Abstract
Natural killer (NK) receptor signaling can lead to reduced cytotoxicity by NK cells and cytolytic T lymphocytes (CTLs) in vitro. Whether T cells are inhibited in vivo remains unknown, since peptide antigen–specific CD8+ T cells have so far not been found to express NK receptors in vivo. Here we demonstrate that melanoma patients may bear tumor-specific CTLs expressing NK receptors. The lysis of melanoma cells by patient-derived CTLs was inhibited by the NK receptor CD94/NKG2A. Thus, tumor-specific CTL activity may be decreased through NK receptor triggering in vivo.
Keywords: cytolytic T lymphocytes, natural killer receptors, melanoma, tumor immunity, peptide antigen
Cytolytic lymphocytes play a central role in the recognition and elimination of abnormal cells. CD8+ T cells are well known to efficiently kill virus-infected cells upon recognition of viral peptides presented by MHC class I molecules on the surface of infected cells 1 2. In recent years, it became clear that CD8+ T cells can also kill tumor cells through the recognition of tumor cell–derived peptides presented by cell surface MHC class I molecules 3 4.
Although many experimental treatment protocols to enhance tumor immunity have been applied, only a minority of the treated patients have experienced tumor regression 5 6 7. Undoubtedly, the development of more efficient immune therapy approaches requires a better understanding of tumor immunity. Several biological mechanisms may account for the failures to achieve efficient immune protection: on the one hand, the activation of tumor-specific T cells may be insufficient in intensity and durability 8 to allow a long-lasting antitumor effect. On the other hand, tumor cells may develop strategies to evade or even counteract immune attack, facilitated by the high degree of genetic instability of advanced tumors. Examples for such immune evasion strategies are mutations of genes encoding MHC, tumor antigens, or molecules that regulate antigen presentation or lymphocyte homing 9 10 11 12.
Another mechanism may be the inhibition of cytolytic function through the recently described NK receptors. Two families of NK receptor molecules have been identified. The first are type I transmembrane proteins belonging to the Ig superfamily, such as p58.2 13 or Ig-like transcript 2 (ILT2)1 14, and the second are the type II transmembrane proteins containing a C-type lectin domain, such as the heterodimer CD94/NKG2 15. Upon ligation with MHC class I recognized on target cells, these NK receptors may inhibit the cytolytic function. Most of the known NK receptors have been identified through studies of NK cells, but subpopulations of CD8+ T cells may also express them. However, only small proportions (0–10%) of human CD8+ T cells are positive for a given NK receptor. Furthermore, the studies showing that NK receptors may inhibit CTL activity have so far only been done with T cell lines or clones 16 17 18 19 20. Therefore, it remains questionable whether NK receptors can significantly inhibit CTLs in vivo and whether this may concern a physiologically relevant proportion of effector CTLs. Thus, further methodological progress is required to successfully address this question experimentally.
Immune protection from melanoma may occur through CD8+ CTLs that are specific for tumor antigens such as Melan-A/MART-1 21 22. In this study, we investigated T cells specific for the immunodominant peptide antigen Melan-A EAAGIGILTV, which is presented by the MHC class I molecule HLA-A*0201 22. To investigate phenotype and function of human Melan-A–specific T cells, we took advantage of the novel “tetramer” technology 23 24 25. As described previously 25, we generated tetramers consisting of four HLA-A*0201 molecules, four Melan-A peptides, and a fluorescent dye. Upon tetramer incubation and flow cytometry analysis, HLA-A2/Melan-A–specific lymphocytes were directly visualized without the need for in vitro expansion. Our study of 10 melanoma patients shows that tumor antigen–specific T cells may express various NK receptors. Furthermore, the lysis of melanoma cells by patient-derived CTLs was inhibited by the NK receptor CD94/NKG2A. Together, these findings strongly suggest that NK receptor triggering may in some instances interfere with tumor-specific immune responses in vivo.
Materials and Methods
Blood and LN Samples, Lymphocytes, and Target Cells.
Blood and LNs were obtained from patients with advanced stage malignant melanoma selected on the basis of HLA-A2 antigen expression. PBLs were separated from heparinized blood by centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech), washed three times, and cryopreserved in RPMI 1640, 40% FCS, and 10% DMSO. Vials containing 5–10 × 106 cells were stored in liquid nitrogen. LNs collected by surgical dissection were dissociated to single cell suspensions in sterile RPMI 1640 supplemented with 10% FCS, washed, and cryopreserved as indicated above for PBLs. Aliquots were placed in 24-well tissue culture plates (Costar Corp.) in 2 ml of IMDM (Life Technologies) supplemented with 0.55 mM Arg, 0.24 mM Asn, 1.5 mM Gln, 10% pooled human A+ serum, recombinant human (rh)IL-2 (100 U/ml), and rhIL-7 (10 ng/ml). The melanoma cell line Me 290 and the A2/Melan-A–specific CD8+ T cell clone 17 were established from surgically excised melanoma metastases from patient LAU 203 as described 26. The cells were maintained in DME supplemented with 0.55 mM Arg, 0.24 mM Asn, 1.5 mM Gln, and 10% FCS.
HLA Typing.
PBLs were typed for HLA-A2 by flow cytometry using the allele-specific mAb BB7.2 27. Complete HLA-A and -B typing was performed by serology, and HLA-A2 allele typing and HLA-C typing were done by PCR using sequence-specific oligonucleotides 28. Since all patients were HLA-A*0201 positive, they all expressed HLA signal peptides able to bind to HLA-E and to serve as ligands for CD94/NKG2A 29 30. All six patients shown in Fig. 1 and Fig. 2 had at least one of the known p58.2 binding ligands Cw1, Cw3, Cw7, and Cw8. In some patients (LAU 181 and LAU 155) both alleles were p58.2 ligands, while in the other patients there was only one p58.2 ligand. The Me 290 melanoma cell line and the Melan-A–specific CD8+ T cell clone 17 were A1, A*0201, B7, B8, Cw07, Cwx.
