Abstract
Disease severity in Plasmodium falciparum infections is a direct consequence of the parasite's efficient evasion of the defense mechanisms of the human host. To date, one parasite-derived molecule, the antigenically variant adhesin P. falciparum erythrocyte membrane protein 1 (PfEMP1), is known to be transported to the infected erythrocyte (pRBC) surface, where it mediates binding to different host receptors. Here we report that multiple additional proteins are expressed by the parasite at the pRBC surface, including a large cluster of clonally variant antigens of 30–45 kD. We have found these antigens to be identical to the rifins, predicted polypeptides encoded by the rif multigene family. These parasite products, formerly called rosettins after their identification in rosetting parasites, are prominently expressed by fresh isolates of P. falciparum. Rifins are immunogenic in natural infections and strain-specifically recognized by human immune sera in immunoprecipitation of surface-labeled pRBC extracts. Furthermore, human immune sera agglutinate pRBCs digested with trypsin at conditions such that radioiodinated PfEMP1 polypeptides are not detected but rifins are detected, suggesting the presence of epitopes in rifins targeted by agglutinating antibodies. When analyzed by two-dimensional electrophoresis, the rifins resolved into several isoforms in the pI range of 5.5–6.5, indicating molecular microheterogeneity, an additional potential novel source of antigenic diversity in P. falciparum. Prominent polypeptides of 20, 22, 76–80, 140, and 170 kD were also detected on the surfaces of pRBCs bearing in vitro–propagated or field-isolated parasites. In this report, we describe the rifins, the second family of clonally variant antigens known to be displayed by P. falciparum on the surface of the infected erythrocyte.
Keywords: P., falciparum, surface antigen, rif gene family, antibodies, strain-specific
The morbidity and mortality associated with Plasmodium falciparum malaria infections occurs exclusively during the erythrocytic phase of the parasite life cycle. Strategies used by P. falciparum for maximizing survival and proliferative capacity in the bloodstream include the receptor-mediated sequestration of P. falciparum–infected erythrocytes (pRBCs) on the vascular endothelium and the constant variation of antigenic epitopes on the pRBC surface to evade antiparasite mechanisms of protection in the human host 1.
A polypeptide exported by the parasite to the outer face of the pRBC membrane, the 200–400-kD P. falciparum erythrocyte membrane protein 1 (PfEMP1),1 is a cytoadherence ligand and undergoes clonal switching 2 3. PfEMP1 polypeptides are encoded by the var gene family 4, and they are expressed on the infected cell surface as the parasite develops from the ring-shaped early forms into the pigmented trophozoite stage, simultaneous with the onset of adhesive capacity and antigenicity of the pRBC 5.
Studies of humoral immune responses in natural infections of P. falciparum highlight the extreme diversity of antigenic determinants on the infected erythrocytes 6 7 8. Although sera from individuals with a history of exposure to the disease may contain antibodies that react with epitopes shared by many parasite isolates, the bulk of the natural or experimentally induced immune response to surface determinants on the pRBC is strain/clone specific 9 10. The variant antigen PfEMP1 has been postulated to be the sole target for specific antibodies that agglutinate pRBCs and confer protection against clinical disease 11 12.
To date, PfEMP1 is the only molecularly characterized protein of P. falciparum shown to be located on the surface of the infected erythrocyte 2. PfEMP1 mediates binding to vascular endothelial receptors such as CD36, intercellular adhesion molecule 1, and thrombospondin 13, as well as to uninfected erythrocytes in the adhesion phenomenon known as rosetting 14 15. Polypeptides of low molecular mass have been radiolabeled on the pRBC surface and termed rosettins after their identification in rosetting strains 16. Based on this and additional observations suggesting that the parasite exports more than one set of polypeptides to the host cell surface (reference 17 and our unpublished data), we set out to systematically reanalyze the surfaces of pRBCs harboring P. falciparum recently isolated from malaria patients or strains and clones adapted to laboratory culture conditions. Here, we have focused the analysis and characterization to parasite-derived products with a molecular size <200 kD, a size distinct from the known PfEMP1 antigens.
Materials and Methods
Parasites and Sera.
