses (Table ) from the actual fluorescence values found. However, such age adjustment did not change the results substantially (results not shown). Most likely the number of observations in our study was insufficient to establish a significant correlation between telomere fluorescence and age in individuals >70 yr of age (see Fig. 2). This consideration may also explain why no correlation was found in the age-matched unrelated pairs in this age group (Table ).
Age-related Changes in T Lymphocyte Subsets.
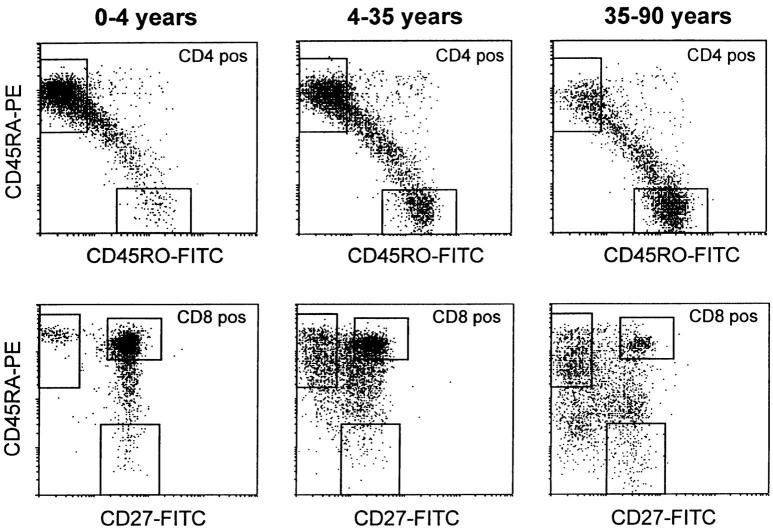
One of the most striking findings was that peripheral blood lymphocytes showed a more pronounced rate of telomere loss than did granulocytes (Fig. 2). Moreover, as shown in Fig. 1C and Fig. D, lymphocytes exhibited more heterogeneous telomere fluorescence signals than did granulocytes. A possible explanation for these observations is a progressive shift from naive to memory T cells with aging, together with the previously described difference in telomere length between these two subsets 22 25. To test this hypothesis, we sorted CD45RA+ naive and CD45RO+ memory CD4+ T lymphocytes as well as CD45RA+CD27+ naive, CD45RA+ CD27− effector, and CD45RA−CD27+ memory CD8+ T cells 9 from 135 individuals in the 0–90 yr age range (Fig. 3, with boxed areas corresponding to sort windows). The relative distribution of naive and memory CD4+ and CD8+ T cell subsets over three age cohorts (0–4, 4–35, and 35–90 yr) is shown in Table . In young children, >70% of CD4+ and CD8+ T lymphocytes had a naive phenotype. A continuous decline with age in the proportion of naive CD4+ and CD8+ lymphocytes was observed, which paralleled an increase in the proportion of memory CD4+ and effector CD8+ cells, and to a lesser extent memory CD8+ cells. As a result, naive CD4+ and CD8+ T lymphocytes represented a minority after the age of 50 yr. This was particularly true for the CD8+ naive T lymphocytes.
Figure 3.
Examples of the changes in cell surface phenotype of CD4+ (top) and CD8+ (bottom) T lymphocytes that occur with age. Note that in early childhood the majority of CD4+ T cells have a CD4+ CD45RA+CD45RO− naive phenotype, whereas in older adults most CD4+ T cells have a CD45RA−CD45RO+ memory phenotype. Similar changes occur in CD8+ T cells. Boxed areas indicate windows that were used to sort cells with the indicated phenotype as well as the gates that were used to calculate the percentage of cells with a given phenotype shown in Table .
Table 3.
The Proportion of Naive and Memory Cells within Subpopulations of CD4+ and CD8+ T Cells Varies with Age
| Subsets | Age | |||
|---|---|---|---|---|
| 0–4 yr (n = 45) | 4–35 yr (n = 45) | 35–90 yr (n = 39) | ||
| CD4+RA+RO− | naive | 74 ± 11 (46–92) | 47 ± 18 (7–78) | 31 ± 13 (5–53) |
| CD4+RA−RO+ | memory | 6 ± 5 (0–23) | 24 ± 12 (8–57) | 35 ± 13 (16–70) |
| CD8+RA+27+ | naive | 72 ± 16 (18–94) | 60 ± 15 (25–88) | 25 ± 13 (2–51) |
| CD8+RA+27− | effector | 9 ± 10 (0–54) | 12 ± 9 (2–42) | 28 ± 15 (5–60) |
| CD8+RA−27+ | memory | 2 ± 3 (0–8) | 5 ± 4 (1–18) | 7 ± 5 (1–21) |
Data is shown as percentage ± SD. The range is shown in parentheses.
Loss of Telomere Fluorescence in T Lymphocyte Subsets.