Figure 1.
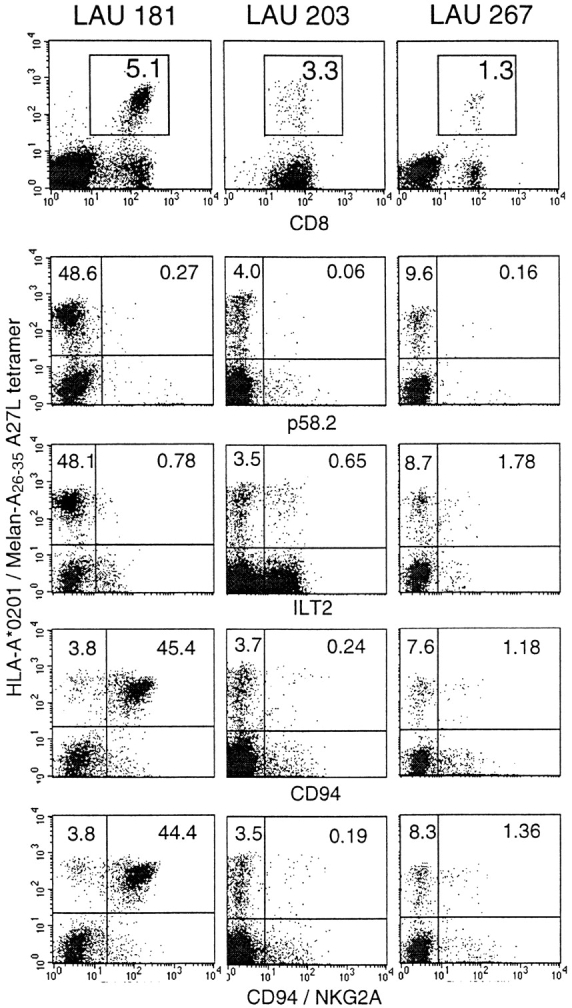
TILNs may contain Melan-A–specific, NK receptor–positive CTLs. TILNs obtained from patients LAU 181, LAU 203, and LAU 267 were cultured in the presence of IL-2 and IL-7 for 2 wk and stained with fluorescent soluble HLA-A*0201/Melan-A26–35 A27L tetramers, anti-CD8, and antibodies specific for the NK receptors p58.2, ILT2, CD94, or CD94/NKG2A. Only cells falling in the lymphocyte gate (see Materials and Methods) are shown. In the three dot plots of the top row, the numbers indicate the percentages of tetramer-positive/CD8+ cells. The dot plots below show the characteristics of lymphocytes in the CD8+ gate, and the numbers indicate the percentages of tetramer-positive/NK receptor–negative or –positive cells among CD8+ cells. Background values obtained using second stage sheep anti–mouse FITC-labeled antibody alone were <0.05% (not shown).
Figure 2.
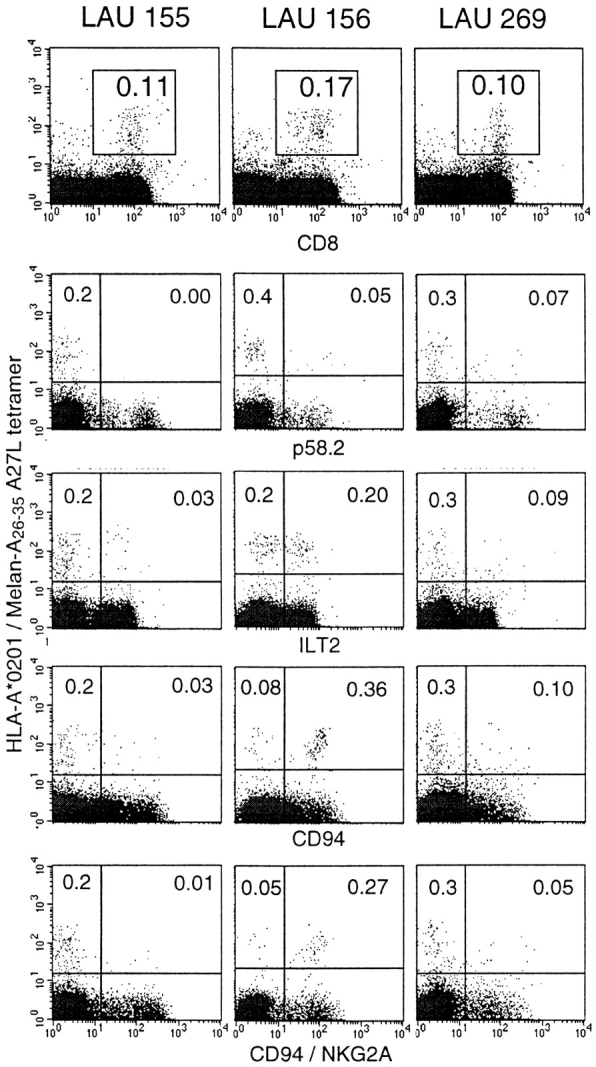
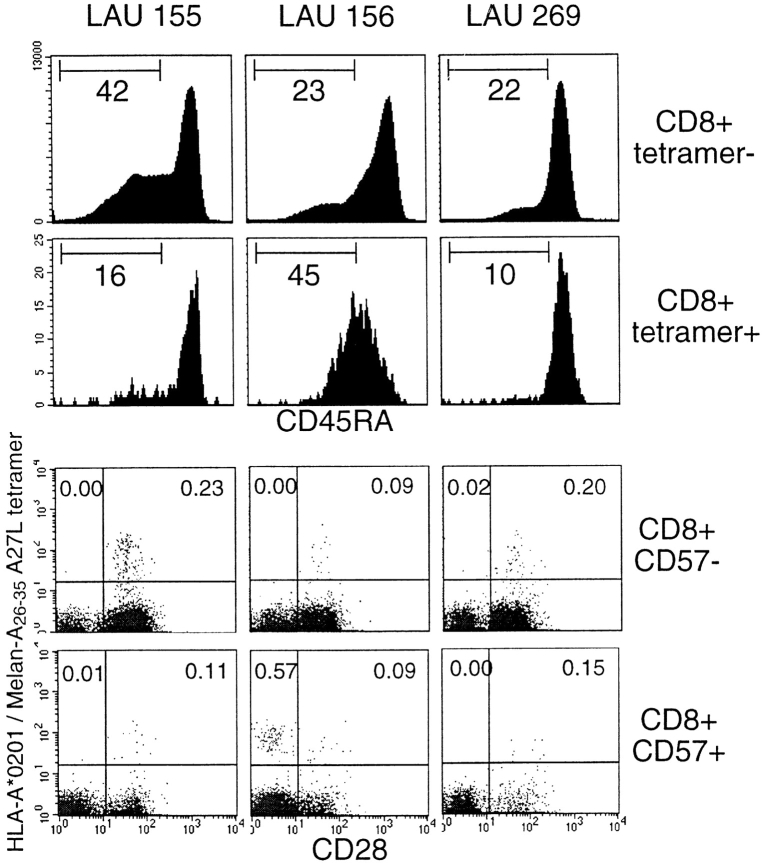
In vivo identification of Melan-A–specific CD8+ cells expressing NK receptors. PBLs from three melanoma patients (LAU 155, LAU 156, and LAU 269) who had vitiligo were analyzed by multiparameter flow cytometry as described in the legend to Fig. 1. The three dot plots in the top row show all cells in the lymphocyte gate; the dot plots below show lymphocytes in the CD8+ gate.
mAbs.
Antibodies specific for the NK receptors p58.2/CD158b (GL183), CD94 (XA185 and Y9), and the heterodimer CD94/NKG2A (ZIN199) were provided by A. Moretta, Università di Genova, Genova, Italy 31. The ILT2-specific antibody HP3F1 14 was obtained from M. López-Botet, University Hospital Princesa, Madrid, Spain. Nonlabeled IgG1 and IgG2a antibodies, and PE-labeled antibodies specific for p58.2 and CD94 were purchased from Immunotech. mAbs specific for human CD3, CD8, CD14, CD16, CD28, CD45RA, and TCR-α/β were obtained from Becton Dickinson. All flow cytometry stainings for CD94 were done with the mAb XA185, while Y9 was used in the cytotoxicity assays. In this study, the term “NKT cell” was used for all NK receptor–positive cells expressing CD3 and/or TCR-α/β.
Tetramers.
