Abstract
TRAIL (TNF-related apoptosis-inducing ligand) is a member of the TNF family that induces apoptosis in a variety of cancer cells. In this study, we demonstrate that human CD11c+ blood dendritic cells (DCs) express TRAIL after stimulation with either interferon (IFN)-γ or -α and acquire the ability to kill TRAIL-sensitive tumor cell targets but not TRAIL-resistant tumor cells or normal cell types. The DC-mediated apoptosis was TRAIL specific, as soluble TRAIL receptor blocked target cell death. Moreover, IFN-stimulated interleukin (IL)-3 receptor (R)α+ blood precursor (pre-)DCs displayed minimal cytotoxicity toward the same target cells, demonstrating a clear functional difference between the CD11c+ DC and IL-3Rα+ pre-DC subsets. These results indicate that TRAIL may serve as an innate effector molecule on CD11c+ DCs for the elimination of spontaneously arising tumor cells and suggest a means by which TRAIL-expressing DCs may regulate or eliminate T cells responding to antigen presented by the DCs.
Keywords: TRAIL, apoptosis, tumor, dendritic cells, human
Dendritic cells (DCs)1 are bone marrow–derived cells that specialize in the uptake, processing, and presentation of foreign and self-antigens 1 2. In humans, two distinct peripheral blood DC subsets have been described 3 4 5 6 7. One subset is characterized by a multilobulated nucleus, the absence of CD3, CD14, CD19, and CD56, the presence of the myeloid-associated antigen CD33, moderate levels of CD11c and CD4, and high levels of MHC class II (HLA-DR). This DC subset, hereafter referred to as CD11c+ DC, also constitutively expresses the high- and low-affinity IgG receptors (FcγRI/CD64 and FcγRII/CD32, respectively), which help mediate the uptake of immune complexes 6 7. DCs with a similar morphology and phenotype are dispersed throughout the germinal center dark and light zones of human tonsils, spleens, and lymph nodes 8. The second blood DC subset, hereafter referred to as IL-3Rα+ pre-DC, is distinguished by an immature appearance containing an oval nucleus, low levels of CD33, and absence of CD3, CD14, CD19, CD56, and CD11c antigens. These DCs express CD4, high levels of HLA-DR and IL-3Rα, low levels of FcγRII/CD32, and no detectable levels of FcγRI/CD64 3 7. A precursor DC with similar phenotype and morphology resides in the extrafollicular T cell–rich regions of the tonsils and lymph nodes 9 10. Another difference between these two DCs is the expression of CD83, which is detected on CD11c+ DCs but not on IL-3Rα+ pre-DCs or monocytes (Mφ) after short-term culture 7 11 12.
Recent reports demonstrate that human DCs can efficiently present antigens derived from apoptotic cells and can cross-present tumor, viral, transplantation, and self-antigens to CD8+ T cells in vitro 13 14. This may correspond to the in vivo phenomenon of cross-priming, where antigens derived from dying tumor cells or transplanted tissue are presented by host APCs to antigen-specific CD8+ cytotoxic T cells 15 16 17. Expression of CD36 and the αvβ5 integrin on the DC appears to be critical for the uptake of antigens from apoptotic cells 14, perhaps through the binding of a bridging molecule such as thrombospondin 18. The fact that DCs can act as efficient APCs in this setting has suggested that these cells may act as “adjuvants” for MHC class I–restricted antitumor immunity 19. Such a protective antitumor immune response can be induced in vivo with DCs that have been incubated in vitro with tumor antigens or peptides derived from tumor antigens 20 21 22. It remains unclear, however, whether DCs can induce cellular apoptosis and selectively process and present antigens from apoptotic bodies in vivo. One possible scenario would involve DCs directly inducing apoptotic cell death of tumor cells or cells within transplanted tissue followed by the uptake of cellular fragments, antigen processing, and eventual cross-priming of naive CD8+ T cells.
Among the molecules known to induce the apoptotic cell death of tumor cells, TRAIL (TNF-related apoptosis-inducing ligand) has received great attention 23. Recombinant, soluble forms of TRAIL are potent mediators of tumor cell apoptosis, while demonstrating little or no cytotoxicity toward normal cells and tissues in vitro and in vivo 24 25 26 27. As with the other death-inducing members of the TNF family (i.e., FasL [ligand] and TNF), cells undergoing TRAIL-induced death exhibit many of the hallmarks of apoptosis, including zeiosis and apoptotic body release, chromatin condensation and DNA fragmentation, expression of prophagocytic signals (i.e., phosphatidylserine) on the cell membrane, and cleavage of multiple intracellular proteins by caspases 23 24 28 29. Soluble TRAIL is tumoricidal for approximately two-thirds of the more than 30 hematopoietic and nonhematopoietic tumor cell lines tested in vitro, suggesting that TRAIL could be a broad-spectrum antitumor molecule in vivo 23 24 30 31. Although a normal biological function for TRAIL remains to be determined, it has been suggested that TRAIL may be important in the activation-induced cell death (AICD) of T cells during HIV infection 32 33. Peripheral blood human T cells express TRAIL after CD3 cross-linking combined with type I IFN stimulation, perhaps also contributing to the AICD of T cells in the natural setting 34. In addition, human Mφ express TRAIL after IFN stimulation, transforming them into potent killers of tumor cells 27.
The aim of these studies was to determine if human DCs are able to induce apoptosis via TRAIL. We demonstrate that human CD11c+ DCs, but not IL-3Rα+ pre-DCs, express TRAIL after stimulation with IFN and are able to induce cellular apoptosis in TRAIL-sensitive cells.
Materials and Methods
Reagents and mAbs.
Reagents and sources were as follows: GM-CSF and leucine zipper (LZ)-CD40L (100 ng/ml; Immunex Corp.); IFN-α and -γ (100 ng/ml; Genzyme Corp.); LPS (5 ng/ml; Difco Labs., Inc.); MOPC-21, nonspecific IgG1 isotype control; M181, IgG1 anti-TRAIL (Immunex Corp.); 7G3, IgG2a anti–IL-3Rα–biotin; G155-178, IgG2a–biotin isotype control (PharMingen); 3.9, IgG1 anti-CD11c–PE; TUK4, IgG2a anti-CD14–FITC; 4D3, IgG2b anti-CD33–FITC; TU39, IgG2b anti–HLA-DR–FITC; IgG1–PE isotype control; IgG2a–FITC isotype control; IgG2b–FITC isotype control; and IgG2b–biotin isotype control (Caltag Labs., Inc.). HB-15a, IgG2b anti-CD83 (a gift of Dr. Thomas F. Tedder, Duke University Medical Center, Durham, NC). The soluble fusion proteins TRAILR2–Fc, Fas–Fc, and TNFR–Fc were produced at Immunex Corp. The LZ-huTRAIL expression plasmid and the production and purification of LZ-huTRAIL (TRAIL) have been previously described 26.
Cell Lines.
The ovarian carcinoma cell line OVCAR3 was obtained from Dr. Richard F. Camalier (Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute, Bethesda, MD). The human prostate carcinoma cell line PC-3 was obtained from Dr. Michael Cohen (University of Iowa, Iowa City, IA). The human melanoma cell lines WM 793 and 164 were obtained from Dr. M. Herlyn (Wistar Institute, Philadelphia, PA). The Jurkat cell line was purchased from American Type Culture Collection. All tumor cell lines were cultured as directed. Normal lung fibroblasts, lung microvascular endothelial cells, and skeletal muscle cells were purchased from Clonetics Corp. and cultured as directed.
