Abstract
Migration of antigen-activated CD4 T cells to B cell areas of lymphoid tissues is important for mounting T cell–dependent antibody responses. Here we show that CXC chemokine receptor (CXCR)5, the receptor for B lymphocyte chemoattractant (BLC), is upregulated on antigen-specific CD4 T cells in vivo when animals are immunized under conditions that promote T cell migration to follicles. In situ hybridization of secondary follicles for BLC showed high expression in mantle zones and low expression in germinal centers. When tested directly ex vivo, CXCR5hi T cells exhibited a vigorous chemotactic response to BLC. At the same time, the CXCR5hi cells showed reduced responsiveness to the T zone chemokines, Epstein-Barr virus–induced molecule 1 (EBI-1) ligand chemokine (ELC) and secondary lymphoid tissue chemokine (SLC). After adoptive transfer, CXCR5hi CD4 T cells did not migrate to follicles, indicating that additional changes may occur after immunization that help direct T cells to follicles. To further explore whether T cells could acquire an intrinsic ability to migrate to follicles, CD4−CD8− double negative (DN) T cells from MRL-lpr mice were studied. These T cells normally accumulate within follicles of MRL-lpr mice. Upon transfer to wild-type recipients, DN T cells migrated to follicle proximal regions in all secondary lymphoid tissues. Taken together, our findings indicate that reprogramming of responsiveness to constitutively expressed lymphoid tissue chemokines plays an important role in T cell migration to the B cell compartment of lymphoid tissues.
Keywords: chemokine, CXCR5, ELC, follicle, T lymphocyte
Tcell–dependent antibody responses require cognate interaction between antigen-presenting B cells and antigen-specific T cells 1 2. Antigen-mediated cross-linking of the B cell receptor promotes B cell localization at the boundary between T zone and follicles (for a review, see reference 3), the location of the earliest encounters between antigen-bearing B cells and antigen-specific T cells 4 5 6. After receiving signals from activated CD4 T cells, some B cells undergo differentiation into plasmablasts, which migrate to the red pulp of spleen or medullary cords of LNs and become antibody-secreting plasma cells. Other activated B cells move into the follicle and form a germinal center (GC)1 1. The GC is strongly dependent on help from antigen-specific T cells that must migrate into the follicle to support this response 7 8 9 10 11 12. Movement of antigen-specific T cells has been studied within lymphoid tissues using an adoptive T cell transfer model 13. When recipients of OVA-specific TCR-transgenic CD4+ T cells are immunized with OVA in adjuvant, there is a short period of transgenic T cell proliferation in the T zone, and then large numbers of these cells are induced to migrate into follicles 13. T cell migration to follicles has also been studied in nontransgenic mice during the response to pigeon cytochrome c (PCC [7, 9, 14]). The predominant responding T cell population expresses TCR containing Vα11 and Vβ3 15 16. When mice are immunized with PCC in adjuvant, a protocol that induces a strong GC response, Vα11Vβ3-expressing T cells are found to begin localizing in follicles by day 5 and to peak in number at day 10, with many of the cells at this time being resident in GCs 7 9 10 14 17. In contrast to these findings, when mice are given antigen in saline intravenously, T cells become activated but fail to migrate into follicles. Many of the cells are quickly eliminated, and those that remain respond poorly to subsequent antigen exposure 13. Therefore, CD4 T cell migration into follicles has been suggested to be important for supporting not only B cell responses, but also a fully developed T cell response and induction of T cell memory.
The understanding of how antigen-specific T cells migrate to follicles is poorly developed. Recently, several chemokines have been characterized that are constitutively expressed in lymphoid tissues and help direct the movements of resting and activated lymphocytes. B lymphocyte chemoattractant (BLC [18]; also called B cell–attracting chemokine 1 [BCA-1; 19]) is a CXC chemokine made by stromal cells in lymphoid follicles that functions as a ligand for CXC chemokine receptor 5 (CXCR5, previously called Burkitt's lymphoma receptor [BLR1]) 20. Gene knockout studies have established that CXCR5 is required for B cell homing to follicles in spleen and Peyer's patches 21. In vitro, BLC was shown to be an efficacious attractant of B cells while attracting few T cells and no myeloid cells 18 19. Two CC chemokines, secondary lymphoid tissue chemokine (SLC, also called 6-Ckine) and EBV-induced molecule 1 (EBI-1) ligand chemokine (ELC, also called macrophage inflammatory protein [MIP]-3β), are constitutively expressed by cells in the T zone 22 23. SLC and ELC are ligands for CC chemokine receptor 7 and both strongly attract resting and in vitro–activated T cells 22 23 24 25 26 27 28 29 30. Mice that lack SLC expression (and have reduced ELC expression) have defective homing of T cells into LNs and splenic T cell areas 31.
Previous studies have shown that while most T cells are negative for CXCR5, a subset of memory phenotype cells are CXCR5+ 20 21. Here we have investigated the relationship between acquisition of CXCR5 expression by CD4 T cells and homing to lymphoid follicles. We establish that CXCR5 upregulation and acquisition of BLC responsiveness of in vivo–activated CD4 T cells occur with a time course consistent with a role in directing T cells to follicles. We also establish that at the same time as upregulating CXCR5, in vivo–activated CD4 T cells downregulate their response to ELC and SLC. Finally, we demonstrate that CD4−CD8− double negative (DN) T cells from MRL-lpr mice express CXCR5 and, upon transfer to normal recipients, migrate to follicle proximal locations in all secondary lymphoid tissues, establishing that T cells can acquire the intrinsic ability to migrate to B cell follicles.
Materials and Methods
Mice.
Six 10-wk-old Balb/cAnN mice were obtained from Charles River Laboratories. C57BL/6 (B6), MRL/MpJ (MRL), and MRL/Mp-lpr/lpr (MRL-lpr) mice were from The Jackson Laboratory. DO11.10 TCR-transgenic mice 32 on the Balb/c background, and aged and control B6 mice for memory T cell analysis were maintained in the University of California at San Francisco animal care facility. Eight 10-wk-old, specific pathogen-free, male B10.BR mice (The Jackson Laboratory) were housed under barrier conditions at the Duke University Vivarium.
Chemokines.
HIS6-tagged murine BLC was prepared by PCR-based insertion of six histidine codons preceding the BLC stop codon. The BLC-his6 construct was inserted into the CMV-based mammalian expression vector pRK5 33 and stably transfected into HEK-293 cells using the Lipotaxi Mammalian Transfection kit (Stratagene) according to the manufacturer's instructions. HIS6-BLC was purified from tissue culture supernatants using an NiNTA column (Qiagen). The protein was eluted in 100 μM imidazole (Fisher Scientific Co.). SDS-PAGE separation of the eluate revealed a band representing >90% of total protein corresponding to the recombinant HIS6-BLC. HIS6-ELC was produced in bacteria and purified as described 23. A similarly constructed vector for bacterial HIS6-SLC production was a gift from M. Gunn (Duke University, Durham, NC). Stromal cell–derived factor (SDF)1α (N33A) produced by chemical ligation (Gryphon Sciences), HIS6-BLC, -ELC, and -SLC were used in all chemotaxis assays, except for MRL-lpr chemotaxis where, due to availability at the time of the experiments, non–HIS-tagged murine SLC (gift of M. Gunn) and human ELC (R&D Systems) were used. We have not observed significant differences in the response of mouse cells to mouse or human ELC.
DO11.10 T Cell Adoptive Transfer, OVA Immunization, and Recipient Analysis.