The telomere fluorescence in naive and memory CD4+ and CD8+ T lymphocytes over the entire age range was analyzed using linear regression (Fig. 4). The mean telomere fluorescence gradually declined with age in all four T lymphocyte subsets. A more pronounced decline in telomere fluorescence was found for memory CD4+ and CD8+ T cells when compared with naive CD4+ and CD8+ T lymphocytes (Table , top). Interestingly, naive CD4+ and CD8+ T lymphocytes as well as granulocytes showed identical rates in telomere loss over the entire age range (Table and Table , tops). Taken together, the overall decline in telomere length in lymphocytes with age appears to reflect telomere shortening in naive and memory T cells as well as a gradual shift from a naive to a memory phenotype.
Figure 4.
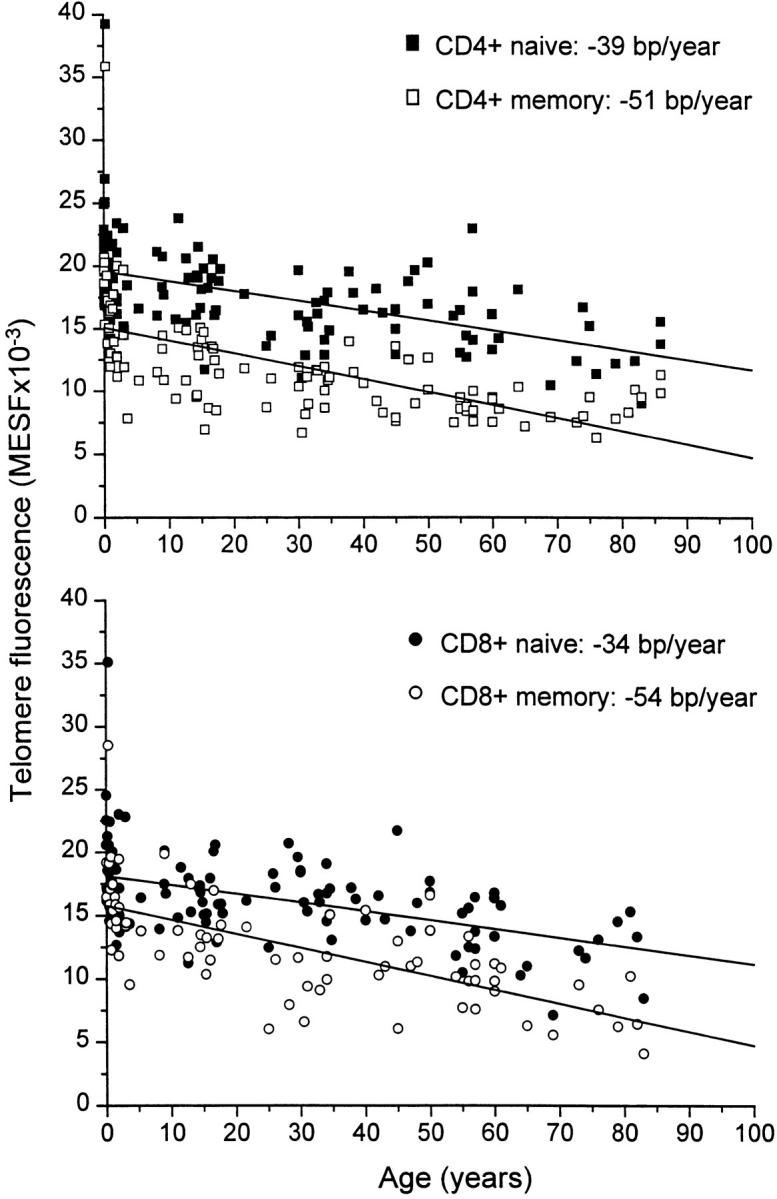
Linear regression analysis of telomere fluorescence and age in subpopulations of peripheral blood T lymphocytes. Subsets of CD4+ and CD8+ T lymphocytes were purified from donors of the indicated age using the sort windows described in Fig. 3. Note the relatively constant difference in telomere fluorescence between naive and memory CD4+ T lymphocytes after the first few years and the increasing difference in telomere fluorescence between CD8+ naive and memory T lymphocytes.
Table 4.