Isolation of Human DCs and Monocytes.
Peripheral blood DCs were enriched using countercurrent elutriation. Cells from leukopheresis packs obtained from healthy volunteers were loaded onto a JE-5 elutriator (Beckman Instruments, Inc.), and 50-ml fractions were collected while increasing the flow rate from 65 to 85 ml/min at 2,000 rpm. Fractions containing the highest blood DC percentages were further enriched using magnetic bead selection. The DC-positive fractions were pooled and incubated with anti-CD3 (OKT3), anti-CD14 (MY23), anti-CD16 (3G8), anti-CD19 (B43), and anti-CD56 (B159) mAbs for 10 min at 20°C. Cells binding these mAbs were then removed using Dynal goat anti–mouse Ig–coated magnetic beads. Remaining cells were recovered and incubated with anti-CD7 (T3-3AI), anti-CD8 (OKT8), anti-CD11b (OKM1), anti-CD34 (MY10), and antiglycophorin A (10F7MN). Cells binding these mAbs were removed using the goat anti–mouse Ig–coated magnetic beads. To separate CD11c+ DCs from IL-3Rα+ DCs, the remaining cells were stained with a PE-labeled anti-CD11c and biotin-labeled anti–IL-3Rα, followed by APC-labeled streptavidin, and sorted on a FACStarPLUS™ (Becton Dickinson) into CD11c+ and IL-3Rα+ populations. Peripheral blood Mφ were enriched in elutriated fractions generated from a flow rate >75 ml/min. The fractions were >93% CD14+ Mφ, as assessed by flow cytometric analysis. Complete medium used for DC and Mφ culturing and functional assays consisted of RPMI 1640 (GIBCO BRL) supplemented with penicillin–streptomycin–glutamine and 10% pooled human serum (Immunex Corp.).
Flow Cytometry.
Cell analysis was performed on a FACScan™ (Becton Dickinson), with >5,000 cells analyzed per sample. For multicolor cell analysis, samples consisting of 20 μl cells were combined in a 96-well flat-bottom plate (Costar Corp.) with 20 μl human IgG (12 mg/ml; Sigma Chemical Co.) to block Fc binding of the mAbs and 20 μl each of the direct PE-labeled, FITC-labeled, and biotin-labeled mAbs (60 μg/ml). Cells were then incubated on a rotator at 4°C for 1 h. After three washes with 200 μl PBS containing 2 mg/ml BSA, 40 μl of APC-labeled streptavidin (1:100 dilution; Molecular Probes, Inc.) was added for an additional 1 h. Cells were analyzed immediately after staining or fixed in 1% paraformaldehyde until analysis.
Morphological Analysis.
Cytospins were performed by centrifuging 2 × 105 sorted DCs at 500 rpm for 5 min onto slides. The DCs were stained with a Hemacolor Stain Set (EM Diagnostic Systems), and photomicrographs were recorded using a Nikon Diaphot microscope with a 40× objective (Carl Zeiss, Inc.).
DC-mediated Killing of Human Tumor Cells.
DCs were cultured for 12 h in medium alone, GM-CSF, LZ-CD40L, LPS, IFN-γ, or IFN-α, washed, and resuspended in complete medium. Tumor cells were labeled with 100 μCi of 51Cr for 1 h at 37°C, washed three times, and resuspended in complete medium. To determine TRAIL-induced death, 51Cr-labeled tumor cells (104 cells/well) were incubated with varying numbers of DC effector cells for 8 h. As a positive control, soluble TRAIL was added to the target cells. Mφ used for comparison were cultured and stimulated under conditions identical to those described for DCs. In some cultures, TRAILR2–Fc, Fas–Fc, or TNFR–Fc (20 μg/ml) was added to the DCs 15 min before adding tumor cell targets. All cytotoxicity assays were performed in 96-well round-bottom plates, and the percent specific lysis was calculated as: 100 × (experimental cpm − spontaneous cpm)/(total cpm − spontaneous cpm). Spontaneous and total 51Cr release values were determined in the presence of either medium alone or 1% NP-40, respectively. The presence of TRAILR2–Fc, Fas–Fc, or TNFR–Fc during the assay had no effect on the level of spontaneous release by the target cells. Apoptotic cell death of tumor cells was measured by flow cytometry using FITC-conjugated annexin V and propidium iodide (apoptosis detection kit; R & D Systems, Inc.) as per the manufacturer's protocol. Light scatter characteristics were used to distinguish the tumor cells from the DCs.
Reverse Transcriptase–PCR.
Total RNA was isolated from DCs with TRIzol reagent (Life Technologies) as per the manufacturer's instructions. RNA samples (1 μg each) were tested for DNA contamination by 30 cycles of PCR with human β-actin primers. After it was shown that there was no DNA contamination, cDNA synthesis was performed using an RNA PCR kit (Perkin-Elmer Corp.) with the supplied oligo d(T)16 primer. Reverse transcription was performed using a thermal program of 25°C for 10 min, 42°C for 30 min, and 95°C for 5 min. PCR reactions were performed using the following primers: human β-actin (forward: 5′-GAAACTACCTTCAACTCCATC-3′, reverse: 5′-CGAGGCCAGGATGGAGCCGCC-3′); and human TRAIL (forward: 5′-CAACTCCGTCAGCTCGTTAGAAAG-3′, reverse: 5′-ttagaccaacaactatttctagcact-3′), giving products of 219 and 443 bp, respectively. β-actin PCR cycle conditions were 95°C for 45 s, 55°C for 1 min, and 72°C for 45 s for 30 cycles. TRAIL cycle conditions were 95°C for 45 s, 55°C for 45 s, and 72°C for 45 s for 30 cycles. Samples were resolved on a 2% agarose gel and visualized with ethidium bromide.
Results
Characterization of Blood DC Subsets.