Adoptive transfer and immunization of recipients were carried out essentially as described 13. Lymphocytes were isolated from LNs or spleen of DO11.10 donor mice, and the percentage of OVA323–339 peptide/I-Ad–specific CD4+ T cells was determined by flow cytometric analysis of an aliquot of cells stained with FITC-conjugated clonotypic mAb KJ1-26 and anti-CD4–PE (Caltag). 2.5 × 106 KJ1-26+CD4+ cells were adoptively transferred into sex-matched Balb/c recipients by intravenous injection. The day after cell transfer, mice were immunized with 300 μg OVA323–339 peptide either emulsified in CFA (Sigma Chemical Co.) and injected subcutaneously in a total volume of 0.1 ml distributed over three points on the back, or in sterile PBS by intravenous injection. Recipients were killed and dissected 2, 3, 5, 7, 10, 14, or 25 d after immunization. In a second immunization protocol 34, mice were injected subcutaneously with 2 mg OVA protein (Sigma Chemical Co.) mixed in 0.2 ml of 250 μg/ml LPS (Sigma Chemical Co.). For subcutaneously injected mice, lymphocytes were isolated from axillary and brachial LNs. For intravenously injected mice, cells from mandibular, cervical, axillary, brachial, inguinal, and in some cases mesenteric nodes were pooled. The remaining peripheral LNs were frozen in OCT (Miles, Inc.) for sectioning. Flow cytometric analysis was performed using affinity-purified anti-CXCR5 rabbit antiserum 35, followed by biotinylated goat anti–rabbit IgG (PharMingen) with normal mouse and rat serum (1:100 dilution), and then streptavidin-Cychrome (PharMingen), KJ1-26–FITC, anti-B220–PE, and anti-CD8–PE (Caltag).
PCC Immunization and Flow Cytometry.
Whole PCC (Sigma Chemical Co.) was diluted into PBS and mixed with the Ribi adjuvant system (RAS; Ribi Immunochem Research). B10.BR mice were immunized with 400 μg of PCC in 200 μl of adjuvant emulsion in two 100-μl doses by subcutaneous injection on either side of the base of the tail. Animals were killed at 3, 5, 7, and 9 d after immunization, and the draining LNs were harvested as described previously 17. In brief, inguinal and periaortic nodes were collected, and using 0.17 M NH4Cl solution for erythrocyte lysis were made into single cell suspensions. Cells were incubated with anti-CXCR5 rabbit antiserum, followed by anti–rabbit IgG-biotin (Santa Cruz Biotechnology). After blocking with normal rabbit and mouse serum (1:100 dilution) for 5 min, staining was completed using streptavidin-PE (PharMingen), anti-Vα11–FITC (PharMingen), anti-Vβ3–allophycocyanin, anti-B220–Cy5PE (PharMingen), anti-CD8–Cy5PE (PharMingen), anti-CD11b–Cy5PE (Caltag), and anti-CD44–Texas red. Finally, cells were resuspended in 2 μg/ml propidium iodide (for dead cell exclusion) in PBS with 5% FCS. The cells were analyzed using a dual laser modified FACStarPLUS™ (Becton Dickinson Immunocytometry Systems; an argon laser as the primary, a tunable dye laser as the secondary) capable of seven-parameter collection. Files were acquired using CELLQuest™ software (Becton Dickinson) and analyzed using FlowJo software (Tree Star, Inc.).
Chemotaxis Assays.
Chemotaxis assays were performed as described 23 using 106 total cells per 5-μm transwell (Corning Costar Corp.). To identify migrating populations, a fraction of transmigrated cells was stained and analyzed by flow cytometry. Transmigrated LN cells from Balb/c recipients of OVA-specific T cells were stained with KJ1-26–FITC and anti-CD4–TriColor (Caltag), or with anti-CD4–PE (Caltag) and KJ1-26–biotin followed by streptavidin-Cychrome (PharMingen). Because BLC causes reversible internalization of CXCR5 36, transmigrated splenocytes from aged B6 mice were washed twice and incubated in RPMI plus 0.5% BSA for 1 h at 37°C, 5% CO2 to allow CXCR5 reexpression before staining with anti-CXCR5 rabbit antiserum/goat anti–rabbit IgG-biotin/streptavidin-Cychrome, anti-CD4–FITC (Caltag), and anti-CD62L–PE (PharMingen). LN suspensions from 5–8-mo-old MRL-lpr mice were stained with anti-B220–PE, anti-Thy1.2–biotin (Caltag), and anti-CXCR5 rabbit antiserum followed by goat anti–rabbit IgG-FITC (Caltag) and streptavidin-Cychrome. To provide an internal control, MRL-lpr splenocytes (70% Thy1+B220+, 9% Thy1+B220−) were mixed 3:1 with B6 splenocytes (<1% Thy1+B220+, 27% Thy1+B220−) for chemotaxis assays. Transmigrated cells were stained with anti-Thy1.2–FITC (Caltag), anti-B220–PE, and anti-CD21–biotin/streptavidin-Cychrome.
MRL-lpr DN T Cell and Aged B6 CXCR5hi T Cell Adoptive Transfers.
DN T cells were purified from LNs of 5–8-mo-old MRL-lpr mice. Total LN cells were incubated with biotinylated mAbs against CD22 (PharMingen), CD4, and CD8 (Caltag) followed by streptavidin-coated magnetic beads, and then passed over a MACS® column (Miltenyi Biotec). Memory phenotype splenocytes from 14-mo-old mice were enriched by MACS® depletion with biotinylated mAbs against CD8, B220, and CD11b (Caltag). For all transfers, 2 × 107 cells were labeled with 5- (and 6-)carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes) as described 35 and transferred by intravenous injection into appropriate syngeneic (MRL or B6) recipients. After ∼24 h, recipients were killed, and spleen, LNs, and Peyer's patches were frozen in OCT for sectioning.
Immunohistochemistry, Immunofluorescence Microscopy, and In Situ Hybridization.
For immunohistochemistry, cryostat sections (7–8 μm) were fixed and stained as described previously 35 with the following reagents: biotinylated or FITC-conjugated KJ1-26, rat anti-CD4 and anti-CD8 (Caltag), and biotinylated peanut agglutinin (PNA; Sigma Chemical Co.). Biotinylated reagents were detected with avidin–alkaline phosphatase (Sigma Chemical Co.), rat mAbs with horseradish peroxidase–conjugated goat anti–rat IgG (Southern Biotechnology Associates), and KJ1-26–FITC with horseradish peroxidase–conjugated antifluorescein (NEN). Enzyme reactions were developed with conventional substrates for peroxidases (diaminobenzidine/H2O2; Sigma Chemical Co.) and alkaline phosphatase (Fast Red/Naphthol AS-MX; Sigma Chemical Co.). Endogenous alkaline phosphatase activity was blocked with levamisole (Sigma Chemical Co.). Some sections were counterstained with hematoxylin (Fisher Scientific Co.). Sections were mounted in crystal mount (Biomeda Corp.). For immunofluorescence microscopy, unfixed sections were air-dried and incubated with biotinylated mAbs against CD8, CD4, or Thy1.2 (Caltag) and CD3∈ (PharMingen) followed by streptavidin-Cy3 (Jackson ImmunoResearch Labs). Three-color staining of spleen sections was achieved by costaining with rat anti–MOMA-1 37 followed by goat anti–rat IgG-aminomethylcoumarin (Jackson ImmunoResearch Labs). Sections were mounted in Fluoromount G (Southern Biotechnology Associates), viewed, and photographed as described 35. In situ hybridization analysis was performed as described 23 using a BLC probe spanning nucleotides 27–1042 of mouse BLC 18.
Results
Immunization Conditions that Promote Antigen-specific CD4 T Cell Homing to Follicles Cause Rapid Upregulation of CXCR5 Expression.