The P. falciparum lines FCR3S/b (K−) and FCR3S1/b (K−) were selected from parasites FCR3S (K−) and FCR3S1 (K−), respectively, for cytoadherence to the CD36 receptor on C32 melanoma cells. FCR3S/a (K−) was obtained from FCR3S by repeated selection for nonrosetting parasites as described elsewhere 18. FCR3S1 was cloned by limiting dilution from FCR3S, which originated from the FCR3 strain isolated in The Gambia, West Africa. The subclones FCR3S1.2 (K−) and FCR3S1.6 (K−) were obtained by micromanipulation from FCR3S1. Clones TM284S2 (K+), TM284S3 (K+), TM284S11 (K+), TM284S12 (K+), TM284S20 (K+), TM284S7 (K−), TM284S9 (K−), and TM284S19 (K−) were derived by micromanipulation from the strain TM284 (K+), which, along with strain TM180 (K−), were isolated from malaria patients in Thailand. The F32 strain was isolated in Tanzania. The parasite 3D7 was obtained by limiting dilution cloning of the isolate NF54, which was derived from a patient who acquired malaria in the airport area in Amsterdam, The Netherlands. R29 (K+) was cloned from ITOR, a parasite from the ITO strain selected for the rosetting phenotype. The parasite Dd2 was originally cloned from the W2-MEF line of the Indochina III isolate. The P. falciparum isolates 186, 199, 341, 347, 352, and 354 were part of a larger panel of field parasites collected from African children infected with malaria. Upon collection, the blood samples were immediately frozen according to standard techniques. For their analysis, the frozen blood samples were thawed and maintained in culture in their own blood for 24–30 h until parasites developed into the mature trophozoite stage, at which time they were harvested and further processed.
Sera were collected from (a) adults living in Yekepa, Liberia, an area characterized by high perennial malaria transmission (denoted 022, 102, 119, 142, 163, 164, 169, 174, 179, 198, 241, and 368), (b) adults from Fajara, The Gambia, a region with seasonal malaria transmission (denoted 072, 100, and 136), and (c) children 1–15 yr old living in Saradidi, an area in western Kenya holoendemic for malaria (denoted 011, 039, 080, 118, and 209). All donors had had repeated malaria attacks; none had symptoms of clinical malaria at the time of sampling. In all cases, informed consent was obtained from the patients and/or their parents. The sera were stored at −70°C and heat inactivated at 56°C before the assays. Control sera were obtained from healthy Swedish blood donors.
Culture of Parasites.
All of the laboratory-adapted parasites used in this study were cultured in human group O Rh+ erythrocytes at 5% hematocrit with 10% AB+ serum added to the malaria culture medium containing RPMI 1640, 25 mM Hepes, 25 mM sodium bicarbonate, and 50 mg/ml gentamycin, pH 7.4. In some cases, cultured parasites were enriched for the rosetting phenotype (R+) as previously described 18.
Surface Analysis of pRBCs.
Surface iodination of pRBCs was performed by the lactoperoxidase/Na125I/H2O2 method under conditions of minimal intracellular labeling. In brief, 2 × 109 cells of a culture at 7–15% parasitemia with a majority of parasites in the trophozoite stage were gently washed in PBS and resuspended to 1 ml in PBS plus 1 mM KI. 1 mCi of Na125I (Amersham) and 100 μl of 2 mg/ml lactoperoxidase (Sigma Chemical Co.) was added, and the reaction was initiated by the addition of 25 μl of 0.03% H2O2. Four subsequent additions of 25 μl of 0.03% H2O2 were administered at 1-min intervals. Radioiodinated cells were washed four times with ice-cold PBS containing 5 mM KI and resuspended in 1 ml of RPMI 1640 plus 5% sorbitol. Labeling of intracellular hemoglobin accounted for <2% of total acid-precipitable incorporated radiolabel. To disrupt rosettes/agglutinates, 100 IU/ml of heparin (Løvens) was added to the cell suspension, and this was passed five times through a 23-gauge (0.6 mm internal diameter) needle using a 1-ml syringe. The cell suspension was overlaid on top of a four-step (40, 60, 70, and 80%) Percoll gradient in RPMI/5% sorbitol and centrifuged in a JA 20 rotor (Beckman Instruments, Inc.) at 10,000 rpm for 30 min at room temperature. Cells floating between the 40 and 60% Percoll layers (>95% mature parasite–containing erythrocytes) were recovered and gently washed with PBS. Enriched pRBCs were first extracted with 1% Triton X-100/PBS containing a cocktail of protease inhibitors (1 mM EDTA-N2, 20 μg/ml leupeptin, 0.7 μg/ml pepstatin, 0.2 mM PMSF, and 50 μg/ml aprotinin) and subsequently extracted in a solution of 2% SDS and protease inhibitors in PBS. The polypeptides in the Triton-insoluble fraction were solubilized in 2% SDS-PAGE sample buffer and separated by a gradient of 5–8.5 or 7.5–17.5% SDS-PAGE. The dried gels were scanned and analyzed using a PhosphorImager and ImageQuant analysis software (Molecular Dynamics).
Immunoprecipitation.