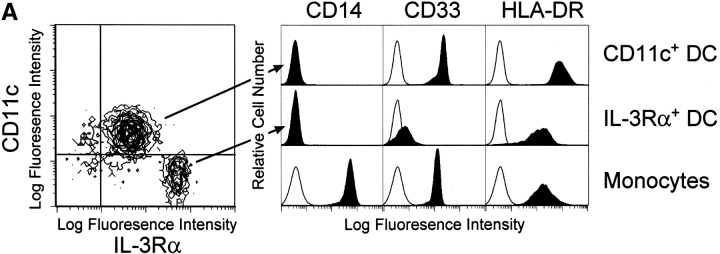
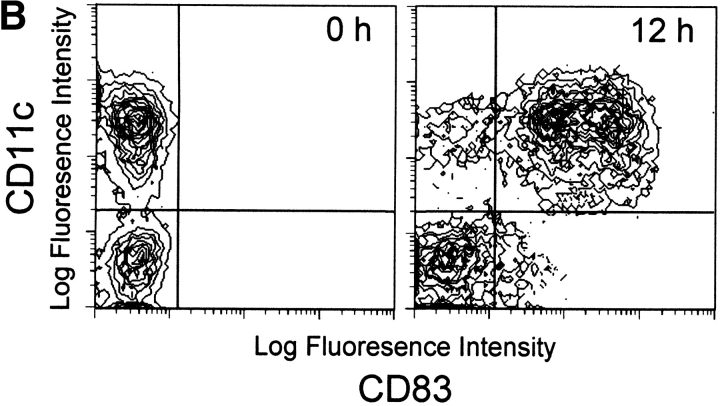
The enriched “bulk” DCs comprised two distinct subsets distinguishable by the surface expression of CD11c and IL-3Rα (Fig. 1 A). The ratio of these two cell populations varied by donor, with some donors demonstrating as high as 70% CD11c+ DCs and 30% IL-3Rα+ pre-DCs, whereas others demonstrated the reverse percentages. Neither CD11c+ DCs nor IL-3Rα+ pre-DCs expressed CD14, but they did differentially express CD33 and HLA-DR. After 12 h of culture in complete medium, the levels of CD11c and CD33 remained relatively constant, whereas CD83 expression was detected on the majority (>90%) of CD11c+ DCs (Fig. 1 B). In contrast, the IL-3Rα+ pre-DCs expressed lower levels of CD33 and HLA-DR and failed to express CD83 after 12-h incubation under identical conditions (Fig. 1a and Fig. b). Distinct morphological differences were also observed between the two DC subsets. The CD11c+ DCs exhibited a characteristic multilobulated nucleus, in contrast to the IL-3Rα+ pre-DCs, which displayed an immature appearance consisting of an oval nucleus (Fig. 2A and Fig. B). Highly pure CD11c+ DCs and IL-3Rα+ pre-DCs were rapidly obtained using this method for functional analysis of TRAIL.
Figure 1.
Surface phenotype of freshly isolated CD11c+ and IL3Rα+ pre-DC subsets. (A) The two blood DCs were distinguishable based on CD11c and IL-3Rα expression as determined by multicolor flow cytometry. The CD11c+ and IL-3Rα+ pre-DCs were compared with Mφ for CD14, CD33, and HLA-DR expression. Filled histograms represent staining with specific antibody; open histograms represent isotype-matched controls. (B) CD11c+ DCs, but not IL-3Rα+ pre-DCs, express CD83 after 12-h incubation in complete medium. Histograms and contour plots represent 104 gated DCs, and viability was >95% as assessed by propidium iodide exclusion.
Figure 2.
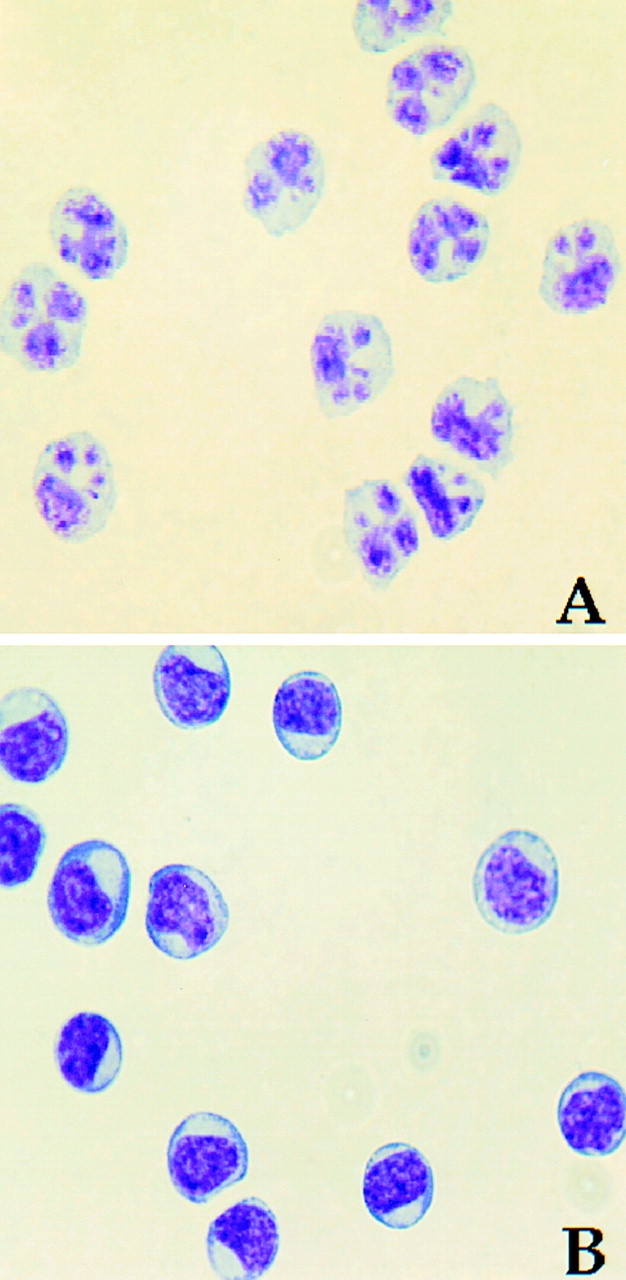
Blood CD11c+ DCs and IL-3Rα+ pre-DCs exhibit distinct morphological differences. Wright-Giemsa staining of freshly sorted CD11c+ DCs (A) and IL-3Rα+ pre-DCs (B). Photomicrographs were captured at a magnification of 40.
IFN-stimulated DCs Kill Tumor Cells via a TRAIL-dependent Mechanism.
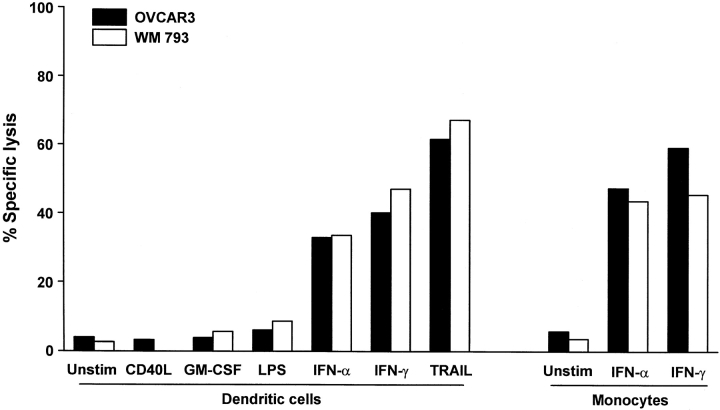
Previous reports have demonstrated that a variety of lymphoid and myeloid cells (T cells, NK cells, Mφ) can express TRAIL and kill TRAIL-sensitive target cells under certain circumstances 27 34 35. To determine if DCs also exhibit tumoricidal activity, unsorted “bulk” DCs were cultured for 12 h with IFN-γ, IFN-α, GM-CSF, CD40L, or LPS and cultured for an additional 8 h in the presence of the TRAIL-sensitive human tumor cell lines OVCAR3, an ovarian carcinoma cell line, and WM 793, a melanoma cell line, at a 50:1 DC/target ratio. Whereas unstimulated, GM-CSF-, CD40L-, and LPS-stimulated DCs demonstrated little or no tumoricidal activity toward the tumor cell targets, DCs stimulated with IFN-γ or -α were potent killers of each of these tumor cells (Fig. 3). The cytotoxic activity of unstimulated, IFN-γ-, and IFN-α-stimulated Mφ is presented for comparison. This cytotoxic activity was seen with IFN-stimulated DCs from multiple donors and with other TRAIL-sensitive tumor cells but not with the TRAIL-resistant melanoma cell line WM 164 or several normal primary cell types (Table ). No cytotoxic effect was observed when normal human lung fibroblasts, microvascular endothelial cells, or skeletal muscle cells were used as targets.