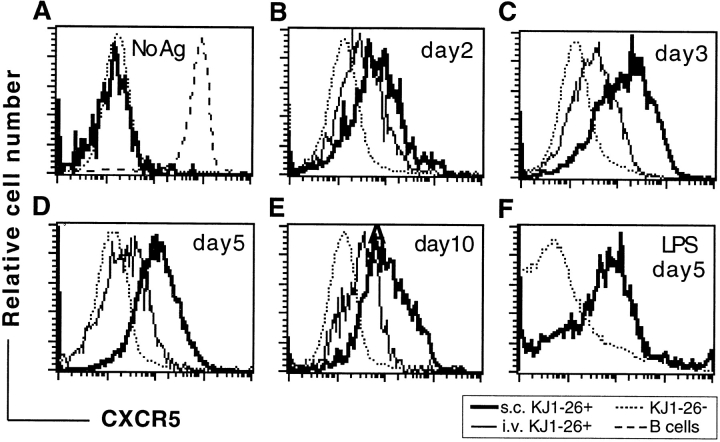
Mice that had received an inoculum of OVA-specific TCR-transgenic (DO11.10) T cells were injected with OVA323–339 peptide either subcutaneously in CFA to promote T cell trafficking to follicles, or intravenously in PBS to promote transient T cell activation without migration to follicles 13. At days 2, 3, 5, and 10 after immunization, LNs were isolated and analyzed by flow cytometry with a clonotypic antibody, KJ1-26, that recognizes the transferred OVA-specific T cells, and with an antiserum specific for CXCR5 35 36. Before immunization, transferred OVA-specific T cells were uniformly CXCR5lo/− (Fig. 1 A). However, within 2 d of immunization with peptide in CFA, when OVA-specific T cell numbers in the draining LNs start to increase 13, a subpopulation of CXCR5+ cells could be identified (Fig. 1 B). By day 3, when the OVA-specific T cell frequency had increased ∼30-fold, as in previous studies 13, most of the antigen-specific cells expressed high levels of CXCR5 (fluorescence intensity at least 10-fold greater than the staining control), and after 5 d the cells were uniformly CXCR5hi (Fig. 1C and Fig. D). Appearance of T cells in follicles followed similar kinetics to the CXCR5 upregulation (Fig. 2). Consistent with previous reports 13 34, KJ1-26+ OVA-specific T cells began appearing in follicles by day 3 after immunization (Fig. 2 A) and reached maximal numbers by day 5 (Fig. 2 B). An enlargement of draining LN B cell areas occurred over this time period, and by day 5 many of the follicles contained nascent GCs (Fig. 2 B). Immunization of mice with OVA protein in LPS, a protocol that has been shown to promote T cell trafficking to follicles 34, also led to increased expression of CXCR5 on CD4 T cells (Fig. 1 F). In contrast to these effects, intravenous injection of OVA peptide in saline led to only weak induction of CXCR5 on OVA-specific T cells in LNs (Fig. 1A–E) and spleen (data not shown), and did not promote KJ1-26+ T cell migration into follicles (Fig. 2 C). When mice immunized with OVA peptide in CFA were followed for longer times, a decline in CXCR5 expression was found to occur, although a significant proportion of KJ1-26+ cells remained CXCR5hi at day 10 after immunization (Fig. 1 E), and CXCR5hi cells could still be detected at day 25 (data not shown). By day 10, many B cell areas had become secondary follicles, comprising a well-developed GC and a surrounding mantle of small resting B cells (Fig. 2 D). Significant numbers of KJ1-26+ T cells were detectable in the secondary follicles, with many residing in the mantle zone and smaller numbers being associated with the outer zone of GCs (Fig. 2 D). BLC in situ hybridization analysis of LNs containing well-developed GCs showed that BLC was highly expressed in the follicular mantle zones (Fig. 2E and Fig. F). Within GCs, only occasional cells could be identified that hybridized with the BLC probe, and these cells tended to be most frequent in the area of the GC distal to the T zone (Fig. 2E and Fig. F). This is also the region of the GC most enriched for CD4 T cells (1; Fig. 2 D).
Figure 1.
CXCR5 upregulation by in vivo–activated OVA-specific T cells. (A–E) Histograms depict CXCR5 expression on non–OVA-specific CD4 cells (KJ1-26−) and on gated KJ1-26+B220−CD8− cells from mice that were treated as follows: (A) unimmunized; (B–E) immunized with OVA peptide subcutaneously in CFA (s.c. KJ1-26+) or intravenously in saline (i.v. KJ1-26+); (F) immunized with OVA protein in LPS. In A, the level of CXCR5 expression on B cells is shown for comparison (dashed line). In B–F, the number of days elapsed between immunization and analysis is displayed in each panel. No Ag, no antigen injected. The experiment shown in F was performed at a different time from A–E, and has a different level of background staining. Results are representative of at least three independent experiments at each time point except panel F, which is representative of two experiments.
Figure 2.
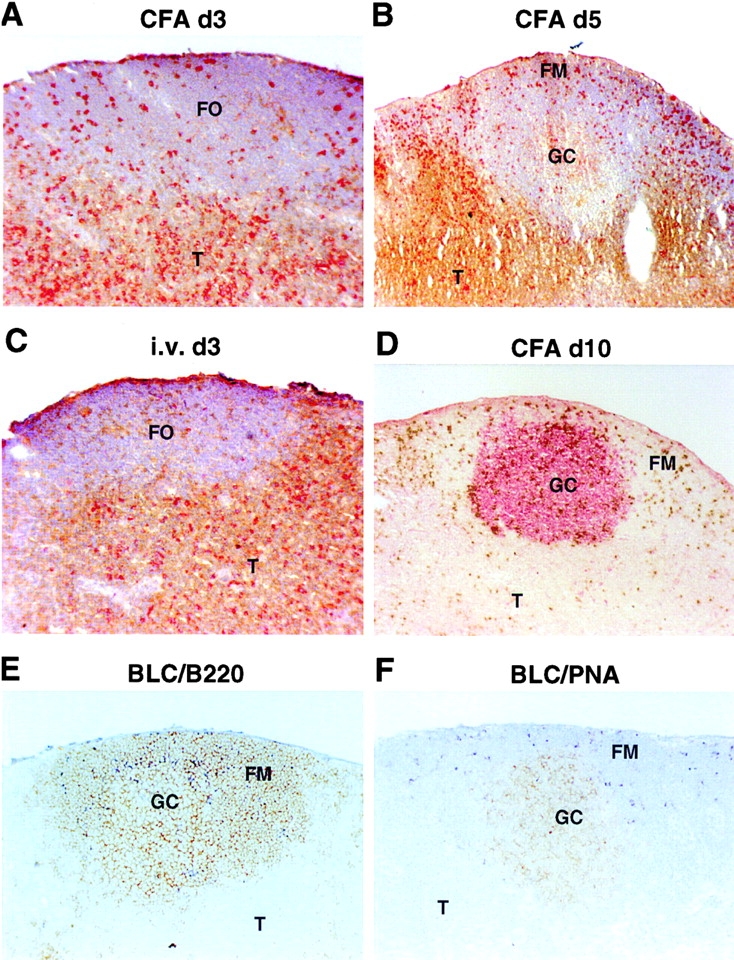
Follicular homing of in vivo–activated OVA-specific T cells and expression pattern of BLC in secondary follicles. (A–C) Staining of LN sections to detect KJ1-26+ OVA-specific T cells in red and CD4 and CD8 in brown. (D) Staining with peanut agglutinin (PNA) to detect GCs in red, and with KJ1-26 to detect OVA-specific T cells in brown. Sections are from draining LNs of mice that received OVA-specific CD4 T cells and were immunized with OVA peptide subcutaneously in CFA 3 (A), 5 (B), or 10 (D) d before isolation, or intravenously in saline (C) 3 d before isolation. (E and F) In situ hybridization analysis of BLC expression pattern in LNs containing secondary follicles. BLC hybridization is seen as dark blue staining, and sections are costained in brown for B220 (E) or PNA (F). The capsular staining in E was also seen in controls and is nonspecific. FO, follicle; T, T cell zone; FM, follicular mantle region. Original magnifications: A, C, E, and F, ×10; B and D, ×5.
CXCR5 Upregulation on Vα11Vβ3-expressing Cells during the PCC Response.
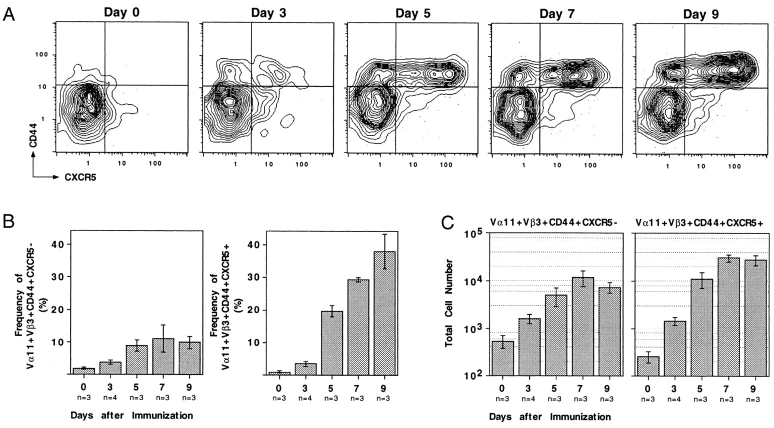
To test whether CXCR5 was upregulated on nontransgenic T cells under conditions not involving T cell transfer, the phenotype of PCC-specific Vα11Vβ3-expressing CD4 T cells was followed in mice immunized subcutaneously with PCC protein in adjuvant. Vα11Vβ3-expressing T cells responding to PCC were detected in immunized, but not unimmunized, animals by upregulation of CD44 (Fig. 3 A). CXCR5 expression became detectable on a subset of PCC-responsive Vα11+Vβ3+ T cells by day 3 after immunization (Fig. 3 A). This subpopulation grew in frequency through day 9 (Fig. 3 B), reaching maximal total numbers by day 7 (Fig. 3 C). These kinetics of CXCR5 expression are in close accord with the kinetics of Vα11Vβ3-expressing T cell accumulation in follicular mantle zones and GCs during the response to PCC 9 17. A subpopulation of the responding CD44+ Vα11Vβ3-expressing T cells did not upregulate CXCR5 (Fig. 3). Such bimodality was not observed in the response of the monoclonal DO11.10 T cells to OVA peptide (Fig. 1 D) and may indicate that T cells with differing affinity for peptide/MHC differ in their propensity to upregulate CXCR5.