Complexes were synthesized as described 24 25. In brief, purified HLA heavy chain and β2-microglobulin were synthesized by means of a prokaryotic expression system (pET; R&D Systems, Inc.). The heavy chain was modified by deletion of the transmembrane cytosolic tail and COOH-terminal addition of a sequence containing the BirA enzymatic biotinylation site. Heavy chain, β2-microglobulin, and peptide were refolded by dilution. The 45-kD refolded product was isolated by fast protein liquid chromatography and then biotinylated by BirA (Avidity) in the presence of biotin, adenosine 5′-triphosphate, and Mg2+ (all from Sigma Chemical Co.). Streptavidin–PE conjugate (Sigma Chemical Co.) was added in a 1:4 molar ratio, and the tetrameric product was concentrated to 1 mg/ml. As the antigenic peptide, the Melan-A26–35 A27L analogue (ELAGIGILTV) was used, which has a higher binding stability to HLA-A*0201 and a higher T cell antigenicity and immunogenicity than the natural Melan-A decapeptide EAAGIGILTV or the nonapeptide AAGIGILTV 26. In this paper, the abbreviation “tetramer” is used for the HLA-A*0201/Melan-A26–35 A27L tetramers produced for this study.
Flow Cytometry.
LN cells were thawed and cultured for 16–20 h in IMDM supplemented with 0.55 mM Arg, 0.24 mM Asn, 1.5 mM Gln, 10% pooled human A+ serum, rhIL-2 (100 U/ml), and rhIL-7 (10 ng/ml). PBLs were thawed and stained after washing. Cells (0.5–1 × 106) were stained with tetramers and FITC-, peridinin chlorophyll protein (PerCP™), and allophycocyanin-labeled mAb conjugates in 50 μl of PBS, 2% BSA, and 0.2% azide for 40 min at 4°C. For indirect fluorescence labeling, cells were incubated (a) with tetramers, (b) with the primary (NK receptor–specific) antibody and washed, (c) with sheep anti–mouse FITC-labeled antibody and washed twice, (d) with IgG1 and IgG2a antibodies, and (e) with PerCP™- and allophycocyanin-labeled antibodies. Cells were washed once in the same buffer and analyzed immediately in a FACSCalibur™ machine (Becton Dickinson). With this method, the percentages of tetramer-positive cells were slightly lower compared with methods without multiple wash steps after tetramer incubation (not shown). Data acquisition and analysis were performed using CELLQuest™ software. Only cells falling in the “lymphocyte gate” were analyzed; this gate was defined by forward/side scatter settings corresponding to a cell population expressing >98% CD45 and <1% CD14 (as determined by control CD45/CD14 stainings).