Loss of Telomere Fluorescence in T Lymphocytes Varies with Age
| Subset | Age range | n | y intercept (MESF × 10−3) | Slope (MESF × 10−3/yr) | Slope(bp/yr) | R value |
|---|---|---|---|---|---|---|
| Linear regression analysis | ||||||
| yr | ||||||
| CD4 + N | 0–90 | 127 | 19.56 | −0.079 | −39 | −0.53 |
| CD4 + M | 0–90 | 109 | 15.07 | −0.103 | −51 | −0.63 |
| CD8 + N | 0–90 | 126 | 18.1 | −0.069 | −34 | −0.49 |
| CD8 + M | 0–90 | 77 | 15.76 | −0.11 | −54 | −0.69 |
| Bisegmented line analysis | ||||||
| CD4 + N | 0–1 | 38 | 20.97 | −2.34 | −1,158 | |
| ≥1 | 89 | 18.7 | −0.06 | −30 | ||
| CD4 + M | 0–3.5 | 32 | 18.81 | −1.93 | −955 | |
| ≥3.5 | 77 | 12.22 | −0.05 | −25 | ||
| CD8 + N | 0–1.5 | 43 | 18.98 | −1.11 | −549 | |
| ≥1.5 | 83 | 17.38 | −0.055 | −27 | ||
| CD8 + M | 0–1.5 | 17 | 19.64 | −3.46 | −1,712 | |
| ≥1.5 | 60 | 14.59 | −0.088 | −44 | ||
In most cases, application of a bisegmented fit analysis on the data resulted in a statistically significant improvement over single-line linear regression analysis (Table , bottom). All T cell subsets showed a rapid decline in telomere length during the first years of life. This was particularly true for CD4+ memory T lymphocytes. The rapid loss of telomere fluorescence in the memory CD4+ T subset lasted for 3.5 yr, whereas bisegmented line analysis revealed a cut-off at earlier time-points for naive CD4+ and CD8+ as well as memory CD8+ T cells (Table , bottom, and Fig. 5). Because no significant differences in telomere length were found between naive and memory T cell subsets from newborns (results not shown), the shift from naive to memory T cells appears to coincide with a rapid and increasing difference in telomere fluorescence, especially in the CD4 compartment. After this initial rapid decline, a more gradual decline in telomere fluorescence in all T cell subsets was observed with a similar rate in naive CD4+ and CD8+ T lymphocytes (30 and 27 bp/yr, respectively). Surprisingly, memory CD8+ T cells showed a higher decline than did memory CD4+ T lymphocytes, with a calculated telomere rate loss of 44 bp/yr versus 25 bp/yr (Table , bottom). As a result, memory CD8+ T lymphocytes showed a progressive and steadily increasing difference in telomere fluorescence between naive and memory subsets with age (Fig. 4 and Table , bottom). In contrast, the difference in telomere fluorescence between naive and memory CD4+ T cells remained relatively constant with age, as has been described previously 22 25.
Figure 5.
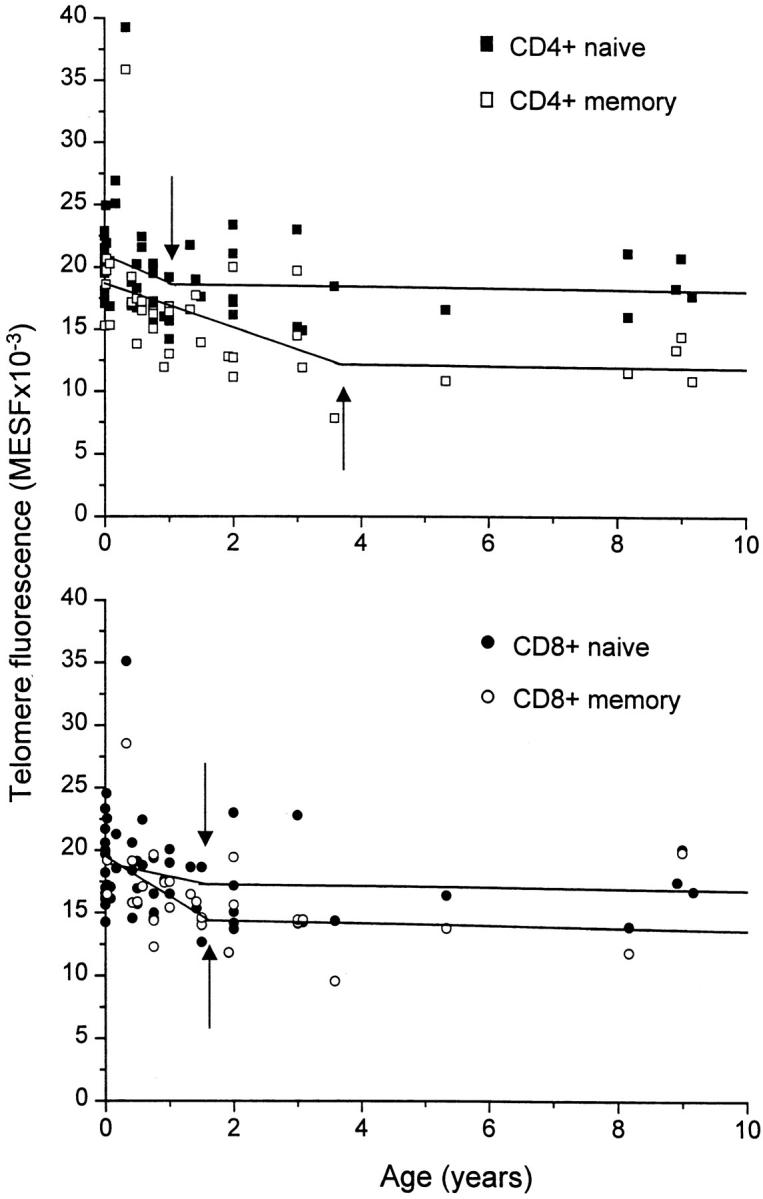
Rapid loss of telomere fluorescence in T cell subsets in early childhood. The data over the whole age range shown in Fig. 4 were subjected to bisegmented fit analysis. Arrows indicate the age that resulted in a significantly better fit for most cell types than was obtained by linear regression. Note the similar telomere fluorescence in naive and memory CD4+ and CD8+ T lymphocytes at birth and the relative rapid loss of telomere fluorescence particularly in CD4+ memory T lymphocytes.