Figure 3.
Antigen-specific CD4 T cell expression of CXCR5 during a primary immune response. (A) Representative probability contours for CD44 and CXCR5 expression on Vα11Vβ3-expressing T cells over the course of a primary response. All profiles are presented as 5% probability contours with outliers, and are propidium iodide− CD8−B220−CD11b−Vα11+Vβ3+. The day after antigen administration is displayed above each panel. The quadrants are defined by the horizontal dotted lines, and indicate the limits of CD44 and CXCR5 regulation that were used to calculate the frequencies of cellular subsets. (B) Frequencies of Vα11Vβ3-expressing T cells that have upregulated CD44 and are either CXCR5− (left panel) or CXCR5+ (right panel). (C) The total number of antigen-activated Vα11Vβ3-expressing T cells in the draining LNs as calculated using the frequencies obtained by flow cytometry and total cell counts estimated when organs were harvested; the left panel represents the CXCR5− subset, the right panel represents the CXCR5+ subset. Each estimation (B and C) is presented as the mean from at least three separate animals ± SEM.
Increased Responsiveness of CXCR5-expressing T Cells to the Follicular Chemokine BLC.
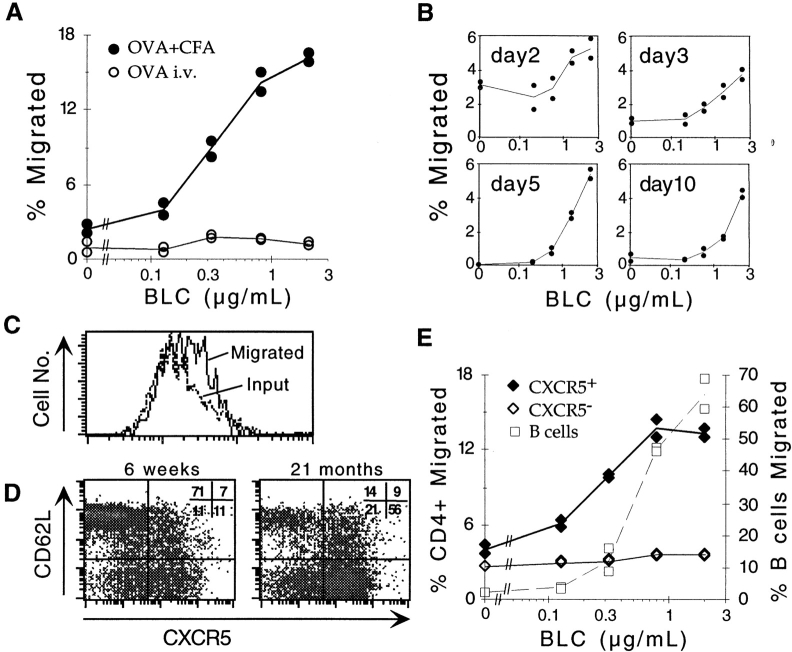
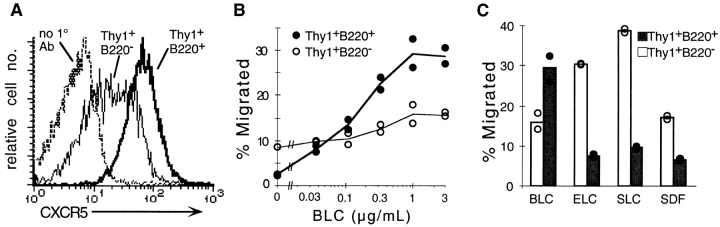
The rapid upregulation of CXCR5 on T cells after injection of antigen in adjuvant and the migration of a fraction of the cells into follicles suggested that these cells had acquired responsiveness to BLC. This was tested directly ex vivo in transwell migration assays. OVA-specific T cells from draining LNs of adoptive transfer recipients immunized 7 d earlier with OVA peptide subcutaneously in CFA showed that a strong dose-dependent response to BLC (Fig. 4 A). By contrast, OVA-specific cells from recipients given the antigen intravenously in the absence of adjuvant did not respond to BLC (Fig. 4 A). Kinetic analysis using cells from mice immunized with OVA peptide in CFA showed the BLC response was detectable by day 2 and was maximal between days 5 and 10 (Fig. 4 B). In addition, flow cytometric analysis of transmigrated OVA-specific cells on day 3 revealed that while most input cells expressed CXCR5 (Fig. 1 C), there was an enrichment for cells expressing the highest levels of CXCR5 in the responding population (Fig. 4 C). These findings provide evidence for a direct relationship between CXCR5 expression on T cells and acquisition of BLC responsiveness, although they do not establish whether the activation state of the cells also affects their ability to respond. To investigate the BLC responsiveness of CXCR5-expressing T cells with a resting phenotype, we took advantage of previous observations that a subset of memory CD4 T cells expresses CXCR5 20 21. CD4 T cells from aged (≥1 yr) mice were characterized as a source of memory phenotype T cells, and a great majority of the L-selectinlo CD4 T cells from these animals were found to express CXCR5 (Fig. 4 D). In addition to low L-selectin expression, the majority of CXCR5hi T cells in young and old mice expressed high levels of CD44 and reduced amounts of CD45RB (data not shown). Most of the CXCR5hi cells were also negative for the activation markers CD69 and CD25, further supporting their designation as memory cells. Interestingly, though a significant proportion of memory phenotype T cells in young mice expresses CXCR5, this proportion was consistently increased in aged mice (Fig. 4 D). In in vitro chemotaxis assays, the CXCR5hi memory T cells showed a very similar dose-sensitive BLC response to the activated OVA-specific T cells characterized above (Fig. 4 E). These results establish that both resting and activated CXCR5hi T cells respond to BLC. Interestingly, although the magnitude of the T cell response to BLC was lower than that observed for B cells, the T cells responded maximally to lower concentrations of BLC than did B cells (Fig. 4 E). This finding is similar to that made previously with transfected Jurkat T cells 18, suggesting that T cells are intrinsically more sensitive than recirculating B cells to CXCR5 signaling. CD4 T cells express severalfold less surface CXCR5 than B cells (Fig. 1), demonstrating that higher surface chemokine receptor expression does not equate to higher chemokine sensitivity.
Figure 4.
BLC chemotactic response of in vivo–activated OVA-specific T cells and memory phenotype CXCR5-expressing T cells. Results are expressed as percentage of transmigrated input cells. Lines represent means of duplicate transwells. (A) Chemotactic response of KJ1-26+CD4+ cells from draining LNs of Balb/c recipients of OVA-specific CD4 T cells immunized 7 d before analysis with OVA peptide subcutaneously in CFA (•) or intravenously in saline (○). Results were similar at day 5 after immunization and are representative of five independent experiments. (B) Kinetic analysis of OVA-specific T cell acquisition of BLC responsiveness. The day after immunization with OVA peptide subcutaneously in CFA is indicated in each panel. Differences in basal migration levels were not reproducible and reflect assay to assay variability. Results are representative of at least two independent experiments at each time point. (C) CXCR5 expression on KJ1-26+CD4+ cells from day 3 draining LNs showing the total input population (Input) and the cells that migrated to BLC (Migrated). (D) CXCR5 and L-selectin (CD62L) expression on CD4+ splenocytes from young (left panel) and 21-mo-old (right panel) B6 mice. Numbers represent the percentage of CD4+ and lymphocyte size-gated cells in each quadrant. Similar results, with progressive accumulation of L-selectinlo and CXCR5hi T cells, were obtained for more than 10 animals <3 or >12 mo of age. (E) Chemotactic response of CXCR5hi (♦) and CXCR5lo/− (⋄) CD4+ T cells, and of B cells (□) from the spleen of a 21-mo-old mouse. The y-axis on the left refers to T cells, and on the right to B cells. Results are representative of eight independent experiments.