Chromium-release Assay.
PBLs were thawed, stained with HLA-A2/Melan-A tetramers, and sorted using a FACStar™ machine (Becton Dickinson). The cells were then cultured in IMDM supplemented with 0.55 mM Arg, 0.24 mM Asn, 1.5 mM Gln, 10% pooled human A+ serum, rhIL-2 (100 U/ml), and rhIL-15 (10 ng/ml) starting at day 2 to upregulate CD94/NKG2A as described 32. 1 wk later, the cells were again FACS® sorted after staining with CD94/NKG2A-specific antibodies and with HLA-A2/Melan-A tetramers. Cytolytic activity was tested in 51Cr-release assays against the HLA-A*0201–expressing melanoma target cell lines Na8 and Me 290 26. Target cells were radiolabeled with Na51CrO4 for 1 h at 37°C, 5% CO2, then washed and coincubated in V-bottomed microwells at the indicated E/T ratio (103 target cells per well) in the presence of the IgM mAbs specific for CD94 (or control CD16). After 4 h at 37°C, supernatants were collected and counted in a Top-count™ (Canberra Packard) gamma counter. Percent specific lysis was calculated as (experimental release − spontaneous release) × 100/(total release − spontaneous release).
Results
Tumor Antigen–specific CD8+ T Cells Expressing Inhibitory NK Receptors.
In PBLs, the frequency of CTLs specific for single MHC/peptide epitopes is usually very low (range 1 in 104–106 CD8+ T cells). Their frequency during acute infections may be much higher (up to 1 in ∼103), but such high frequencies are not observed for tumor-specific CD8+ T cells because immune responses to tumors involve much lower antigen-specific T cell numbers compared with acute microbial infections. The frequencies of tumor-specific CD8+ T cells may be significantly higher in tumor-infiltrated lymph nodes (TILNs) than in PBLs, since TILNs may contain up to 3% HLA-A2/Melan-A tetramer–positive cells among CD8+ cells when analyzed ex vivo 25. To study the phenotype of tumor-specific CTLs in more detail, we cultured the TILNs for 2 wk in the presence of IL-2 and IL-7. This procedure induced expansion of the tumor antigen–specific T cells, allowing us to perform multiparameter FACS® analysis. Fig. 1 shows the results of TILNs stained with tetramers and antibodies specific for CD8 and one of four different NK receptors. The TILNs from the three patients contained high percentages of CD8+ tetramer-positive cells (1.3–5.1%). In patient LAU 181, these cells were largely negative for the NK receptors p58.2 and ILT2, but they expressed CD94 and CD94/NKG2A at high percentages. The Melan-A–specific lymphocytes from patient LAU 203 expressed some ILT2 but only low levels of the other three receptors. Finally, ILT2, CD94, and CD94/NKG2A were also expressed by some Melan-A–specific T cells from patient LAU 267. The Melan-A–specific TILNs from four further patients (data not shown) expressed no or only low levels (<0.5%) of NK receptors.
In Vivo Identification of Tumor Antigen–specific CD8+ T Cells Expressing NK Receptors in PBLs from Melanoma Patients with Vitiligo.
It has been demonstrated that melanoma patients develop vitiligo more frequently than individuals without melanoma, and that vitiligo is associated with an ongoing immune response directed against melanoma cells 33 34. We have recently reported that patients with vitiligo have high frequencies of Melan-A–specific circulating CTLs which were detectable ex vivo using HLA-A2/Melan-A tetramers 24 34a. The relatively high percentages of tetramer-positive circulating CTLs provided us the unique opportunity of investigating NK receptor expression by specific CTLs in vivo. We obtained PBLs from three HLA-A2–positive melanoma patients with vitiligo, and found 0.10–0.17% CD8+ tetramer-positive cells (Fig. 2). These cells expressed the following NK receptors: in patient LAU 155, practically all tetramer-positive cells were negative for each of the four NK receptors analyzed (p58.2, ILT2, CD94, and CD94/NKG2A). In contrast, in patient LAU 156, most of the tumor antigen–specific CTLs expressed CD94 and CD94/NKG2A, and about half of them expressed ILT2. Finally, in the third patient (LAU 269), there were low percentages of NK receptor–positive, tetramer-positive T cells. In conclusion, some tumor antigen–specific T cells may express NK receptors in vivo, and there are situations where this is the case for a large fraction of a given CTL population.
T Cells That Express NK Receptors In Vivo Have an Activated Phenotype.
The relatively high frequency of tumor-specific CTLs in the patients with vitiligo allowed us to investigate the in vivo phenotype of these cells in more detail. We investigated the expression of CD45RA, a marker for naive T cells 35 36. In PBLs of patients LAU 155 and LAU 269, the tetramer-positive cells expressed high levels of CD45RA (Fig. 3). In contrast, a reduced level of CD45RA was found in patient LAU 156, i.e., in the cells with high levels of NK receptor expression. We also analyzed the costimulatory molecule CD28 and the “adhesion” molecule CD57, since downregulation of CD28 and upregulation of CD57 have been shown to occur in activated “effector” CTLs 37 38, and we have recently demonstrated that NK receptor expression by T cells is primarily confined to the CD28− population 39. Interestingly, the tetramer-positive CD8+ cells of the patient with high percentages of NK receptor–expressing CTLs (patient LAU 156) were predominantly CD28− and CD57+ (Fig. 3). In contrast, the tumor-specific CTLs of the other two patients were mostly CD28+ and CD57−.
Figure 3.