Discussion
Does the Telomere Length in Blood Cells Reflect Their Replicative History?
A major unresolved issue in mammalian telomere biology is whether the loss of telomere repeats in somatic cells occurs at a constant rate with each cell division or whether it is variable and dependent on the cellular levels of positive and negative regulatory factors. Based on the observation that telomeric sequences are not lost at a constant rate throughout life in normal human leukocytes, Frenck et al. concluded that developmental stage-dependent changes in the rate of telomere loss must occur and that telomere length cannot be a direct reflection of cellular turnover 19. Our more extensive data set on the rate of telomere shortening in granulocytes and (sub)populations of T cells do not contradict the results of this previous study. However, in contrast to Frenck et al., we believe that known ontogeny-related functional differences in primitive hematopoietic cells 2 and a relatively simple model of telomere erosion in stem cells most easily explain the data. According to this model, each postnatal division in stem cells results in more or less constant losses of telomere repeats. Consequently, heritable differences in telomere length and the number of cell divisions will determine the telomere length in stem cells and their progeny. The model implies that the telomerase activity present in purified “candidate” stem cells 26 27 and T cells 28 29 30 is not capable of elongating or maintaining 2 the length of telomeres in these cells under normal physiological conditions in vivo. This simple telomere loss model may not be valid for all somatic cells in humans. For example, primary human B cells appear to be capable of extending their telomeres in the germinal center of lymph nodes 30 31.
Variation in Telomere Length.
Previous studies using Southern blot analysis have documented considerable variation in the average length of telomeric DNA at any given age 14 16 17. In studies of MZ and DZ twins, this variation was found to be to a large extent genetically determined 21. The results reported here confirm and extend these previous studies. A large variation in the telomere fluorescence of granulocytes as well as lymphocytes was observed (Fig. 2). We assume that method as well as cell sample–related variables will have contributed to the observed variation (see Materials and Methods). However, analysis of blood samples from MZ and DZ twins as well as pairs of unrelated individuals (Table ) suggests that genetic differences are the major contributors to the observed variation in telomere fluorescence values. Despite the relatively small number and the relative old age of the twins studied (all between 73 and 88 yr old), the MZ correlation for telomere fluorescence in lymphocytes was significantly higher than the DZ correlation (P < 0.05), confirming the influence of genetic factors on the variation in telomere fluorescence. In granulocytes, both the MZ and DZ correlations were very high and statistically significant, but there was only a marginal and statistically insignificant difference between the two. This result does not exclude a major influence of genetic factors on the telomere length in granulocytes and their precursors. Previous studies in the mouse have shown that telomere length adjustments do occur in somatic cells under the control of a gene that was mapped to a distal region of chromosome 2q 32. Most likely the primary determinants of telomere length in humans are heritable chromosome-specific factors 33 as well as (genetic) factors that adjust the length of telomeres in diploid cells of the developing embryo 32. Interestingly, similarities in telomere length were maintained throughout life in both MZ and DZ twins. This observation suggests that the total number of cell divisions (and the amount of telomeric DNA lost per cell division) in granulocytes and lymphocytes from related individuals is remarkably similar.
Implications of Variations in Telomere Length.
The nature of the heritable variation in telomere length and its consequences are intriguing. At first glance, the variation in telomere fluorescence values in childhood appears to be larger than in adults (Fig. 2). In a previous study, we observed that telomere fluorescence values within normal adult bone marrow metaphases are not normally distributed 34, suggesting that a minimum number of repeats is maintained at each telomere. Recently, telomere length measurements by Southern blot analysis as well as FISH were found to best fit a lognormal distribution and it was suggested that this may relate to the breaking and recombination of telomeres in normal somatic cells 35. Given the overall decline in telomere length with age, it is tempting to speculate that, at any given age, cells from individuals with long telomeres may have a larger replicative potential than cells from individuals with short telomeres. However, it should be kept in mind that the actual mechanism by which telomere shortening triggers cell senescence in human cells is currently not known (for review see references 36 and 37). One possibility is that the telomere length on individual chromosomes 33 is a better predictor of replicative senescence than is the average telomere length measured here. In view of the significant decline in telomere length with age, a relationship between this parameter and life span in general also seems possible. Ideally, longitudinal studies should be performed to address this issue, as the telomere length kinetics in individuals may be different from the population kinetics described here. For example, the turnover rate of stem cells may vary between individuals, complicating the relationship between age and telomere length. Interestingly, in mice the genetically controlled fraction of actively cycling hematopoietic progenitor cells correlates significantly with the life span of the animal 38, whereas the overall telomere length clearly does not 32. Further studies in all these areas are needed to fully understand the value and potential of telomere length measurements in studies of aging and the (patho)physiology of stem cells and their myeloid and lymphoid progeny.