CXCR5-expressing T Cells Have Reduced Responsiveness to the T Zone Chemokines ELC and SLC.
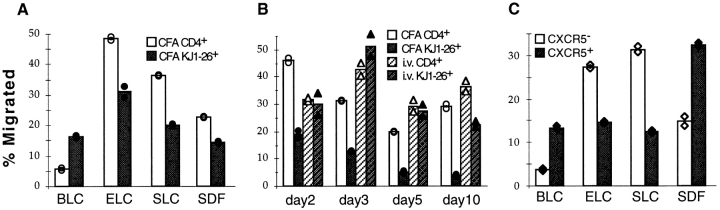
T cells have been shown to respond strongly to ELC and SLC in in vitro chemotaxis assays 22 23 24 27 28 29 30. Since these chemokines are expressed in the T zone and might be able to counteract the ability of a cell to respond to a chemokine made in follicles, we tested whether CXCR5-expressing T cells were altered in their responsiveness to ELC and SLC. In striking contrast to the elevated ELC and SLC responsiveness of in vitro–activated T cells 28 29 30, OVA-specific T cells activated in vivo by subcutaneous peptide/CFA injection showed a significant downregulation in responsiveness to these T zone chemokines (Fig. 5 A). The time course of this decreased responsiveness to ELC (Fig. 5 B) was similar to the time course over which the cells became responsive to BLC (Fig. 4 B). In contrast, OVA-specific T cells from mice immunized intravenously with peptide in saline maintained their ability to respond to ELC throughout the 10-d time course (Fig. 5 B). Responsiveness to SDF1 was also decreased in the in vivo–activated T cells (Fig. 5 A), consistent with the recent in vitro finding that anti-CD3 stimulation reduces responsiveness to SDF1 38. CXCR5-expressing memory phenotype cells from aged mice showed similarly low responsiveness to ELC and SLC (Fig. 5 C). Interestingly, in these cells the responsiveness to SDF1 was elevated (Fig. 5 C).
Figure 5.
Chemotactic response profiles of in vivo–activated OVA-specific T cells and memory phenotype CXCR5-expressing T cells. Results are expressed as percentage of transmigrated input cells. Bars represent means of duplicate transwells. (A) Chemotaxis of KJ1-26+CD4+ OVA-specific (black bars) and nonspecific CD4+ (white bars) cells from draining LNs of OVA-specific T cell transfer recipients immunized 7 d previously with OVA peptide subcutaneously in CFA. Chemokine concentrations were: BLC, 2 μg/ml; ELC, 0.2 μg/ml; SLC, 0.2 μg/ml; and SDF1, 0.3 μg/ml. Results were similar at day 5 after immunization and are representative of at least three independent experiments for each chemokine. (B) Response of KJ1-26+CD4+ OVA-specific (black bars) and nonspecific CD4+ (white bars) cells to 0.2 μg/ml ELC. Cells are from draining LNs of transfer recipients immunized with OVA peptide subcutaneously in CFA (solid bars) or intravenously in saline (hatched bars). The day after immunization is indicated on the x-axis. Data at day 5 are representative of five experiments for subcutaneous immunization in CFA and two experiments for intravenous immunization in saline. (C) Chemotaxis of CXCR5lo/− (white bars) and CXCR5hi (black bars) CD4+ T cells from the spleen of a 21-mo-old mouse. Chemokine concentrations were: BLC, 2 μg/ml; ELC, 0.5 μg/ml; SLC, 0.8 μg/ml; and SDF1, 0.3 μg/ml. Results are representative of at least four independent experiments for each chemokine. CXCR5hi T cells exhibited reduced responsiveness to ELC (0.02–1.5 μg/ml) and SLC (0.08–1.2 μg/ml) at all chemokine concentrations tested.
T Cells Can Acquire the Intrinsic Ability to Migrate to Regions of Lymphoid Tissues Proximal to Follicles.
The studies above demonstrate a tight relationship between CD4 T cell upregulation of CXCR5 expression, acquisition of BLC responsiveness, and migration into lymphoid follicles during an immune response. However, they do not establish whether intrinsic changes in the T cell are sufficient to direct these cells to follicles or whether additional (extrinsic) changes that accompany the adjuvant-induced immune response are also needed. Although many of the CD4 T cells in aged mice express CXCR5 (see Fig. 4 C), immunohistochemical analysis did not reveal a significantly greater number of T cells in follicles in aged mice compared with young mice (data not shown). When CXCR5hi CD4 cells were transferred from aged to young mice, they were found to localize within the T zone, with only occasional cells migrating into follicles (Fig. 6 A). These findings indicate that expression of CXCR5 is not sufficient to direct all types of T cells into B cell follicles. However, in contrast to aged normal mice, aged MRL-lpr mice contain very large numbers of T cells in a follicular distribution 39 40. These CD3+ T cells are unusual in lacking CD4 and CD8 and in expressing B220 39 40. Flow cytometric analysis showed that they also express high surface CXCR5 (Fig. 7 A), and in in vitro chemotaxis assays they demonstrated a robust response to BLC (Fig. 7 B) and a reduced response to ELC and SLC (Fig. 7 C). Importantly, the BLC dose–response curve of the DN T cells was typical of CXCR5+ CD4 T cells (compare Fig. 7 B and Fig. 4A and Fig. D) and not of B cells (see Fig. 4 D). Although the follicular location of the DN T cells in MRL-lpr mice suggests these cells have acquired the intrinsic ability to migrate to follicles, MRL-lpr mice have multiple immunological abnormalities, and it was possible that homing of DN T cells to follicles was dependent on extrinsic changes in the lymphoid tissues. To test this directly, DN T cells were purified from LNs of MRL-lpr mice, labeled with CFSE, and transferred to normal syngeneic MRL mice. Strikingly, the transferred T cells migrated to regions proximal to B cell areas in all secondary lymphoid tissues of the recipient mice (Fig. 6). Differences in the distribution of the cells were noted in the different tissues. In the spleen, the T cells homed to the outer rim of the follicles, especially near the marginal zone bridging channels, and often the cells appeared in contact with marginal metallophilic macrophages (Fig. 6 B). In LNs the T cells homed to perifollicular and interfollicular locations (Fig. 6 C), and in Peyer's patches the cells were seen to circle the whole follicular area (Fig. 6 D). These observations demonstrate that T cells can acquire the intrinsic ability to migrate to the boundaries of lymphoid follicles.
Figure 6.
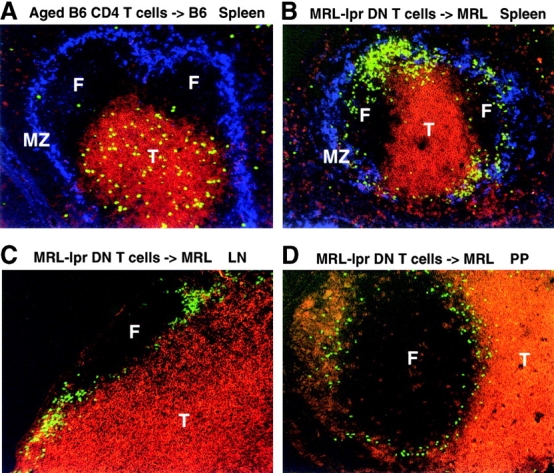
Transferred MRL-lpr DN T cells, but not memory phenotype CXCR5hi T cells, home towards B cell follicles in secondary lymphoid organs of unimmunized nonautoimmune mice. Spleen (A and B), LN (C), and Peyer's patch (PP; D) sections from syngeneic recipients of CFSE-labeled (A) CD4+ CXCR5+-enriched splenocytes from 12–15-mo-old mice or (B–D) purified DN T cells from 8-mo-old MRL-lpr mice. Recipient tissues were isolated 1 d after cell transfer. CFSE-labeled cells are green. T cells are stained in red using mAbs to CD3∈ and Thy1.2 (A and B), CD8 (C), or CD4 (D). MOMA-1+ marginal zone metallophilic macrophages are stained blue. A is representative of five and B–D of three mice. F, follicle; T, T cell zone; MZ, marginal zone. Original magnification: ×10.