NK receptor expression correlating with reduced intensity of CD45RA and CD28, and upregulation of CD57. PBLs from the three melanoma patients with vitiligo were analyzed with tetramers and antibodies specific for CD8, CD28, CD45RA, and CD57. The histograms show the results obtained with CD45RA-specific antibody, for cells gated for CD8+ tetramer-negative (top row) and CD8+ tetramer-positive (second row) cells. The dot plots show the characteristics of lymphocytes gated for CD8+CD57− (third row) or CD8+CD57+ (bottom row).
CD94/NKG2A-mediated Inhibition of Melanoma Cell Lysis.
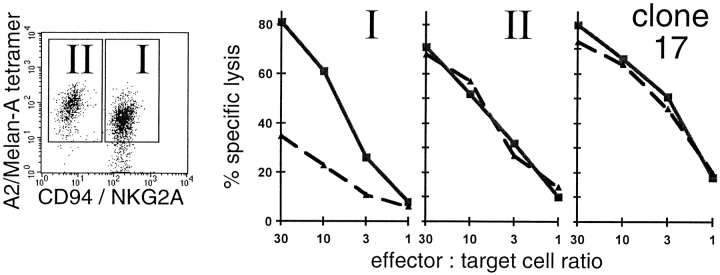
It has been shown that antigen-specific T cell cytotoxicity can indeed be inhibited through triggering of CD94/NKG2A 20 or p58.2 16 18 19. However, these results were obtained with selected T cell lines or clones, leaving the question open whether NK receptor inhibition is functional in polyclonal T cell responses. Therefore, we investigated the cytotoxicity by tetramer-sorted PBLs. Several of our attempts failed, since we did not obtain enough tetramer-positive, NK receptor–positive cells through FACS® sorting. However, one of our patients (LAU 156) had exceptionally high percentages of A2/Melan-A tetramer–positive, CD94/NKG2A-positive cells in the peripheral blood (Fig. 2). After FACS® sorting, the tetramer-positive cells were cultured for 1 wk and sorted again to obtain pure A2/Melan-A tetramer CD94/NKG2A double positive CTLs. These cells were tested in cytotoxicity assays against the melanoma cell line Me 290, previously stimulated with IFN-γ for 48 h to increase MHC expression and inhibitory receptor triggering 40. Indeed, the killing was enhanced in the presence of blocking anti-CD94 antibody Y9 (Fig. 4, solid line) compared with the killing in the presence of isotype-matched control anti-CD16 antibody (dashed line). The target cells were efficiently killed by the NK receptor–negative CTLs of the patient, and by the HLA-A*0201/Melan-A–specific CTL clone 17, both to a similar extent in the presence of anti-CD94 or control antibody. The killing of the Me 290 melanoma cell line (HLA-A*0201 and Melan-A positive) was antigen specific, since all of the CTLs did not lyse the Melan-A–negative melanoma cells Na8 unless synthetic Melan-A peptide was added (data not shown). In summary, the data demonstrate that the lysis of melanoma cells was inhibited due to the inhibitory receptor CD94/NKG2A expressed by the Melan-A–specific effector CTLs.
Figure 4.
CD94/NKG2A-mediated inhibition of melanoma cell lysis by Melan-A–specific CTLs. PBLs from patient LAU 156 were FACS® sorted to obtain purified HLA-A2/Melan-A tetramer–positive cells, then in vitro expanded for 1 wk in the presence of IL-2 and IL-15. Subsequently, a second FACS® sorting was performed to obtain tetramer-positive cells that were positive (I) or negative (II) for CD94/NKG2A (dot plot). These cells were tested against the HLA-A*0201–positive melanoma target cells Me 290 previously stimulated with IFN-γ for 48 h to increase MHC expression. The addition of the blocking mAb Y9 (anti-CD94; solid line) enhanced lysis by the CD94/NKG2A–positive (I) but not by the negative (II) population. In the former, only low cytolytic activity was observed in the presence of the irrelevant isotype-matched control anti-CD16 mAb (dashed line). Lysis by the CD94/NKG2A-negative cells of the patient (II) and by the A2/Melan-A–specific clone 17 were not altered in the presence of either of the two mAbs. Cultures without mAbs gave similar results as with the anti-CD16 control mAb (not shown). The results are representative for two experiments.
Discussion
TILNs of seven melanoma patients were investigated. In one patient, the Melan-A–specific CD8+ T cells were mostly CD94/NKG2A positive. In other patients, they were partly ILT2 positive. Similar NK receptor–positive tumor antigen–specific T cells were also found in peripheral blood from at least one melanoma patient with vitiligo. Furthermore, NK receptor triggering inhibited the cytolytic activity of T cells from one patient. Together, these results show that CD8+ T cells may express NK receptors in vivo and that this may lead to inhibition of melanoma cell lysis.
The early observation that NK cells preferentially lyse target cells that lack MHC class I molecules led to the formulation of the “missing self” hypothesis 41. This surmised that NK cells were endowed with the ability to recognize and destroy cells lacking expression of MHC class I, whereas normal cells positive for MHC class I would be protected from NK cell lysis. Only after the identification and cloning of several NK receptors could this hypothesis be confirmed 13 14 15. It is now established that the binding of inhibitory NK receptors to MHC class I molecules on target cells can lead to the delivery of signals that inhibit the cytolytic function of NK cells. As a consequence, abnormal (infected or malignant) cells lacking MHC class I may be destroyed preferentially.