Figure 3.
Cytolytic activity by human DCs after stimulation. Bulk DCs were incubated for 12 h in the absence (Unstim.) or presence of CD40L, GM-CSF, LPS, IFN-α, or IFN-γ and then cultured for 8 h with 51Cr-labeled OVCAR3 or WM 793 target cells at an E/T ratio of 50:1. As a positive control, soluble TRAIL was added to target cells at 1 μg/ml. For comparison, the cytolytic activity of unstimulated (Unstim.), IFN-α–, or IFN-γ–stimulated human monocytes at 50:1 E/T is also included. Data represent the mean of triplicate wells, and experiments were repeated at least three times with similar results. SD bars were omitted from the graphs but were <10% of the value of all points.
Table 1.
Tumoricidal Activity of Cytokine-stimulated CD11c+ DCs
| Target cell | No. donors tested | CD11c+ DCs | TRAIL | |||
|---|---|---|---|---|---|---|
| Unstimulated | GM-CSF | IFN-γ | IFN-α | |||
| Jurkat (T cell lymphoma) | 2 | 0.2 ± 0.1 | 4.3 ± 0.8 | 33.4 ± 2.6 | 23.0 ± 2.1 | 35.9 ± 2.5 |
| OVCAR3 (ovarian carcinoma) | 4 | 4.6 ± 1.3 | 5.4 ± 1.3 | 47.3 ± 4.7 | 38.7 ± 3.1 | 59.2 ± 2.0 |
| PC-3 (prostate carcinoma) | 3 | 5.5 ± 1.4 | 4.8 ± 0.9 | 49.1 ± 4.2 | 33.7 ± 2.0 | 68.3 ± 1.9 |
| WM 164 (melanoma) | 2 | 0.4 ± 0.1 | 1.5 ± 0.7 | 3.4 ± 1.1 | 1.7 ± 0.6 | 1.7 ± 1.2 |
| WM 793 (melanoma) | 3 | 6.2 ± 2.6 | 6.4 ± 0.9 | 42.3 ± 3.9 | 38.6 ± 2.7 | 55.2 ± 2.8 |
| Normal lung fibroblasts | 2 | 1.0 ± 0.2 | 1.2 ± 0.7 | 2.9 ± 1.3 | 2.4 ± 0.6 | 1.1 ± 0.2 |
| Lung microvascular endothelium | 2 | 1.0 ± 0.4 | 0.6 ± 0.3 | 0.1 ± 0.1 | 1.1 ± 0.5 | 3.6 ± 1.0 |
| Skeletal muscle cells | 2 | 2.1 ± 0.4 | 1.4 ± 1.2 | 2.4 ± 0.9 | 1.6 ± 1.1 | 3.9 ± 0.7 |
Means were calculated from experiments performed with sorted CD11c+ DCs from the indicated numbers of donors.
CD11c+ DCs, but Not IL-3Rα+ Pre-DCs, Exhibit TRAIL-dependent Tumoricidal Activity.
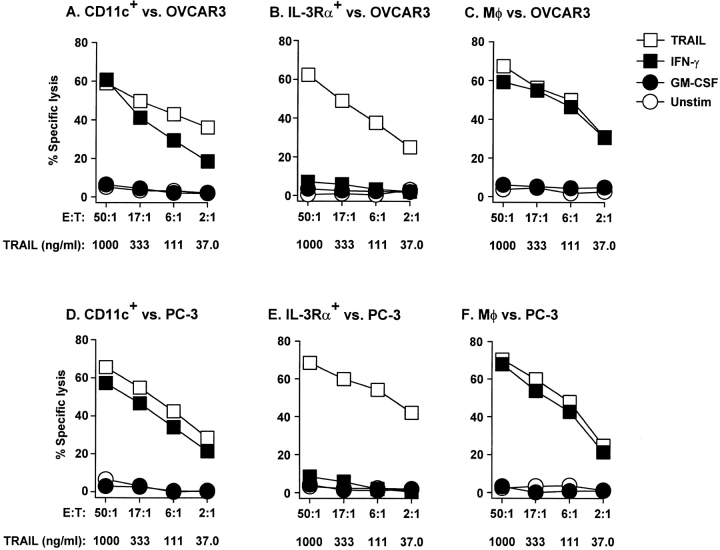
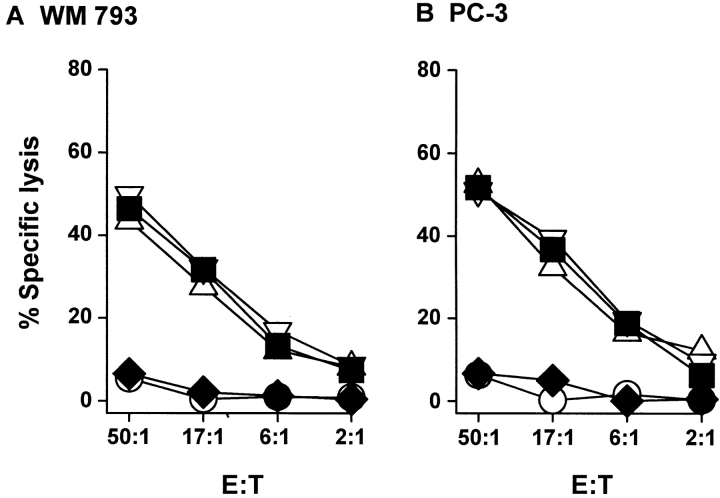
Once it was determined that bulk DCs can kill TRAIL-sensitive tumor cell targets, we examined the tumoricidal activity of the CD11c+ DC and IL-3Rα+ pre-DC subsets. Sorting the bulk DCs into these subsets revealed that nearly all of the cytotoxic activity of the IFN-γ–stimulated DCs (Fig. 3) was attributable to the CD11c+ DC subset (Fig. 4). Furthermore, the observed tumoricidal activity was dependent upon the number of IFN-γ–stimulated DCs, as decreasing the E/T ratio decreased the amount of target cell death. The tumoricidal activity of the IFN-γ–stimulated CD11c+ DCs was nearly equivalent to that of IFN-γ–stimulated Mφ. Similar results were observed with IFN-α–stimulated DCs and Mφ (data not shown). To confirm that the observed cytotoxicity of the CD11c+ DCs was specific to TRAIL and not other apoptosis-inducing molecules (i.e., FasL and TNF), IFN-γ–stimulated CD11c+ DCs were treated with recombinant soluble receptors for TRAIL (TRAILR2–Fc), Fas (Fas–Fc), or TNF (TNFR–Fc) before incubation with the tumor cell targets. Pretreating the IFN-γ–stimulated CD11c+ DCs with TRAILR2–Fc reduced target cell death to control (unstimulated DC effector cells) levels, whereas Fas–Fc or TNFR–Fc did not alter the cytolytic ability of the IFN-γ–stimulated DCs (Fig. 5). These results demonstrate that the TRAIL expressed on the CD11c+ DCs induces the killing of TRAIL-sensitive targets.