Turnover of Blood Cells Estimated from Telomere Fluorescence Data.
The results of this study have far-reaching implications for models of the cellular turnover in stem cells and T cells. Both granulocytes and naive T lymphocytes showed a dramatic and parallel decline in telomere fluorescence in the first 2 yr after birth, followed by a 30-fold lower rate in telomere shortening after 4 yr of age (Table and Table ). These telomere length kinetics most likely reflect the turnover of the hematopoietic stem cells, the progenitors of both cell types. Other explanations such as a similar turnover in lineage-restricted precursors or a physiological change in telomere length unrelated to cell division affecting multiple lineages in a similar manner cannot be excluded at this point but seem less likely. Assuming a loss of between 50 and 100 bp per cell division 39 40, our observations are compatible with 15–30 stem cell divisions in the first half year followed by less than one stem cell division per year in the following years.
In previous studies we showed that hematopoietic progenitor cells, including purified “candidate” stem cells, lose telomeric DNA with proliferation in vitro and in vivo 3, and we suggested that telomere shortening in stem cells could explain the telomere shortening in leukocytes that has been observed by others 14 15 16 17 18 19 21. In separate studies we showed that the proliferative potential of purified “candidate” stem cells in humans and mice is subject to pronounced ontogeny-related changes 2 41. On the basis of these findings, we proposed the intrinsic timetable (IT) model of stem cell biology, which incorporates both ontogeny-related functional changes and the loss of telomere repeats 1. Similar to the clonal succession 42 and generational age 43 44 models, the intrinsic timetable model assumes that stem cells have a restricted capacity for proliferation in vivo and that life-long production of blood cells is derived from a population of stem cells with limited replicative potential. Limitations in the number of times that stem cells can divide may require special control mechanisms to avoid replicative exhaustion. We recently reported that purified “candidate” stem cells from human fetal liver maintain long-term in vitro hematopoiesis through asymmetric cell divisions 45. In these studies we observed that daughter cells resulting from the cell division of purified single precursors do not enter mitosis simultaneously. Strikingly, slowly dividing daughter cells were endowed with the largest numerical expansion potential. Asymmetric divisions at the level of stem cells are expected to result in a hierarchy within the stem cell compartment in which cells differ in replicative history and associated self-renewal properties. Such a hierarchy could explain the large “self-renewal” potential of hematopoietic tissues that is apparent from marrow regeneration after stem cell transplantation or marrow injury. The principle of an extensive replicative hierarchy may also be applicable to stem cells from other tissues with continued cell turnover. Comparisons between the telomere fluorescence in the progeny of single purified stem cells and their functional properties can be used to test this hypothesis.
Telomere Length Dynamics in Lymphocyte Subpopulations.
Relative to naive T cells and granulocytes, memory T lymphocytes showed an even higher loss of telomere fluorescence in the first 4 yr. Most likely the rapid and sustained loss of telomere repeats in memory CD4+ T cells in early childhood reflects repeated antigenic challenges to the infant immune system in the form of infections and vaccination. Together with previously observed differences in the telomere length between naive and memory T cells 22 25, these observations support the notion that each cell division in T cells results in the loss of telomere repeats. In view of these observations, the readily detectable presence of telomerase in T cells (for review see references 46 and 47) is puzzling. One possibility is that telomeres in T cells are relatively inaccessible to the enzyme or that levels of telomerase are insufficient to increase telomere length. Interestingly, the loss of telomere fluorescence in CD8 cells was less pronounced than that in CD4 cells in early childhood (Fig. 5 and Table ). However, after the age of four, the fluorescence of CD8+ memory T cells gradually declined more than in CD4+ memory T cells (Fig. 4 and Table ). As a result, relatively short telomeres were observed in both cell types in subjects >60 yr old. At this time most circulating T cells are of the “memory” type (Table ) and CD8+ T cells are increasingly oligoclonal 48 49. The relationship among T cell clonality, telomere length, and immune senescence deserves further study.