NK receptors were also found to be expressed by T cells. However, although NK cells frequently express these receptors, only small percentages of NK receptor–positive T cells (NKT cells) were identified 16. In addition, some NKT cell populations were found to be mono- or oligoclonal, as indicated by very limited diversity of T cell receptor rearrangements 42. Based on these findings, it was postulated that NKT cells are a small subpopulation and may represent particular lymphocyte lineages with special antigen specificity and/or special function.
Recently, Ikeda et al. described a melanoma-specific CD8+ T cell clone that was inhibited by p58.2 recognizing HLA-Cw7 on autologous melanoma cells 18. Noppen et al. characterized sister T cell clones bearing the same T cell receptor specific for a melanoma-associated antigen 20. Some clones were NK receptor negative, but others expressed the inhibitory receptor CD94/NKG2A and showed reduced cytotoxicity to melanoma cells. These results demonstrated that cloned NKT cells may bear functional T cell receptors specific for classical peptide antigens presented by MHC class I.
The above-mentioned studies were done with selected T cell clones that may not be representative, since the majority of T cell lines and clones are NK receptor negative (data not shown). To investigate whether NKT cells are rare or frequent in vivo, we applied the recently developed tetramer technology which allowed us to visualize and phenotype peptide antigen–specific, yet polyclonal CD8+ T cells without in vitro cultivation. Our data show that tumor-specific T cells expressing NK receptors can indeed be found at high percentages in some patients. Thus, NKT cells may not necessarily represent separate cell lineages, but can belong to the large pool of T cells with classical peptide/MHC class I specificity.
Why should the CTL activity be modulated through this pathway? Current evidence suggests that CTLs which are activated over a prolonged period may need and have inhibitory mechanisms, and that human NKT cells may fall in this category of activated CTLs. Some NK receptor–expressing T cells have been described to be CD28 negative and express activation markers such as CD56 43, CD18, and CD45RO 42. Using a large panel of different NK receptor–specific mAbs, we have recently demonstrated that the majority of NKT cells in the circulation are TCR-α/β1, CD8+, and CD28−. Furthermore, these cells account for a large fraction of CD28− T cells, which make up 10–80% of circulating CD8+ T cells in melanoma patients 39. CD28− T cells have also been shown to be strongly cytotoxic and to proliferate poorly in vitro 37. Thus, T cells may downregulate CD28 and upregulate NK receptors in association with prolonged activation for cytolytic effector function. It is likely that NK receptors are involved in peripheral regulatory mechanisms avoiding overwhelming immune responses and immunopathology, particularly in situations of strong and/or long-lasting immune activation. The increased numbers of CD28− T cells in prolonged infections such HIV 44 or CMV 45 may support this notion. The function of NK receptors as inhibitory pathways has the advantage that abnormal infected or tumor cells lacking MHC class I may still be efficiently eliminated by the CTLs.
In summary, we found tumor-specific TCR-α/β1CD8+ T cells expressing NK receptors that could inhibit the lysis of melanoma cells. These data strongly suggest that NK receptors may modulate T cell activities in vivo. NK receptor expression by T cells varies depending on the cellular activation status and cytokines such as IL-12, IL-15, and TGF-β 31 32 46. Possibly, new therapeutic strategies modifying NK receptor expression or function may help improve the efficacy of immunotherapy in infectious disease, cancer, autoimmunity, and transplantation.
Acknowledgments
We would like to thank Alessandro Moretta and Miguel López-Botet for their kind gifts of NK receptor–specific antibodies. We particularly acknowledge the excellent technical assistance of Andrée Porret, Danielle Minaïdis, Nicole Montandon, and Renate Milesi, and the secretarial help of Martine van Overloop. We also thank Christian Knabenhans and Pierre Zaech for flow cytometry, Vincent Aubert and Jean-Marie Tiercy for HLA typing, and Ferdy Lejeune, Serge Leyvraz, and Giuseppe Pantaleo for their help.
Footnotes
1used in this paper: ILT2, Ig-like transcript 2; NKT cell, NK receptor–positive cell expressing CD3 and/or TCR-α/β; TILN, tumor-infiltrated LN
References
- Zinkernagel R.M., Doherty P.C. Restriction of in vitro T cell mediated cytotoxicity in lymphocytic choriomeningitis within a syngeneic or semiallogeneic system. Nature. 1974;248:701–702. doi: 10.1038/248701a0. [DOI] [PubMed] [Google Scholar]
- Townsend A.R.M., Rothbard J., Gotch F.M., Bahadur G., Wraith D., McMichael A.J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Robbins P.F., Kawakami Y. Human tumor antigens recognized by T cells. Curr. Opin. Immunol. 1996;8:628–636. doi: 10.1016/s0952-7915(96)80078-1. [DOI] [PubMed] [Google Scholar]