Figure 7.
CXCR5 expression and chemotactic response profile of DN T cells from MRL-lpr mice. (A) CXCR5 expression on DN (Thy1+B220+) and conventional (Thy1+B220−) T cells. DN T cells stained with the secondary antibody alone (no 1° Ab) are shown as a control. (B and C) Chemotaxis of a 3:1 mixture of MRL-lpr and B6 splenocytes in response to (B) BLC and (C) a panel of lymphoid chemokines. Results are expressed as percentage of input cells transmigrated for DN T cells (•) and conventional T cells (○). Chemokine concentrations in C: BLC, ELC, and SLC, 1 μg/ml; SDF1, 0.3 μg/ml. MRL-lpr mice were old 5 mo at the time of analysis. Lines (B) and bars (C) represent means of duplicate transwells. Results in A are representative of three, and in B and C of two independent experiments.
Discussion
The findings above establish that immunization with antigen in adjuvant causes antigen-specific T cells to upregulate CXCR5 expression and acquire responsiveness to the follicular chemokine, BLC, while simultaneously becoming less responsive to the T zone chemokines, ELC and SLC. We propose that this reprogramming of responsiveness to B and T zone chemokines is part of the mechanism by which antigen-activated T cells home to follicles to help initiate T-dependent antibody responses.
In the adoptive transfer studies of Jenkins and co-workers, it was observed that antigen needed to be injected in adjuvant for activated T cells to migrate to follicles 13 34. When antigen was injected in the absence of adjuvant, T cell activation was transient and the activated cells failed to home to follicles. Our results provide a basis for understanding the different trafficking patterns of the activated cells as they show that CXCR5 upregulation and acquisition of BLC responsiveness only occurs after injection of antigen in adjuvant. Many studies have indicated that the effectiveness of adjuvants is through their potent activation of dendritic cells (DCs 41), and it is therefore reasonable to suggest that effective induction of CXCR5 expression on T cells requires interaction with appropriately activated antigen-presenting DCs within the lymphoid tissue. OX40L is expressed by a subset of activated DCs 42, and recent studies by Lane and co-workers provide evidence that stimulation of T cells through OX40 can promote upregulation of CXCR5 43 and homing of T cells to follicles 44. Further studies are needed to define whether additional costimulatory molecules can regulate CXCR5 expression on T cells.
In vivo activation by antigen in adjuvant decreases T cell responsiveness to ELC and SLC at the same time as increasing responsiveness to BLC. This contrasts with findings in vitro, where PHA- and IL-2–activated T cells responded more strongly than unactivated cells to SLC and ELC 28 29 30 and again indicates that the mode of T cell activation can strongly influence chemokine responsiveness. Recently, it has been shown that plt/plt mice, which exhibit defective homing of T cells to splenic T zones and LNs 45, have a compound defect that causes a deficiency in SLC expression and markedly reduced ELC expression 31. This finding provided strong evidence that SLC and ELC are needed for T cell homing to lymphoid T cell areas. Therefore, reduced responsiveness of CXCR5hi T cells to ELC and SLC may allow the cells to more readily leave the T zone and enter follicles. Reciprocally, the failure of T cells activated after intravenous injection of antigen to downregulate their SLC and ELC response might contribute to their inability to migrate to follicles. Since SLC appears important for cells to enter LNs via high endothelial venules or lymphatics 22 31 46, decreased responsiveness to this chemokine is also likely to influence the recirculation pattern of the cells.
Our studies provide strong evidence that altered responsiveness to constitutively expressed chemokines is part of the mechanism by which antigen-activated CD4 T cells migrate towards and into B cell follicles. This conclusion is also supported by the transfer experiments showing that DN T cells from MRL-lpr mice are intrinsically capable of migrating to areas proximal to follicles (Fig. 6). However, the failure of CXCR5hi CD4 T cells to migrate to follicles after adoptive transfer suggests that additional factors might normally help guide antigen-activated CD4 T cells. The migration of only a subset of OVA-activated T cells to follicles also suggests that CXCR5hi cells may be heterogeneous in their responsiveness to these factors. Several studies have shown that B cell receptor–stimulated B cells upregulate expression of chemokines, including MIP-1α, MIP-1β 47 48, and macrophage-derived chemokine (MDC 49), that can attract subsets of activated T cells 50 51 52 53. Since antigen-activated B cells move to the boundary of B and T zones 3, it is likely that chemokines produced by activated B cells work together with constitutively expressed chemokines to bring antigen-activated T cells to the outer T zone. Whether further cues are needed to direct cells from the outer T zone into follicles remains unclear, although the failure of MRL-lpr DN T cells to migrate to the inner regions of follicles suggests this is the case. Possibilities include changes in the responsiveness of the T cells to other unknown chemokines, and changes in the relative adhesiveness of the cells for features of the B or T cell compartments. Several examples of cell sorting occurring as a result of differential adhesiveness of cells have been reported 54 55 56.
A major role of T cells inside follicles is to support the GC response 1 2. 9 d after immunization with PCC in adjuvant, a large majority of the PCC-responsive Vα11Vβ3-expressing T cells express CXCR5 (Fig. 3), and at this time point, ∼75% of the cells are localized within GCs 17. Therefore, at least a subset of PCC-responsive CXCR5hi T cells acquires the ability to enter GCs. Similarly, in the DO11.10 adoptive transfer system, 10 d after immunization with OVA peptide in CFA, the majority of KJ1-26+ T cells were CXCR5hi, and many cells were found in follicular mantle zones and GCs (Fig. 2 D). The strong expression of BLC in primary follicles 18 and in follicular mantle zones of secondary follicles (Fig. 2E and Fig. F) is consistent with BLC playing a role in attracting T cells to these sites. The presence of only small numbers of BLC-expressing cells within GCs, predominantly in the region distal to the T zone (most likely corresponding to the GC light zone [1]) suggests that while BLC/CXCR5 might have a role in helping position cells within GCs, additional cues are likely to be needed. These findings also establish that there is substantial heterogeneity among follicular stromal cells in terms of BLC expression levels, with GC follicular DCs expressing relatively little of this chemokine. Perhaps by being concentrated predominantly outside the T zone–distal pole of the GC, BLC helps polarize the GC light and dark zone compartments. The notion that cues other than BLC play important roles in GC organization is supported by the finding that GCs are able to form in CXCR5-deficient mice 21. Furthermore, although spleens of CXCR5-deficient mice lack polarized follicles and contain aberrantly located GCs, the follicular disruption in LNs appeared to be minimal 21. Although this suggests that the role of CXCR5/BLC in B and T cell homing to LN follicles is redundant to other chemokine/receptor systems, studies in mice lacking lymphotoxin or TNF have shown that effects on follicular organization in LNs are more difficult to detect than in spleen or Peyer's patches 57 58, yet these effects can still be substantial 59 60. As we have shown here, BLC is expressed in LN follicles, and CXCR5 is strongly upregulated on activated T cells in LNs. Future studies of BLC-deficient mice should help further dissect the contribution of BLC and CXCR5 to follicular organization and GC formation in LNs.
T cell homing to follicles may be important not only for providing help to B cells, but also for providing activated T cells with growth and survival signals. This possibility is suggested by the finding that antigen injected in the absence of adjuvant fails to promote T cell migration to follicles, and also fails to promote survival of activated or memory T cells 13 34. The selective accumulation of the CXCR5-expressing subset of CD4 T cells in aged mice (Fig. 4 C) and in HIV-infected humans during disease progression 61 is consistent with the notion that trafficking through B cell areas plays a role in long-term survival of memory T cells. This possibility is also supported by the finding of a defect in CD4 T cell memory in B cell–deficient mice 62. Although we did not observe trafficking of CXCR5hi memory T cells to follicles in short-term transfer experiments, studies in rats have suggested that memory T cells migrate through follicles at greater frequency than naive T cells 63.