A major finding in this study was that the loss of telomere fluorescence in granulocytes was parallel to that of naive T cells over the whole age range. Again assuming a simple relation between telomere length and replicative history, this observation suggests that the number of cell divisions between granulocytes, naive T cells, and their common precursors (stem cells) is relatively constant throughout life. According to this model, the telomere loss rates in granulocytes and naive T cells are expected to be equal, even if these cells and their immediate precursors are dividing at different rates. Recent data indicating that although thymic function declines with age, substantial output of naive T cells is maintained into late adulthood 12 support this model. Because telomere fluorescence values in naive T cells were always higher than those in granulocytes, the data further suggest that the number of cell divisions between granulocytes and stem cells is higher than that between naive T cells and stem cells. The differences in telomere length between granulocytes and lymphocyte subpopulations reported here are large relative to the reported differences in telomere length between the donors and recipients of allogeneic bone marrow transplants 4 5. In these previous studies, the telomere length was measured using DNA extracted from unfractionated circulating leukocytes. Our data suggest that similar studies using subpopulations of cells are needed to address questions about stem cell turnover and immune reconstitution in transplant recipients. Such studies are currently in progress.
Although lymphocytes showed higher telomere fluorescence values than did granulocytes at birth, the opposite was true after the age of 60 in the majority of individuals (Fig. 2 and Table ). This observation is most likely explained by the age-related increase in the percentage of memory T cells, which have shorter telomeres than naive T cells (Table and Fig. 4). In general, our data support the notion that telomere-related restrictions in replicative potential are more likely to occur in (memory) T cells than in other cells of the hematopoietic system, such as the precursors of granulocytes.
We conclude that studies of telomere length in different cell types by flow cytometry provide important insights into the organization and turnover of hematopoietic cells including T cells. Adaptation of telomere FISH to tissue sections may provide similar information about the less readily accessible cells in solid organs.
Acknowledgments
We thank Connie Eaves, Gerry Krystal, and Claudia Bos for critical reading of the manuscript. Debra Gindis and Karin Batt are thanked for help with the collection and coding of blood samples. We also thank Visia Dragowska, Giovanna Cameron, Rick Zapf, and Gayle Thornbury for help with the sorting of cells.
This work was supported by grants AI29524 and GM56162 from the National Institutes of Health and by a grant from the National Cancer Institute of Canada with funds from the Terry Fox Run. N. Rufer was a recipient of a fellowship from the Fonds National Suisse. T.H. Brümmendorf is funded by a grant from the Deutsche Forschungsgemeinschaft. S. Kolvraa and C. Bischoff are supported through the Danish Center for Molecular Gerontology under the Danish National Research Foundation. K. Christensen is supported by National Institute on Aging research grant NIA-PO1-AG08761 and the Danish National Research Foundation.
Footnotes
2The maintenance of short telomeres in a serially passaged memory T cell clone (22) is not an exception. Recently we found a clonal chromosomal abnormality in these cells (t(1,19), that was already present before cloning (results not shown)
1used in this paper: DZ, dizygotic; FISH, fluorescence in situ hybridization; MESF units, molecules of equivalent soluble fluorochrome units; MZ, monozygous
N. Rufer and T.H. Brümmendorf contributed equally to this work.
References
- Lansdorp P.M. Self-renewal of stem cells. Biol. Blood Marrow Transplant. 1997;3:171–178. [PubMed] [Google Scholar]
- Lansdorp P.M., Dragowska W., Mayani H. Ontogeny-related changes in proliferative potential of human hematopoietic cells. J. Exp. Med. 1993;178:787–791. doi: 10.1084/jem.178.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaziri H., Dragowska W., Allsopp R.C., Thomas T.E., Harley C.B., Lansdorp P.M. Evidence for a mitotic clock in human hematopoietic stem cellsloss of telomeric DNA with age. Proc. Natl. Acad. Sci. USA. 1994;91:9857–9860. doi: 10.1073/pnas.91.21.9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notaro R., Cimmino A., Tabarini D., Rotoli B., Luzzatto L. In vivo telomere dynamics of human hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 1997;94:13782–13785. doi: 10.1073/pnas.94.25.13782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn R.F., Cross M.A., Hatton C., Will A.M., Lashford L.S., Dexter T.M., Testa N.G. Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet. 1998;351:178–181. doi: 10.1016/S0140-6736(97)08256-1. [DOI] [PubMed] [Google Scholar]