Figure 4.
TRAIL-mediated tumoricidal activity of blood DCs is restricted to CD11c+ DCs. CD11c+ DCs, IL-3Rα+ pre-DCs, and Mφ were incubated for 12 h in the absence or presence of GM-CSF or IFN-γ and then cultured for 8 h with 51Cr-labeled OVCAR3 (A–C) or PC-3 (D–F) target cells at the indicated E/T ratios. As a positive control, soluble TRAIL was added to the target cells at the indicated concentrations. Data represent the mean of triplicate wells, and experiments were repeated at least three times with similar results. SD bars were omitted from the graphs but were <10% of the value of all points.
Figure 5.
Specificity of CD11c+ DC cytolytic activity is restricted to TRAIL. Inclusion of the fusion protein TRAILR2–Fc (20 μg/ml), but not Fas–Fc (20 μg/ml) or TNFR–Fc (20 μg/ml), to 12-h IFN-γ–stimulated CD11c+ DCs inhibited killing of WM 793 (A) or PC-3 (B) tumor cell targets. Data points represent the mean of triplicate wells, and experiments were repeated at least three times with similar results. For clarity, SD bars were omitted from the graphs but were <10% of the value of all points. ○, unstimulated; ▪, IFN-γ; ♦, IFN-γ + TR2–Fc; ▵, IFN-γ + Fas–Fc; ▿, IFN-γ + TNFR–Fc.
IFN-stimulated CD11c+ DCs Induce Apoptotic Cell Death of Tumor Cells.
The results presented in Fig. 3 Fig. 4 Fig. 5 clearly demonstrate that IFN-stimulated CD11c+ DCs kill tumor cells via a TRAIL-dependent mechanism. However, these experiments only measure the release of 51Cr-labeled intracellular proteins into the culture supernatant, which is an event that can occur with either necrotic or apoptotic cell death. Previous reports have shown that TRAIL induces apoptotic death, as measured by DNA fragmentation, caspase activation, and annexin V binding 23 24 28. Thus, to demonstrate that the TRAIL-expressing CD11c+ DCs were inducing the apoptotic cell death of the target tumor cells, the binding of FITC-conjugated annexin V to the tumor cells was measured 36 37. Light scatter characteristics were used to distinguish the tumor cells from the DCs, such that only the tumor cells were counted in the analysis. After 8-h incubation, only those tumor cells incubated with soluble TRAIL or IFN-stimulated DCs (E/T ratio, 4:1) were positive for annexin V binding (Fig. 6). This apoptosis-inducing, tumoricidal activity of the IFN-stimulated DCs was seen with DCs from multiple donors and with other tumor cell targets (data not shown). These results demonstrate that TRAIL-expressing human DCs can kill TRAIL-sensitive tumor cells by inducing apoptosis.
Figure 6.
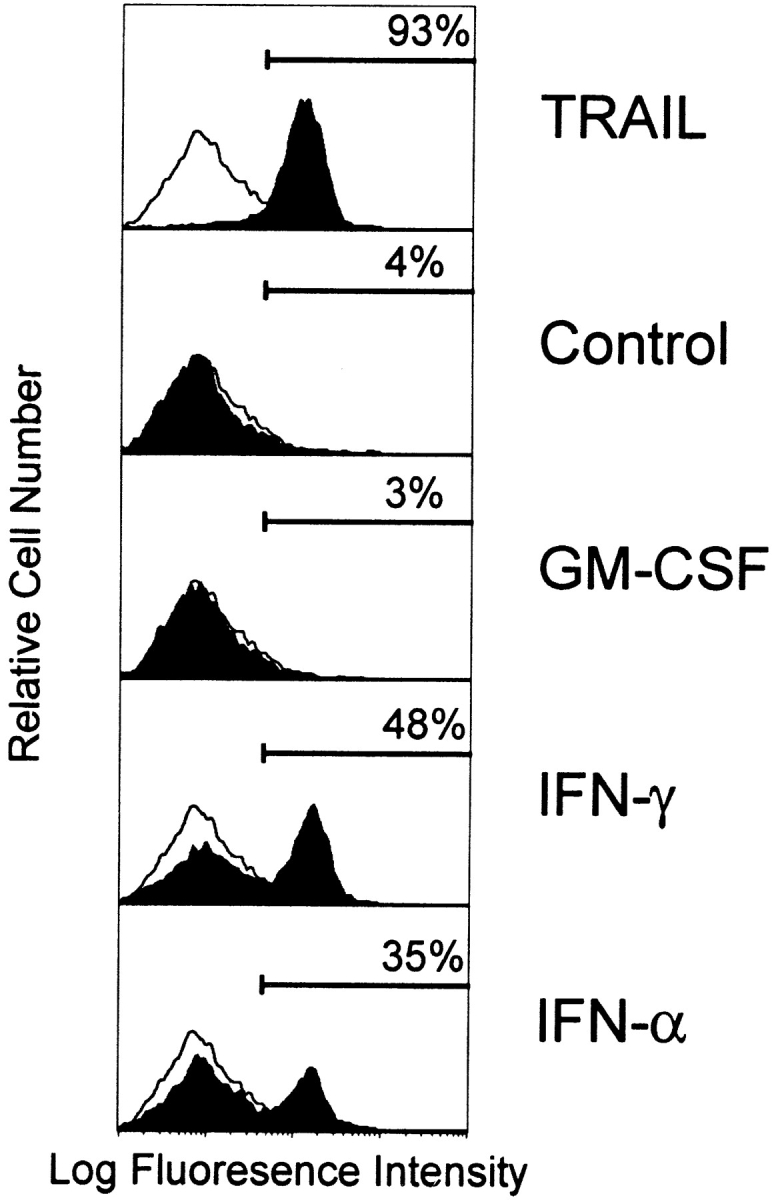
Tumor cell targets undergo apoptotic cell death when cultured with IFN-stimulated DCs as determined by phosphatidylserine externalization. OVCAR3 tumor cells were cultured for 8 h in complete medium alone or in the presence of LZ-TRAIL (1 μg/ml), unstimulated DCs, or cytokine (GM-CSF, IFN-γ, or IFN-α [100 ng/ml for 12 h])-stimulated DCs (E/T ratio 4:1). Cells were then stained with FITC–annexin V and analyzed by flow cytometry. The percentage of FITC–annexin V–positive tumor cells is indicated for each condition. Histograms represent 104 gated tumor cells. Similar results were seen with DCs from three other donors.