In summary, our findings suggest a model for how helper T cells home to follicles. After engagement of peptide/MHC complexes on appropriately activated T zone DCs, CD4 T cells upregulate CXCR5, acquire responsiveness to the follicular chemokine BLC, and simultaneously downregulate responsiveness to the T zone chemokines ELC and SLC. Together with additional presently undefined changes, this reprogrammed chemokine responsiveness helps propel T cells toward B cell areas. Further cues, such as those emanating from activated B cells and from GC cells, can then act upon these T cells to more precisely control their positioning and facilitate their ability to act as B cell helpers.
Acknowledgments
We thank Wei Bai for generating the BLC-his6 construct, Paul Hyman for purifying chemokines, and Lucy Tang for help with some of the adoptive transfer experiments. We also thank Peter Lane, Sanjiv Luther, and Mary Keir for comments on the manuscript.
K.M. Ansel is a Howard Hughes Medical Institute predoctoral fellow, and J.G. Cyster is a Pew Scholar. This work was supported by National Institutes of Health grants AI40098 and AI45073.
Footnotes
1used in this paper: B6, C57BL/6; BLC, B lymphocyte chemoattractant; BLR, Burkitt's lymphoma receptor; CFSE, 5- (and 6-) carboxyfluorescein succinimidyl ester; CXCR, CXC chemokine receptor; DC, dendritic cell; DN, double negative; EBI-1, EBV-induced molecule 1; ELC, EBI-1 ligand chemokine; GC, germinal center; MIP, macrophage inflammatory protein; PCC, pigeon cytochrome c; SDF, stromal cell–derived factor; SLC, secondary lymphoid tissue chemokine
References
- MacLennan I.C.M. Germinal centers. Annu. Rev. Immunol. 1994;12:117–139. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- Kelsoe G. The germinal centera crucible for lymphocyte selection. Semin. Immunol. 1996;8:179–184. doi: 10.1006/smim.1996.0022. [DOI] [PubMed] [Google Scholar]
- Cyster J.G. Signaling thresholds and interclonal competition in preimmune B-cell selection. Immunol. Rev. 1997;156:87–101. doi: 10.1111/j.1600-065x.1997.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Van den Eertwegh A.J.M., Noelle R.J., Roy M., Shepherd D.M., Aruffo A., Ledbetter J.A., Boersma W.J.A., Claassen E. In vivo CD40–gp39 interactions are essential for thymus-dependent humoral immunity. I. In vivo expression of CD40 ligand, cytokines, and antibody production delineates sites of cognate T–B cell interactions. J. Exp. Med. 1993;178:1555–1565. doi: 10.1084/jem.178.5.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toellner K.M., Gulbranson-Judge A., Taylor D.R., Sze D.M., MacLennan I.C. Immunoglobulin switch transcript production in vivo related to the site and time of antigen-specific B cell activation. J. Exp. Med. 1996;183:2303–2312. doi: 10.1084/jem.183.5.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garside P., Ingulli E., Merica R.R., Johnson J.G., Noelle R.J., Jenkins M.K. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- Fuller K.A., Kanagawa O., Nahm M.H. T cells within germinal centers are specific for the immunizing antigen. J. Immunol. 1993;151:4505–4512. [PubMed] [Google Scholar]
- Casamayor-Palleja M., Khan M., MacLennan I.C. A subset of CD4+ memory T cells contains preformed CD40 ligand that is rapidly but transiently expressed on their surface after activation through the T cell receptor complex. J. Exp. Med. 1995;181:1293–1301. doi: 10.1084/jem.181.4.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulbranson-Judge A., MacLennan I. Sequential antigen-specific growth of T cells in the T zones and follicles in response to pigeon cytochrome c. Eur. J. Immunol. 1996;26:1830–1837. doi: 10.1002/eji.1830260825. [DOI] [PubMed] [Google Scholar]
- Zheng B., Han S., Kelsoe G. T helper cells in murine germinal centers are antigen-specific emigrants that downregulate Thy-1. J. Exp. Med. 1996;184:1083–1091. doi: 10.1084/jem.184.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther S.A., Gulbranson-Judge A., Acha-Orbea H., MacLennan I.C. Viral superantigen drives extrafollicular and follicular B cell differentiation leading to virus-specific antibody production. J. Exp. Med. 1997;185:551–562. doi: 10.1084/jem.185.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutloff A., Dittrich A.M., Beier K.C., Eljaschewitsch B., Kraft R., Anagnostopoulos I., Kroczek R.A. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- Kearney E.R., Pape K.A., Loh D.Y., Jenkins M.K. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- Zheng B., Han S., Zhu Q., Goldsby R., Kelsoe G. Alternative pathways for the selection of antigen-specific peripheral T cells. Nature. 1996;384:263–266. doi: 10.1038/384263a0. [DOI] [PubMed] [Google Scholar]
- Hedrick S.M., Engel I., McElligott D.L., Fink P.J., Hsu M.L., Hansburg D., Matis L.A. Selection of amino acid sequences in the beta chain of the T cell antigen receptor. Science. 1988;239:1541–1544. doi: 10.1126/science.2832942. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M.G., Davis M.M. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams L.J., Panus J.F., Mikszta J.A., McHeyzer-Williams M.G. Evolution of antigen-specific T cell receptors in vivopreimmune and antigen-driven selection of preferred complementarity-determining region 3 (CDR3) motifs. J. Exp. Med. 1999;189:1823–1838. doi: 10.1084/jem.189.11.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn M.D., Ngo V.N., Ansel K.M., Ekland E.H., Cyster J.G., Williams L.T. A B-cell-homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- Legler D.F., Loetscher M., Roos R.S., Clark-Lewis I., Baggiolini M., Moser B. B cell–attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J. Exp. Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R., Emrich T., Kremmer E., Lipp M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- Forster R., Mattis A.E., Kremmer E., Wolf E., Brem G., Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Tangemann K., Tam C., Cyster J.G., Rosen S.D., Williams L.T. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc. Natl. Acad. Sci. USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo V.N., Tang H.L., Cyster J.G. Epstein-Barr virus–induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J. Exp. Med. 1998;188:181–191. doi: 10.1084/jem.188.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe S., Lu Z., Luo Y., Quackenbush E.J., Berman M.A., Collins-Racie L.A., Mi S., Reilly C., Lo D., Jacobs K.A., Dorf M.E. Identification of a new mouse beta-chemokine, thymus-derived chemotactic agent 4, with activity on T lymphocytes and mesangial cells. J. Immunol. 1997;159:5671–5679. [PubMed] [Google Scholar]
- Yoshida R., Nagira M., Kitaura M., Imagawa N., Imai T., Yoshie O. Secondary lymphoid-tissue chemokine is a functional ligand for the CC chemokine receptor CCR7. J. Biol. Chem. 1998;273:7118–7122. doi: 10.1074/jbc.273.12.7118. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Bowman E.P., Murphy K., Youngman K.R., Siani M.A., Thompson D.A., Wu L., Zlotnik A., Butcher E.C. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3β receptor CCR7. J. Cell Biol. 1998;141:1053–1059. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.H., Pelus L.M., White J.R., Applebaum E., Johanson K., Broxmeyer H.E. CK beta-11/macrophage inflammatory protein-3 beta/EBI1-ligand chemokine is an efficacious chemoattractant for T and B cells. J. Immunol. 1998;160:2418–2424. [PubMed] [Google Scholar]