SDS or Triton X-100 extracts of surface-radioiodinated pRBCs (25 μl) were mixed with 375 μl of NETT (50 mM Tris, pH 7.4, 150 mM NaCl, 5 mM disodium EDTA, 20 μg/ml leupeptin, 50 μg/ml aprotinin, 0.02% sodium azide, and 0.5% Triton X-100) buffer/10 mg/ml IgG-free BSA (Sigma Chemical Co.) and 100 μl of 10% Triton X-100/PBS. 15 μl of serum was added to 500 μl of reconstituted extract, and the samples were incubated for 12 h at 4°C with rotation. Protein A–Sepharose (100 μl of a 50% suspension in NETT buffer/10 mg/ml IgG-free BSA) was added, and the samples were further incubated for 1 h at room temperature with rotation. The adsorbent was then washed twice with 1 ml of NETT, twice with 1 ml of NETT/0.35 M NaCl, and twice with 1 ml of NETT. The Sepharose beads were boiled for 4 min in the presence of 5% SDS-PAGE sample buffer, and the solubilized polypeptides were separated and analyzed as described above.
Trypsin Treatment of the PRBC Surface.
Intact or radiolabeled pRBCs purified on Percoll gradients were digested with trypsin (Sigma Chemical Co.) at the indicated concentrations (see Fig. 5 and Table ) for 10 min at 37°C. The reaction was terminated by the addition of 1 ml soybean of trypsin inhibitor (Sigma Chemical Co.) at 1 mg/ml in RPMI 1640/10% AB+ serum. Cells were washed with PBS and either resuspended in RPMI–Hepes/10% AB+ serum for binding and agglutination assays or extracted with Triton X-100/SDS and analyzed by SDS-PAGE as described above.
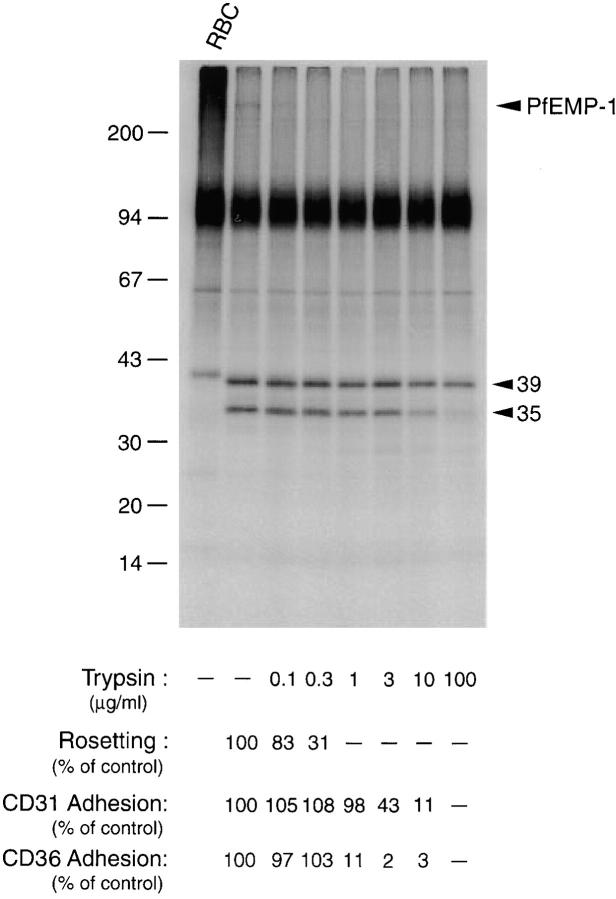
Figure 5.
Trypsin sensitivity of rifins and binding phenotypes. 125I-labeled pRBCs containing FCR3S1 parasites at the trophozoite stage were digested for 10 min at 37°C at the indicated concentrations of enzyme. The Triton X-100–insoluble fraction was analyzed by SDS-PAGE in a 7.5–17.5% gradient acrylamide gel. Treated cells were washed in PBS, resuspended in culture medium supplemented with 10% AB+ serum, and either mixed with uninfected RBCs of the blood group O at a final hematocrit of 5% and allowed to reform rosettes for 45 min or assayed for cytoadherence on monolayers of PECAM1/CD31-transfected L cells or CHO cells transfected with the CD36 receptor, as described in Materials and Methods. Results represent the mean of three experiments.
Table 1.
Reactivity of Malaria Immune Sera with Trypsin-sensitive and Trypsin-resistant Antigens on the pRBC Surface
| Sera | Agglutination of trypsin-digested pRBCs (μg/ml trypsin) | |||
|---|---|---|---|---|
| 0 | 1 | 10 | 100 | |
| 011K | 4+ | 4+ | 3+ | 1+ |
| 022L | 1+ | – | – | – |
| 080K | 4+ | 4+ | – | – |
| 118K | 1+ | 1+ | 1+ | – |
| 119L | 4+ | 3+ | – | – |
| 142L | 2+ | 1+ | 1+ | 1+ |
| 163L | 4+ | 2+ | 2+ | 2+ |
| 174L | 2+ | 1+ | 1+ | – |
| 241L | – | – | – | – |
| Antigen | Amount detected on pRBC surface | |||
| % | ||||
| PfEMP1 | 100 | 0 | 0 | 0 |
| 39-kD rifin | 100 | 97 | 71 | 65 |
| 35-kD rifin | 100 | 74 | 37 | 15 |
Assay of Serum-induced Agglutination of pRBCs.