- Boon T., Cerottini J.-C., Van den Eynde B., van der Bruggen P., Van Pel A. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- Rosenberg S.A., Yang J.C., Schwartzentruber D.J., Hwu P., Marincola F.M., Topalian S.L., Restifo N.P., Dudley M.E., Schwarz S.L., Spiess P.J. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat. Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle F.O., Alijagic S., Gilliet M., Yuansheng S., Grabbe S., Dummer R., Burg G., Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat. Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- Marchand M., Van Baren N., Weynants P., Brichard V., Dréno B., Tessier M.H., Rankin E., Parmiani G., Arienti F., Humblet Y. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int. J. Cancer. 1999;80:219–230. doi: 10.1002/(sici)1097-0215(19990118)80:2<219::aid-ijc10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Speiser D.E., Miranda R., Zakarian A., Bachmann M.F., McKall-Faienza K., Odermatt B., Hanahan D., Zinkernagel R.M., Ohashi P.S. Self antigens expressed by solid tumors do not stimulate naive or activated T cellsimplications for immunotherapy. J. Exp. Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X., McCarrick J., Jewett L., Knowles B.B. Timely immunization subverts the development of peripheral nonresponsiveness and suppresses tumor development in simian virus 40 tumor antigen-transgenic mice. Proc. Natl. Acad. Sci. USA. 1994;91:3916–3920. doi: 10.1073/pnas.91.9.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen E.P., Kim T.S. Neoplastic cells that express low levels of MHC class I determinants escape host immunity. Semin. Cancer Biol. 1994;5:419–428. [PubMed] [Google Scholar]
- Cromme F.V., Airey J., Heemels M.-T., Ploegh H.L., Keating P.J., Stern P.L., Meijer C.J.L.M., Walboomers J.M.M. Loss of transporter protein, encoded by the TAP-1 gene, is highly correlated with loss of HLA expression in cervical carcinomas. J. Exp. Med. 1994;179:335–340. doi: 10.1084/jem.179.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onrust S.V., Hartl P.M., Rosen S.D., Hanahan D. Modulation of L-selectin ligand expression during an immune response accompanying tumorigenesis in transgenic mice. J. Clin. Invest. 1996;97:54–64. doi: 10.1172/JCI118406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Sivori S., Vitale M., Pende D., Morelli L., Augugliaro R., Bottino C., Moretta L. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J. Exp. Med. 1995;182:875–884. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M., Navarro F., Bellon T., Llano M., Garcia P., Samaridis J., Angman L., Cella M., López-Botet M. A common inhibitory receptor for major histocompatibility complex class I molecules on human lymphoid and myelomonocytic cells. J. Exp. Med. 1997;186:1809–1818. doi: 10.1084/jem.186.11.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Vitale M., Sivori S., Bottino C., Morelli L., Augugliaro R., Bararesi M., Pende D., Ciccone E., López-Botet M., Moretta L. Human natural killer cell receptors for HLA-class I molecules. Evidence that the Kp43 (CD94) molecule functions as receptor for HLA-B alleles. J. Exp. Med. 1994;180:545–555. doi: 10.1084/jem.180.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J.H., Gumperz J.E., Parham P., Lanier L.L. Superantigen-dependent, cell-mediated cytotoxicity inhibited by MHC class I receptors on T lymphocytes. Science. 1995;268:403–405. doi: 10.1126/science.7716542. [DOI] [PubMed] [Google Scholar]
- Ferrini S., Cambiaggi A., Meazza R., Sforzini S., Marciano S., Mingari M.C., Moretta L. T cell clones expressing the natural killer cell-related p58 receptor molecule display heterogeneity in phenotypic properties and p58 function. Eur. J. Immunol. 1994;24:2294–2298. doi: 10.1002/eji.1830241005. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Lethé B., Lehmann F., Van Baren F., Baurain J.-F., De Smet C., Chambost H., Vitale M., Moretta A., Boon T., Coulie P.G. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. doi: 10.1016/s1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- Bakker A.B.H., Phillips J.H., Figdor C.G., Lanier L.L. Killer cell inhibitory receptors for MHC class I molecules regulate lysis of melanoma cells mediated by NK cells, gamma delta cells, and antigen-specific CTL. J. Immunol. 1998;160:5239–5245. [PubMed] [Google Scholar]
- Noppen C., Schaefer C., Zajac P., Schütz A., Kocher T., Kloth J., Heberer M., Colonna M., De Libero G., Spagnoli G.C. C-type lectin-like receptors in peptide-specific HLA class I-restricted cytotoxic T lymphocytesdifferential expression and modulation of effector functions in clones sharing identical TCR structure and epitope specificity. Eur. J. Immunol. 1998;28:1134–1142. doi: 10.1002/(SICI)1521-4141(199804)28:04<1134::AID-IMMU1134>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Coulie P.G., Brichard V., Van Pel A., Wölfel T., Schneider J., Traversari C., Mattei S., De Plaen E., Lurquin C., Szikora J.P., Renauld J.C., Boon T. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 1994;180:35–42. doi: 10.1084/jem.180.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y., Eliyahu S., Delgado C.H., Robbins P.F., Sakaguchi K., Appella E., Yannelli J.R., Adema G.J., Miki T., Rosenberg S.A. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc. Natl. Acad. Sci. USA. 1994;91:6458–6462. doi: 10.1073/pnas.91.14.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J.D., Moss P.A.H., Goulder P.J.R., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Ogg G.S., Dunbar P.R., Romero P., Chen J., Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J. Exp. Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero P., Dunbar P.R., Valmori D., Pittet M., Ogg G.S., Rimoldi D., Chen J.L., Liénard D., Cerottini J.-C., Cerundolo V. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmori D., Fonteneau J.F., Marañón-Lizana C., Gervois N., Liénard D., Rimoldi D., Jongeneel C.V., Jotereau F., Cerottini J.-C., Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogs. J. Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- Parham P., Brodsky F.M. Partial purification and some properties of BB7.2, a cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum. Immunol. 1981;3:277–284. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- Speiser D.E., Tiercy J.-M., Rufer N., Grundschober C., Gratwohl A., Chapuis B., Helg C., Löliger C.-C., Siren M.-K., Roosnek E., Jeannet M. High resolution HLA matching associated with decreased mortality after unrelated bone marrow transplantation. Blood. 1996;87:4455–4462. [PubMed] [Google Scholar]