- Nagira M., Imai T., Yoshida R., Takagi S., Iwasaki M., Baba M., Tabira Y., Akagi J., Nomiyama H., Yoshie O. A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur. J. Immunol. 1998;28:1516–1523. doi: 10.1002/(SICI)1521-4141(199805)28:05<1516::AID-IMMU1516>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Willimann K., Legler D.F., Loetscher M., Roos R.S., Delgado M.B., Clark-Lewis I., Baggiolini M., Moser B. The chemokine SLC is expressed in T cell areas of lymph nodes and mucosal lymphoid tissues and attracts activated T cells via CCR7. Eur. J. Immunol. 1998;28:2025–2034. doi: 10.1002/(SICI)1521-4141(199806)28:06<2025::AID-IMMU2025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Yoshida R., Nagira M., Imai T., Baba M., Takagi S., Tabira Y., Akagi J., Nomiyama H., Yoshie O. EBI1-ligand chemokine (ELC) attracts a broad spectrum of lymphocytesactivated T cells strongly up-regulate CCR7 and efficiently migrate toward ELC. Int. Immunol. 1998;10:901–910. doi: 10.1093/intimm/10.7.901. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Kyuwa S., Tam C., Kakiuchi T., Matsuzawa A., Williams L.T., Nakano H. Mice lacking expression of secondary lymphoid-organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med. 1999;189:451–460. doi: 10.1084/jem.189.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K.M., Heimberger A.B., Loh D.Y. Induction by antigen of intrathymic apoptosis of CD4+CD8+ TCRlo thymocytes in vivo. Science. 1990;250:1720–1723. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- Lamarche N., Tapon N., Stowers L., Burbelo P.D., Aspenstrom P., Bridges T., Chant J., Hall A. Rac and Cdc42 induce actin polymerization and G1 cell cycle progression independently of p65pak and the JNK/SAPK MAP kinase cascade. Cell. 1996;87:519–529. doi: 10.1016/s0092-8674(00)81371-9. [DOI] [PubMed] [Google Scholar]
- Pape K.A., Khoruts A., Mondino A., Jenkins M.K. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J. Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- Schmidt K.N., Hsu C.W., Griffin C.T., Goodnow C.C., Cyster J.G. Spontaneous follicular exclusion of SHP1-deficient B cells is conditional on the presence of competitor wild-type B cells. J. Exp. Med. 1998;187:929–937. doi: 10.1084/jem.187.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyster J.G., Ngo V.N., Ekland E.H., Gunn M.D., Sedgwick J.D., Ansel K.M. Chemokines and B-cell homing to follicles. Curr. Top. Microbiol. Immunol. 1999;246:87–92. doi: 10.1007/978-3-642-60162-0_11. [DOI] [PubMed] [Google Scholar]
- Kraal G., Janse M. Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology. 1986;58:665–669. [PMC free article] [PubMed] [Google Scholar]
- Peacock J.W., Jirik F.R. TCR activation inhibits chemotaxis toward stromal cell-derived factor-1evidence for reciprocal regulation between CXCR4 and the TCR. J. Immunol. 1999;162:215–223. [PubMed] [Google Scholar]
- Lieberum B., Hartmann K.U. Successive changes of the cellular composition in lymphoid organs of MRL-Mp/lpr-lpr mice during the development of lymphoproliferative disease as investigated in cryosections. Clin. Immunol. Immunopathol. 1988;46:421–431. doi: 10.1016/0090-1229(88)90061-x. [DOI] [PubMed] [Google Scholar]
- Usui T., Yoshioka H., Ko K., Sung M.E., Nagata N., Okamoto T., Ohshio G., Kita T., Inada M. Age associated changes in the distribution of lpr gene-induced B220-positive T cells in lymphoid organs of MRL/Mp-lpr/lpr mice using dual exposure microphotographs of double immunofluorescence staining. Biotech. Histochem. 1996;71:182–189. doi: 10.3109/10520299609117157. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Tanaka Y., Tozawa H., Takahashi Y., Maliszewski C., Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J. Immunol. 1997;159:3838–3848. [PubMed] [Google Scholar]
- Flynn S., Toellner K.M., Raykundalia C., Goodall M., Lane P. CD4 T cell cytokine differentiationthe B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J. Exp. Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocker T., Gulbranson-Judge A., Flynn S., Riedinger M., Raykundalia C., Lane P. CD4 T cell traffic controlin vivo evidence that ligation of OX40 on CD4 T cells by OX40-ligand expressed on dendritic cells leads to the accumulation of CD4 T cells in B cell follicles. Eur. J. Immunol. 1999;29:1610–1616. doi: 10.1002/(SICI)1521-4141(199905)29:05<1610::AID-IMMU1610>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Nakano H., Tamura T., Yoshimoto T., Yagita H., Miyasaka M., Butcher E.C., Nariuchi H., Kakiuchi T., Matsuzawa A. Genetic defect in T lymphocyte-specific homing into peripheral lymph nodes. Eur. J. Immunol. 1997;27:215–221. doi: 10.1002/eji.1830270132. [DOI] [PubMed] [Google Scholar]
- Campbell J.J., Hedrick J., Zlotnik A., Siani M.A., Thompson D.A., Butcher E.C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- Zipfel P.F., Balke J., Irving S.G., Kelly K., Siebenlist U. Mitogenic activation of human T cells induces two closely related genes which share structural similarities with a new family of secreted factors. J. Immunol. 1989;142:1582–1590. [PubMed] [Google Scholar]
- Krzysiek R., Lefevre E.A., Zou W., Foussat A., Bernard J., Portier A., Galanaud P., Richard Y. Antigen receptor engagement selectively induces macrophage inflammatory protein-1α (MIP-1α) and MIP-1β chemokine production in human B cells. J. Immunol. 1999;162:4455–4463. [PubMed] [Google Scholar]
- Schaniel C., Pardali E., Sallusto F., Speletas M., Ruedl C., Shimizu T., Seidl T., Andersson J., Melchers F., Rolink A.G., Sideras P. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J. Exp. Med. 1998;188:451–463. doi: 10.1084/jem.188.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loetscher P., Seitz M., Baggiolini M., Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J. Exp. Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Mackay C.R., Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonecchi R., Bianchi G., Bordignon P.P., D'Ambrosio D., Lang R., Borsatti A., Sozzani S., Allavena P., Gray P.A., Mantovani A., Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H.L., Cyster J.G. Chemokine upregulation and activated T cell attraction by maturing dendritic cells. Science. 1999;284:819–822. doi: 10.1126/science.284.5415.819. [DOI] [PubMed] [Google Scholar]
- Steinberg M.S. Reconstitution of tissues by dissociated cells. Science. 1963;141:401–408. doi: 10.1126/science.141.3579.401. [DOI] [PubMed] [Google Scholar]
- Nose A., Nagafuchi A., Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Xu Q., Mellitzer G., Robinson V., Wilkinson D.G. In vivo cell sorting in complementary segmental domains mediated by Eph receptors and ephrins. Nature. 1999;399:267–271. doi: 10.1038/20452. [DOI] [PubMed] [Google Scholar]
- Neumann B., Luz A., Pfeffer K., Holzmann B. Defective Peyer's patch organogenesis in mice lacking the 55-kD receptor for tumor necrosis factor. J. Exp. Med. 1996;184:259–264. doi: 10.1084/jem.184.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasparakis M., Alexopoulou L., Episkopou V., Kollias G. Immune and inflammatory responses in TNF alpha-deficient micea critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner H., Cook M., Riminton D.S., Lemckert F.A., Hoek R.M., Ledermann B., Kontgen F., Fazekas de St B., Groth, Sedgwick J.D. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol. 1997;27:2600–2609. doi: 10.1002/eji.1830271020. [DOI] [PubMed] [Google Scholar]
- Fu Y.X., Huang G., Matsumoto M., Molina H., Chaplin D.D. Independent signals regulate development of primary and secondary follicle structure in spleen and mesenteric lymph node. Proc. Natl. Acad. Sci. USA. 1997;94:5739–5743. doi: 10.1073/pnas.94.11.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R., Schweigard G., Johann S., Emrich T., Kremmer E., Nerl C., Lipp M. Abnormal expression of the B-cell homing chemokine receptor BLR1 during the progression of acquired immunodeficiency syndrome. Blood. 1997;90:520–525. [PubMed] [Google Scholar]
- Homann D., Tishon A., Berger D.P., Weigle W.O., von Herrath M.G., Oldstone M.B.A. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell deficient micefailure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from μMT/μMT mice. J. Virol. 1998;72:9208–9216. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann J., Geismar U., Sponholz A., Bode U., Sparshott S.M., Bell E.B. CD4+ T cells of both the naive and the memory phenotype enter rat lymph nodes and Peyer's patches via high endothelial venuleswithin the tissue their migratory behavior differs. Eur. J. Immunol. 1997;27:3174–3181. doi: 10.1002/eji.1830271214. [DOI] [PubMed] [Google Scholar]