- Akiyama M., Hoshi Y., Sakurai S., Yamada H., Yamada O., Mizoguchi H. Changes of telomere length in children after hematopoietic stem cell transplantation. Bone Marrow Transplant. 1998;21:167–171. doi: 10.1038/sj.bmt.1701060. [DOI] [PubMed] [Google Scholar]
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219–228. doi: 10.1016/0092-8674(94)90330-1. [DOI] [PubMed] [Google Scholar]
- Smith S.H., Brown M.H., Rowe D., Callard R.E., Beverley P.C. Functional subsets of human helper-inducer cells defined by a new monoclonal antibody, UCHL-1. Immunology. 1986;58:63–70. [PMC free article] [PubMed] [Google Scholar]
- Hamann D., Baars P.A., Rep M.H.G., Hooibrink B., Kerkhof-Garde S.R., Klein M.R., van Lier R.A.W. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie C.A., McLean A., Alcock C., Beverley P.C. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- Tough D.F., Sprent J. Turnover of naive- and memory-phenotype T cells. J. Exp. Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek D.C., McFarland R.D., Keiser P.H., Gage E.A., Massey J.M., Haynes B.F., Polis M.A., Haase A.T., Feinberg M.B., Sullivan J.L. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- Harley C.B., Futcher A.B., Greider C.W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hastie N.D., Dempster M., Dunlop M.G., Thompson A.M., Green D.K., Allshire R.C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990;346:866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Lindsey J., McGill N.I., Lindsey L.A., Green D.K., Cooke H.J. In vivo loss of telomeric repeats with age in humans. Mutat. Res. 1991;256:45–48. doi: 10.1016/0921-8734(91)90032-7. [DOI] [PubMed] [Google Scholar]
- Vaziri H., Schachter F., Uchida I., Wei L., Zhu X., Effros R., Cohen D., Harley C.B. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am. J. Hum. Genet. 1993;52:661–667. [PMC free article] [PubMed] [Google Scholar]
- Iwama H., Ohyashiki K., Ohyashiki J.H., Hayashi S., Yahata N., Ando K., Toyama K., Hoshika A., Takasaki M., Mori M., Shay J.W. Telomeric length and telomerase activity vary with age in peripheral blood cells obtained from normal individuals. Hum. Genet. 1998;102:397–402. doi: 10.1007/s004390050711. [DOI] [PubMed] [Google Scholar]
- Jeanclos E., Krolewski A., Skurnick J., Kimura M., Aviv H., Warram J.H., Aviv A. Shortened telomere length in white blood cells of patients with IDDM. Diabetes. 1998;47:482–486. doi: 10.2337/diabetes.47.3.482. [DOI] [PubMed] [Google Scholar]
- Frenck R.W., Jr., Blackburn E.H., Shannon K.M. The rate of telomere sequence loss in human leukocytes varies with age. Proc. Natl. Acad. Sci. USA. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp R.C., Harley C.B. Evidence for a critical telomere length in senescent human fibroblasts. Exp. Cell Res. 1996;219:130–136. doi: 10.1006/excr.1995.1213. [DOI] [PubMed] [Google Scholar]
- Slagboom P.E., Droog S., Boomsma D.I. Genetic determination of telomere size in humansa twin study of three age groups. Am. J. Hum. Genet. 1994;55:876–882. [PMC free article] [PubMed] [Google Scholar]
- Rufer N., Dragowska W., Thornbury G., Roosnek E., Lansdorp P.M. Telomere length dynamics in human lymphocyte subpopulations measured by flow cytometry. Nat. Biotechnol. 1998;16:743–747. doi: 10.1038/nbt0898-743. [DOI] [PubMed] [Google Scholar]
- Henderson L.O., Marti G.E., Gaigalas A., Hannon W.H., Vogt R.F., Jr. Terminology and nomenclature for standardization in quantitative fluorescence cytometry. Cytometry. 1998;33:97–105. doi: 10.1002/(sici)1097-0320(19981001)33:2<97::aid-cyto3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Draper N.R., Smith H. Applied Regression Analysis. 3rd Edition. John Wiley and Sons; NY: 1998. [Google Scholar]
- Weng N.-P., Levine B.L., June C.H., Hodes R.J. Human naive and memory T lymphocytes differ in telomeric length and replictive potential. Proc. Natl. Acad. Sci. USA. 1995;92:11091–11094. doi: 10.1073/pnas.92.24.11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu C.-P., Dragowska W., Kim N.W., Vaziri H., Yui J., Thomas T.E., Harley C.B., Lansdorp P.M. Differential expression of telomerase activity in hematopoietic progenitors from adult human bone marrow. Stem Cells. 1996;14:239–248. doi: 10.1002/stem.140239. [DOI] [PubMed] [Google Scholar]
- Yui J., Chiu C.-P., Lansdorp P.M. Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood. 1998;91:3255–3262. [PubMed] [Google Scholar]