- Braud V.M., Allan D.S.J., O'Callaghan C.A., Söderström K., D'Andrea A., Ogg G.S., Lazetic S., Young N.T., Bell J.I., Phillips J.H. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- Borrego F., Ulbrecht M., Weiss E.H., Coligan J.E., Brooks A.G. Recognition of human histocompatibility leukocyte antigen (HLA)-E complexed with HLA class I signal sequence–derived peptides by CD94/NKG2 confers protection from natural killer cell–mediated lysis. J. Exp. Med. 1998;187:813–816. doi: 10.1084/jem.187.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A., Biassoni R., Bottino C., Pende D., Vitale M., Poggi A., Mingari M.C., Moretta L. Major histocompatibility complex class I-specific receptors on human natural killer and T lymphocytes. Immunol. Rev. 1997;155:105–117. doi: 10.1111/j.1600-065x.1997.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Mingari M.C., Ponte M., Bertone S., Schiavetti F., Vitale C., Bellomo R., Moretta A., Moretta L. HLA class I-specific inhibitory receptors in human T lymphocytesinterleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc. Natl. Acad. Sci. USA. 1998;95:1172–1177. doi: 10.1073/pnas.95.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund J.J., Kirkwood J.M., Forget B.M., Milton G., Albert D.M., Lerner A.B. Vitiligo in patients with metastatic melanomaa good prognostic sign. J. Am. Acad. Derm. 1983;9:689–696. doi: 10.1016/s0190-9622(83)70182-9. [DOI] [PubMed] [Google Scholar]
- Song Y.-H., Connor E., Li Y., Zorovich B., Balducci P., Maclaren N. The role of tyrosinase in autoimmune vitiligo. Lancet. 1994;344:1049–1052. doi: 10.1016/s0140-6736(94)91709-4. [DOI] [PubMed] [Google Scholar]
- Pittet, M.J., D. Valmori, P.R. Dunbar, D.E. Speiser, D. Liénard, F. Lejeune, K. Fleischhauer, V. Cerundolo, J.-C. Cerottini, and P. Romero. 1999. High frequencies of naive Melan-A/MART-1–specific CD8+ T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. 190:705–715. [DOI] [PMC free article] [PubMed]
- Merkenschlager M., Beverley P.C. Evidence for differential expression of CD45 isoforms by precursors for memory-dependent and independent cytotoxic responseshuman CD8 memory CTLp selectively express CD45RO (UCHL1) Int. Immunol. 1989;1:450–459. doi: 10.1093/intimm/1.4.450. [DOI] [PubMed] [Google Scholar]
- de Jong R., Brouwer M., Miedema F., van Lier R.A. Human CD8+ T lymphocytes can be divided into CD45RA+ and CD45RO+ cells with different requirements for activation and differentiation. J. Immunol. 1991;146:2088–2094. [PubMed] [Google Scholar]
- Azuma M., Phillips J.H., Lanier L.L. CD28− T lymphocytes. Antigenic and functional properties. J. Immunol. 1993;150:1147–1159. [PubMed] [Google Scholar]
- Hamann D., Baars P.A., Rep M.H., Hooibrink B., Kerkhof-Garde S.R., Klein M.R., van Lier R.A. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speiser D.E., Valmori D., Rimoldi D., Pittet M.J., Liénard D., MacDonald H.R., Cerottini J.-C., Romero P. CD28-negative cytolytic effector T cells frequently express NK receptors and are present at variable proportions in circulating lymphocytes from healthy donors and melanoma patients. Eur. J. Immunol. 1999;29:1990–1999. doi: 10.1002/(SICI)1521-4141(199906)29:06<1990::AID-IMMU1990>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Pende D., Accame L., Pareti L., Mazzocchi A., Moretta A., Parmiani G., Moretta L. The susceptibility to natural killer cell-mediated lysis of HLA class I-positive melanomas reflects the expression of insufficient amounts of different HLA class I alleles. Eur. J. Immunol. 1998;28:2384–2394. doi: 10.1002/(SICI)1521-4141(199808)28:08<2384::AID-IMMU2384>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Ljunggren H.-G., Kärre K. In search of the ‘missing self’MHC molecules and NK cell recognition. Immunol. Today. 1990;11:237–244. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Mingari M.C., Schiavetti F., Ponte M., Vitale C., Maggi E., Romagnani S., Demarest J., Pantaleo G., Fauci A.S., Moretta L. Human CD8+ T lymphocyte subsets that express HLA class I-specific inhibitory receptors represent oligoclonally or monoclonally expanded cell populations. Proc. Natl. Acad. Sci. USA. 1996;93:12433–12438. doi: 10.1073/pnas.93.22.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin C., Foster B. TCR Vα24 and Vβ11 coexpression defines a human NK1 T cell analog containing a unique Th0 subpopulation. J. Immunol. 1997;159:5862–5870. [PubMed] [Google Scholar]
- Borthwick N.J., Bofill M., Gombert W.M., Akbar A.N., Medina E., Sagawa K., Lipman M.C., Johnson M.A., Janossy G. Lymphocyte activation in HIV-1 infection. II. Functional defects of CD28− T cells. AIDS. 1994;8:431–441. doi: 10.1097/00002030-199404000-00004. [DOI] [PubMed] [Google Scholar]
- Wang E.C.Y., Moss P.A., Frodsham P.M., Lehner P.J., Bell J.I., Borysiewicz L.K. CD8high CD57+ T lymphocytes in normal, healthy individuals are oligoclonal and respond to human cytomegalovirus. J. Immunol. 1995;155:5046–5056. [PubMed] [Google Scholar]
- Poggi A., Costa P., Tomasello E., Moretta L. IL-12-induced up-regulation of NKRP1A expression in human NK cells and consequent NKRP1A-mediated down-regulation of NK cell activation. Eur. J. Immunol. 1998;28:1611–1616. doi: 10.1002/(SICI)1521-4141(199805)28:05<1611::AID-IMMU1611>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]