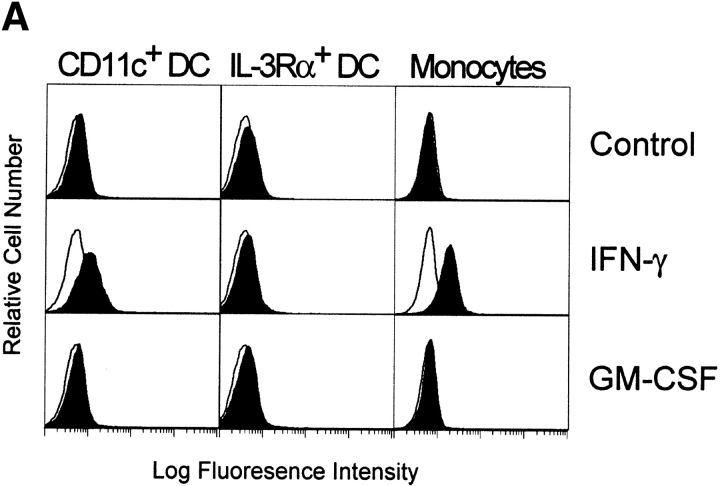
IFN Stimulation Upregulates TRAIL Expression on the CD11c+ DCs.
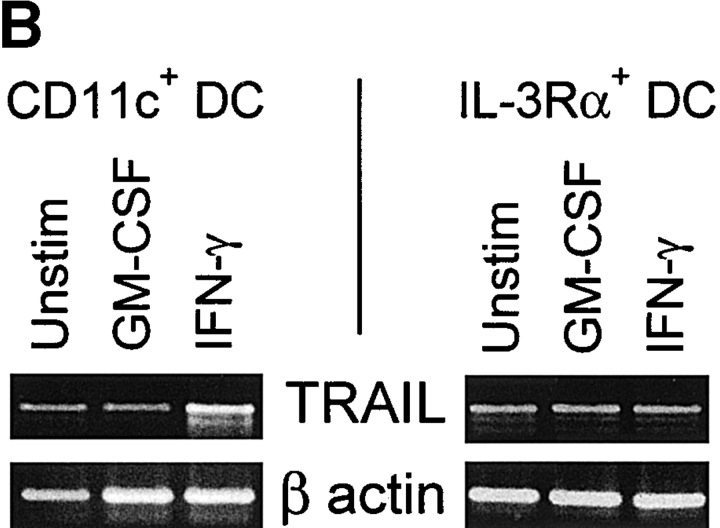
The results obtained thus far functionally describe the tumoricidal activity of IFN-stimulated CD11c+ DCs to be via a TRAIL-dependent mechanism. However, we wanted to correlate this functional activity with TRAIL protein expressed on the surfaces of these cells. Thus, DCs were cultured in medium or stimulated with GM-CSF or IFN-γ for 12 h and then analyzed for TRAIL expression using flow cytometry. Whereas Mφ express significant levels of TRAIL on their surfaces after stimulation with IFN-γ 27, lower levels of TRAIL expression were detected on CD11c+ DCs after IFN-γ stimulation (Fig. 7 A). TRAIL was undetectable on unstimulated or GM-CSF–stimulated CD11c+ DCs (Fig. 7 A), as well as on cells stimulated with CD40L or LPS (data not shown). In contrast, TRAIL was not detected on the unstimulated or cytokine-stimulated IL-3Rα+ pre-DCs. Analysis of the IFN-γ–stimulated CD11c+ DCs by reverse transcriptase (RT)-PCR revealed that TRAIL mRNA levels increased during the culture period as compared with unstimulated or GM-CSF–stimulated CD11c+ DCs (Fig. 7 B). Conversely, whereas TRAIL mRNA was detected in unstimulated IL-3Rα+ pre-DCs, the levels were unaltered after GM-CSF or IFN-γ stimulation (Fig. 7 B).
Figure 7.
TRAIL expression on IFN-stimulated human CD11c+ DCs and Mφ but not IL-3Rα+ pre-DCs. (A) Flow cytometric analysis of TRAIL protein expression. DCs and Mφ were incubated for 12 h in the absence or presence of IFN-γ or GM-CSF and then analyzed for TRAIL expression. Open histograms represent staining by the FITC-labeled M181 (anti-TRAIL mAb); filled histograms represent staining by the FITC-labeled isotype control. Histograms represent 104 gated cells in all conditions. (B) RT-PCR analysis of TRAIL mRNA levels in CD11c+ and IL-3Rα+ pre-DCs. Sorted CD11c+ and IL-3Rα+ pre-DCs were incubated for 12 h in the absence or presence of IFN-γ or GM-CSF. β-actin was used as a control over the same time period.
Discussion
The data presented here demonstrate that human blood DCs express the apoptosis-inducing molecule TRAIL after stimulation with either IFN-γ or IFN-α and acquire the ability to kill TRAIL-sensitive target cells. The TRAIL-specific activity was restricted to CD11c+ DCs and correlated with the increased levels of TRAIL mRNA and protein after IFN-γ stimulation. The low number (<8 × 106) of blood CD11c+ DCs obtained after enrichment from each donor restricted our ability to examine TRAIL expression and cytotoxicity after stimulation with the various cytokines. The 12-h stimulation time used in these studies was based on previous studies where TRAIL was maximally expressed on monocytes 27. Although the TRAIL surface expression was lower in comparison to that on IFN-γ–stimulated human Mφ, the IFN-stimulated CD11c+ DCs were able to mediate apoptosis with comparable efficiency, suggesting that even low levels of membrane-bound TRAIL result in potent tumoricidal activity. Moreover, the cytotoxic activity of the IFN-γ–stimulated CD11c+ DCs was completely inhibited by soluble TRAILR2–Fc and not by Fas–Fc or TNFR–Fc. These blocking studies provide additional evidence that the cellular apoptosis elicited by the IFN-stimulated CD11c+ DCs is a TRAIL-specific phenomenon.
In contrast to the CD11c+ DCs, IFN-stimulated IL-3Rα+ pre-DCs did not demonstrate significant cytotoxic activity against the same TRAIL-sensitive targets. We elected to positively select for IL-3Rα+ pre-DCs by sorting with an anti–IL-3R mAb, as this appears not to alter the functional capacity of these cells 10. We cannot rule out the possibility, however, that IL-3R triggering by the anti–IL-3R mAb did, in part, enhance cell viability. We routinely observed >90% cell viability of the IL-3Rα+ pre-DCs for prolonged periods (up to 20 h) when cultured in the presence of human serum. This is significantly longer than previous reports, where IL-3Rα+ pre-DCs cultured in the presence of FBS underwent rapid cell death 9. Thus, the lack of IL-3Rα+ pre-DC cytotoxicity in this study was not due to poor viability.
It is interesting that these two blood DCs respond differently with regard to induced TRAIL expression and TRAIL-mediated death. Previous reports have demonstrated that these two DC subsets differ with regard to phagocytic capacity, T cell stimulation capacity, and cell surface phenotype before and after stimulation (9 10; Fanger, N.A., unpublished observations). The differential expression of TRAIL after IFN-γ stimulation suggests further that these two DC subsets have different roles in directing antitumor responses via TRAIL. It is possible, however, that the IL-3Rα+ pre-DCs are able to express TRAIL upon further maturation/differentiation or with stimuli not examined in this study. It has been shown that when IL-3Rα+ pre-DCs are cultured with IL-3 ± GM-CSF and CD40L, a phenotypically and functionally different cell results by selective proliferation and death 9. Ongoing studies will determine whether these more mature/differentiated IL-3Rα+ pre-DCs can express TRAIL after IFN activation or other stimuli.