- Weng N.-P., Levine B.L., June C.H., Hodes R.J. Regulated expression of telomerase activity in human T lymphocyte development and activation. J. Exp. Med. 1996;183:2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng N.-P., Levine B.L., June C.H., Hodes R.J. Regulation of telomerase RNA template expression in human T lymphocyte development and activation. J. Immunol. 1997;158:3215–3220. [PubMed] [Google Scholar]
- Weng N.-P., Hathcock K.S., Hodes R.J. Regulation of telomere length and telomerase in T and B cellsa mechanism for maintaining replicative potential. Immunity. 1998;9:151–157. doi: 10.1016/s1074-7613(00)80597-x. [DOI] [PubMed] [Google Scholar]
- Weng N.-P., Granger L., Hodes R.J. Telomere lengthening and telomerase activation during human B cell differentiation. Proc. Natl. Acad. Sci. USA. 1997;94:10827–10832. doi: 10.1073/pnas.94.20.10827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Hathcock K.S., Hande P., Lansdorp P.M., Seldin M.F., Hodes R.J. Telomere length regulation in mice is linked to a novel chromosome locus. Proc. Natl. Acad. Sci. USA. 1998;95:8648–8653. doi: 10.1073/pnas.95.15.8648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens U.M., Zijlmans J.M., Poon S.S., Dragowska W., Yui J., Chavez E.A., Ward R.K., Lansdorp P.M. Short telomeres on human chromosome 17p. Nat. Genet. 1998;18:76–80. doi: 10.1038/ng0198-018. [DOI] [PubMed] [Google Scholar]
- Lansdorp P.M., Verwoerd N.P., van de Rijke F.M., Dragowska V., Little M.T., Dirks R.W., Raap A.K., Tanke H.J. Heterogeneity in telomere length of human chromosomes. Hum. Mol. Genet. 1996;5:685–691. doi: 10.1093/hmg/5.5.685. [DOI] [PubMed] [Google Scholar]
- Oexle K. Telomere length distribution and Southern blot analysis. J. Theor. Biol. 1998;190:369–377. doi: 10.1006/jtbi.1997.0559. [DOI] [PubMed] [Google Scholar]
- Campisi J. The biology of replicative senescence. Eur. J. Cancer. 1997;33:703–709. doi: 10.1016/S0959-8049(96)00058-5. [DOI] [PubMed] [Google Scholar]
- Sedivy J.M. Can ends justify the means? Telomeres and the mechanisms of replicative senescence and immortalization in mammalian cells. Proc. Natl. Acad. Sci. USA. 1998;95:9078–9081. doi: 10.1073/pnas.95.16.9078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan G., Van Zant G. Intrinsic and extrinsic control of hemopoietic stem cell numbersmapping of a stem cell gene. J. Exp. Med. 1997;186:529–536. doi: 10.1084/jem.186.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp R.C., Vaziri H., Patterson C., Goldstein S., Younglai E.V., Futcher A.B., Greider C.W., Harley C.B. Telomere length predicts replicative capacity of human fibroblasts. Proc. Natl. Acad. Sci. USA. 1992;89:10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp P.M. Telomere length and proliferation potential of hematopoietic stem cells. J. Cell Sci. 1995;108:1–6. doi: 10.1242/jcs.108.1.1. [DOI] [PubMed] [Google Scholar]
- Rebel V.I., Miller C.L., Thornbury G.R., Dragowska W.H., Eaves C.J., Lansdorp P.M. A comparison of long-term repopulating hematopoietic stem cells in fetal liver and adult bone marrow from the mouse. Exp. Hematol. 1996;24:638–648. [PubMed] [Google Scholar]
- Kay H.E.M. How many cell-generations? Lancet. 1965;2:418–419. doi: 10.1016/s0140-6736(65)90763-4. [DOI] [PubMed] [Google Scholar]
- Hellman S., Botnick L.E., Hannon E.C., Vigneulle R.M. Proliferative capacity of murine hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 1978;75:490–494. doi: 10.1073/pnas.75.1.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendaal M., Hodgson G.S., Bradley T.R. Organization of haemopoietic stem cellsthe generation-age hypothesis. Cell Tissue Kinet. 1979;12:17–29. doi: 10.1111/j.1365-2184.1979.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Brummendorf T.H., Dragowska W., Zijlmans J.M., Thornbury G., Lansdorp P.M. Asymmetric cell divisions sustain long-term hematopoiesis from single-sorted human fetal liver cells. J. Exp. Med. 1998;188:1117–1124. doi: 10.1084/jem.188.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R.B., Pawelec G. Replicative senescence of T cellsdoes the Hayflick Limit lead to immune exhaustion? Immunol. Today. 1997;18:450–454. doi: 10.1016/s0167-5699(97)01079-7. [DOI] [PubMed] [Google Scholar]
- Weng N.-P., Palmer L.D., Levine B.L., Lane H.C., June C.H., Hodes R.J. Tales of tailsregulation of telomere length and telomerase activity during lymphocyte development, differentiation, activation, and aging. Immunol. Rev. 1997;160:43–54. doi: 10.1111/j.1600-065x.1997.tb01026.x. [DOI] [PubMed] [Google Scholar]
- Hingorani R., Choi I.-H., Akolkar P., Gulwani-Akolkar B., Pergolizzi R., Silver J., Gregersen P.K. Clonal predominance of T cell receptors within the CD8+CD45RO+ subset in normal human subjects. J. Immunol. 1993;151:5762–5769. [PubMed] [Google Scholar]
- Posnett D.N., Sinha R., Kabak S., Russo C. Clonal populations of T cells in normal elderly humansthe T cell equivalent to “benign monoclonal gammapathy”. J. Exp. Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]