The melanoma cell line WM 164 and normal primary cells were resistant to both TRAIL-mediated apoptosis and TRAIL-expressing DCs, even though they express one and both of the apoptosis-inducing TRAIL receptors (TRAILR1 and -R2), respectively (29; Griffith, T.S., unpublished observation). The mechanism(s) that regulate sensitivity and resistance to TRAIL-induced apoptosis remain unclear. It was initially hypothesized that the expression of the non–death-inducing, or “decoy,” TRAIL receptors (TRAILR3 and -R4) were responsible for resistance to TRAIL 38 39 40. However, we have examined the expression of the four TRAIL receptors at both the mRNA and protein level in a variety of human tumor cell lines and found there to be no correlation between decoy receptor expression and TRAIL sensitivity/resistance 24 29. Likewise, additional studies have failed to clearly show a link between decoy receptor expression and resistance 41 42. Thus, it is unlikely that the decoy receptor hypothesis is the sole explanation for resistance to TRAIL-induced apoptosis. One component of the cell death machinery that appears to play a role in determining sensitivity and resistance to TRAIL is FLICE (Fas-associated death domain–like IL-1β–converting enzyme)-inhibitory protein (FLIP). It is believed that FLIP prevents the binding of caspase-8 to the death domain of cross-linked death receptor, inhibiting any downstream apoptotic signaling events. Intracellular levels of FLIP are high in the TRAIL-resistant melanoma WM 164 and in the normal cells used in this study (24; Griffith, T.S., unpublished observations). High FLIP levels have also been shown to correlate with resistance to Fas-mediated apoptosis in naive peripheral T cells 43 44. Although FLIP may have a protective function in these cells, it is likely to be one of several intracellular proteins that cooperate with other proteins (both intracellular and at the cell surface) to regulate sensitivity to TRAIL-induced death.
The tumoricidal activity reported here suggests that CD11c+ DCs may be one of several cells responsible for the active killing and removal of spontaneously arising tumors in the body. To date, NK cells, Mφ, T cells, and now DCs have been shown to express TRAIL under certain conditions, providing tumoricidal capability to a variety of effector cells that patrol the body 27 34 35. Whereas all these cell types may employ TRAIL to induce cell death outright, DCs may use TRAIL to generate apoptotic bodies for subsequent uptake, processing, and presentation of target cell antigens to CD8+ T cells, resulting in a stimulatory (i.e., CTL) or tolerogenic response. Indeed, several reports have demonstrated that DCs engulf apoptotic bodies and present antigen derived from these cell fragments in an MHC class I–restricted fashion, resulting in CTL activity 13 14. T cell tolerance is important in preventing the induction of autoimmunity. It can occur in the thymus, resulting in the deletion of self-reactive thymocytes before they are released into the periphery 45. Peripheral tolerance can also occur when specialized, tissue-specific antigens are encountered 17 46 47. In this situation, a DC presenting self-antigen may also be expressing TRAIL, or some other apoptosis-inducing molecule, which would kill the antigen-specific T cell once it came into contact with the DC. Recent studies have demonstrated that DCs can kill CD4+ T cells through the expression of FasL 48 49. Thus, the expression of TRAIL and/or FasL on DCs may prove to be a mechanism for deleting T cells in vivo.
The data presented here also suggest that DC TRAIL expression may contribute to other physiologic and pathologic situations, such as HIV infection. DCs are one of the first cells to encounter antigen at areas of inflammation in mucous membranes 50, which are the major sites of initiation of HIV infection. After the interaction between DCs and the virus at these sites, the DCs migrate to the draining lymph nodes, where they stimulate CD4+ T cells. In the process, the T cells become infected, leading to the replication and spread of the virus 12 51. The viral infection may also induce the production of various cytokines, such as IFN-γ and -α, activating an antiviral immune response. It could be hypothesized from our results that IFN production would stimulate the DCs to express TRAIL, which could potentially kill any activated T cells in the area.
Acknowledgments
We thank Drs. David Cosman, Raymond Goodwin, David Lynch, Craig Smith, Michael Widmer, Steven Wiley, and Douglas Williams for careful reading of the manuscript, Gary Carlton for figure preparation, and Anne Aumell for editorial assistance.
Footnotes
1used in this paper: DCs, dendritic cells; FLIP, FLICE (Fas-associated death domain–like IL-1β–converting enzyme)-inhibitory protein; L, ligand; LZ, leucine zipper; Mφ, monocytes; RT, reverse transcriptase; TRAIL, TNF-related apoptosis-inducing ligand
References
- Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu. Rev. Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Banchereau J., Steinman R.M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- O'Doherty U., Steinman R.M., Peng M., Cameron P.U., Gezelter S., Kopeloff I., Swiggard W.J., Pope M., Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J. Exp. Med. 1993;178:1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U., Peng M., Gezelter S., Swiggard W.J., Betjes M., Bhardwaj N., Steinman R.M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994;82:487–493. [PMC free article] [PubMed] [Google Scholar]
- Nijman H.W., Kleijmeer M.J., Ossevoort M.A., Oorschot V.M.J., Vierboom M.P.M., van de Keur M., Kenemans P., Kast W.M., Geuze H.J., Melief C.J.M. Antigen capture and major histocompatibility class II compartments of freshly isolated and cultured human blood dendritic cells. J. Exp. Med. 1995;182:163–174. doi: 10.1084/jem.182.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger N.A., Wardwell K., Shen L., Tedder T.F., Guyre P.M. Type I (CD64) and type II (CD32) Fcγ receptor-mediated phagocytosis by human blood dendritic cells. J. Immunol. 1996;157:541–548. [PubMed] [Google Scholar]
- Fanger N.A., Voigtlaender D., Liu C., Swink S., Wardwell K., Fisher J., Graziano R.F., Pfefferkorn L.C., Guyre P.M. Characterization of expression, cytokine regulation, and effector function of the high affinity IgG receptor FcγRI (CD64) expressed on human blood dendritic cells. J. Immunol. 1997;158:3090–3098. [PubMed] [Google Scholar]
- Grouard G., Durand I., Filgueira L., Banchereau J., Liu Y.J. Dendritic cells capable of stimulating T cells in germinal centres. Nature. 1996;384:364–367. doi: 10.1038/384364a0. [DOI] [PubMed] [Google Scholar]
- Grouard G., Rissoan M.-C., Filgueira L., Durand I., Banchereau J., Liu Y.-J. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olweus J., BitMansour A., Warnke R., Thompson P.A., Carballido J., Picker L.J., Lund-Johansen F. Dendritic cell ontogenya human dendritic cell lineage of myeloid origin. Proc. Natl. Acad. Sci. USA. 1997;94:12551–12556. doi: 10.1073/pnas.94.23.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L.J., Tedder T.F. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]
- Weissman D., Li Y., Orenstein J.M., Fauci A.S. Both a precursor and a mature population of dendritic cells can bind HIV. However, only the mature population that expressed CD80 can pass infection to unstimulated CD4+ T cells. J. Immunol. 1995;155:4111–4117. [PubMed] [Google Scholar]
- Albert M.L., Sauter B., Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- Albert M.L., Pearce S.F.A., Francisco L.M., Sauter B., Roy P., Silverstein R.L., Bhardwaj N. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 1998;188:1359–1368. doi: 10.1084/jem.188.7.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A.Y.C., Golumbek P., Ahmadzadeh M., Jaffee E., Pardoll D., Levitsky H. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- Kurts C., Heath W.R., Carbone F.R., Allison J., Miller J.F., Kosaka H. Constitutive class I–restricted exogenous presentation of self-antigens in vivo. J. Exp. Med. 1996;184:923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savill J., Hogg N., Ren Y., Hasslet C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J.W., Inaba K. Dendritic cells as adjuvants for class I major histocompatibility complex–restricted antitumor immunity. J. Exp. Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L., Mayordomo J.I., Tjandrawan T., DeLeo A.B., Clarke M.R., Lotze M.T., Storkus W.J. Therapy of murine tumors with tumor peptide–pulsed dendritic cellsdependence on T cells, B7 costimulation, and T helper cell 1–associated cytokines. J. Exp. Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celluzzi C.M., Mayordomo J.I., Storkus W.J., Lotze M.T., Falo L.D., Jr. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J. Exp. Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglia P., Chiodoni C., Rodolfo M., Colombo M.P. Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J. Exp. Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley S.R., Schooley K., Smolak P.J., Din W.S., Huang C.-P., Nicholl J.K., Sutherland G.R., Davis Smith T., Rauch C., Smith C.A. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Griffith T.S., Chin W.A., Jackson G.C., Lynch D.H., Kubin M.Z. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J. Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- Schneider P., Holler N., Bodmer J.L., Hahne M., Frei K., Fontana A., Tschopp J. Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak H., Miller R.E., Gliniak B., Ariail K., Griffith T.S., Kubin M., Chin W., Jones J., Woodward A., Le T. Tumoricidal activity of TRAIL in vivo. Nat. Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- Griffith T.S., Wiley S.R., Kubin M.Z., Sedger L.M., Maliszewski C.R., Fanger N.A. Monocyte-mediated tumoricidal activity via the tumor necrosis factor–related cytokine, TRAIL. J. Exp. Med. 1999;189:1343–1353. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitti R.M., Marsters S.A., Ruppert S., Donahue C.J., Moore A., Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J. Biol. Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- Griffith T.S., Rauch C.T., Smolak P.J., Waugh J.Y., Boiani N., Lynch D.H., Smith C.A., Goodwin R.G., Kubin M.Z. Functional analysis of TRAIL receptors using monoclonal antibodies. J. Immunol. 1999;162:2597–2605. [PubMed] [Google Scholar]
- Degli-Esposti M.A., Dougall W.C., Smolak P.J., Waugh J.Y., Smith C.A., Goodwin R.G. The novel receptor TRAIL-R4 induces NFκB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- Griffith T.S., Lynch D.H. TRAILa molecule with multiple receptors and control mechanisms. Curr. Opin. Immunol. 1998;10:559–563. doi: 10.1016/s0952-7915(98)80224-0. [DOI] [PubMed] [Google Scholar]
- Katsikis P.D., Garcia-Ojeda M.E., Wunderlich E.S., Smith C.A., Yagita H., Okumura K., Kayagaki N., Alderson M., Herzenberg L.A., Herzenberg L.A. Activation-induced peripheral blood T cell apoptosis is Fas independent in HIV-infected individuals. Int. Immunol. 1996;8:1311–1317. doi: 10.1093/intimm/8.8.1311. [DOI] [PubMed] [Google Scholar]
- Katsikis P.D., Garcia-Ojeda M.E., Torres-Roca J.F., Tijoe I.M., Smith C.A., Herzenberg L.A., Herzenberg L.A. Interleukin-1β converting enzyme–like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J. Exp. Med. 1997;186:1365–1372. doi: 10.1084/jem.186.8.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayagaki N., Yamaguchi N., Nakayama M., Eto H., Okumura K., Yagita H. Type I interferons (IFNs) regulate tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) expression on human T cellsa novel mechanism for the antitumor effects of type I IFNs. J. Exp. Med. 1999;189:1451–1460. doi: 10.1084/jem.189.9.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamai L., Ahmad M., Bennett I.M., Azzoni L., Alnemri E.S., Perussia B. Natural killer (NK) cell–mediated cytotoxicitydifferential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J. Exp. Med. 1998;88:2375–2380. doi: 10.1084/jem.188.12.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok V.A., Voelker D.R., Campbell P.A., Cohen J.J., Bratton D.L., Henson P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- Martin S.J., Reutelingsperger C.P., McGahon A.J., Rader J.A., van Schie R.C., LaFace D.M., Green D.R. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulusinhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 1995;182:1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G., Ni J., Wei Y.-F., Yu G.-I., Gentz R., Dixit V.M. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- Sheridan J.P., Marsters S.A., Pitti P.M., Gurney A., Skubatch M., Baldwin D., Ramakrishnan L., Gray C.L., Baker K., Wood W.I. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors. Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- Marsters S.A., Sheridan J.P., Pitti R.M., Huang A., Skubatch M., Baldwin D., Yuan J., Gurney A., Goddard A.D., Godowski P. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr. Biol. 1997;7:1003–1006. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- Keane M.M., Ettenberg S.A., Nau M.M., Russell E.K., Lipkowitz S. Chemotherapy augments TRAIL-induced apoptosis in breast cell lines. Cancer Res. 1999;59:734–741. [PubMed] [Google Scholar]
- Frank S., Kohler U., Schackert G., Schackert H.K. Expression of TRAIL and its receptors in human brain tumors. Biochem. Biophys. Res. Commun. 1999;257:454–459. doi: 10.1006/bbrc.1999.0493. [DOI] [PubMed] [Google Scholar]
- Refaeli Y., Van Parijs L., London C.A., Tschopp J., Abbas A.K. Biochemical mechanisms of IL-2 regulated Fas-mediated T cell apoptosis. Immunity. 1998;8:615–623. doi: 10.1016/s1074-7613(00)80566-x. [DOI] [PubMed] [Google Scholar]
- Algeciras-Schimnich A., Griffith T.S., Lynch D.H., Paya C.V. Cell cycle-dependent regulation of FLIP levels and susceptibility to Fas-mediated apoptosis. J. Immunol. 1999;162:5205–5211. [PubMed] [Google Scholar]
- Brocker T., Karjalainen K. Targeted expression of MHC class II molecules demonstrates dendritic cells can induce negative but no positive selection of thymocytes in vivo. J. Exp. Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurts C., Kosaka H., Carbone F.R., Miller J.F., Heath W.R. Class I–restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8+ T cells. J. Exp. Med. 1997;186:239–245. doi: 10.1084/jem.186.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster I., Lieberam I. Peripheral tolerance of CD4 T cells following local activation in adolescent mice. Eur. J. Immunol. 1996;26:3194–3202. doi: 10.1002/eji.1830261253. [DOI] [PubMed] [Google Scholar]
- Suss S., Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas ligand–induced apoptosis. J. Exp. Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Qian S., Hershberger P.A., Rudert W.A., Lynch D.H., Thomson A.W. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J. Immunol. 1997;158:5676–5684. [PubMed] [Google Scholar]
- McWilliam A.S., Nelson D., Thomas J.A., Holt P.G. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J. Exp. Med. 1994;179:1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P.U., Freudenthal P.S., Barker J.M., Gezelter S., Inaba K., Steinman R.M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992;257:383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]