Agglutination of parasitized red blood cells was assayed as previously described 19 with some modifications. pRBCs cultured to trophozoite and late schizont stages were washed three times in malaria culture medium and then resuspended at 20% hematocrit. Aliquots of 25 μl were mixed with an equal volume of prediluted or nonprediluted serum to a final dilution of 1:2, 1:5, or 1:10. The pRBC–serum mixture was incubated at 37°C for 1 h under a constant rotation of 3 rpm. A 10-μl aliquot was mixed with 5 μl of acridine orange and mounted on a glass slide, and 50 consecutive fields of vision were examined diagonally using a 40× lens in an incident UV light microscope. Control sera from healthy Swedish blood donors were used. Semiquantitative scoring was used for analysis. The agglutination assay was scored negative when no agglutinates of 4 pRBCs were detected; 1+, 1–5 agglutinates of 4–10 pRBCs; 2+, >5 agglutinates of 4–10 pRBCs or 1–5 agglutinates of 11–20 pRBCs; 3+, >5 agglutinates of 11–20 pRBCs or 1–5 agglutinates of >20 pRBCs; 4+, >5 agglutinates of >20 pRBCs.
Rosetting, Autoagglutination, and Cytoadherence.
Rosetting of uninfected red cells and autoagglutination of parasitized erythrocytes was assessed by direct staining of cultures with acridine orange (Sigma Chemical Co.) and examination using an epifluorescence microscope. Rosetting rates were measured as previously described 20. Spontaneous autoagglutination of pRBCs in the P. falciparum cultures was measured as described elsewhere 18. Adherence of pRBCs to unfixed C32 melanoma cells, human umbilical vein endothelial cells (HUVECs), chinese hamster ovary (CHO) cells transfected with CD36 or intercellular adhesion molecule 1, or L cells transfected with platelet/endothelial cell adhesion molecule (PECAM)1/CD31 was performed as described 21. In some cytoadherence assays on HUVECs, C32, and L cells, rosettes were first disrupted by adding 100 IU/ml of heparin (Løvens) to the culture and passage (five times) through a 23-gauge (0.6 mm internal diameter) needle using a 1-ml syringe. The cell suspension was overlaid on a four-step (40, 60, 70, and 80%) Percoll gradient, and the trophozoite-bearing red cells were selected as described above, washed twice in RPMI, and used for the assays in serum-free medium.
Surface Immunofluorescence.
The assay of the binding of human nonimmune normal Swedish serum Igs to pRBCs was performed by direct labeling of the cells using FITC-conjugated sheep anti–human Ig antibodies (State Bacteriology Laboratory, Sweden) as described elsewhere 22.
Results
Novel Variable Polypeptides Identified on the pRBC Surface.
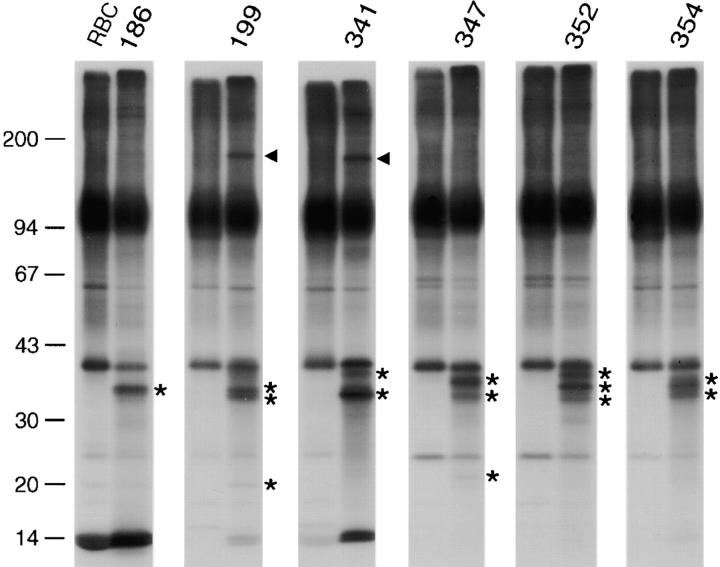
Frozen blood samples from P. falciparum malaria patients were thawed, and the cells were maintained in culture for 30 h. The intact cells were mildly radioiodinated under conditions ensuring >98% isotope incorporation to surface-exposed molecular moieties, and the labeled polypeptides were analyzed by protein gel electrophoresis. In all six isolates that developed into the mature stages, we found, in addition to PfEMP1 bands (>200 kD), several polypeptides with an estimated molecular mass between 33 and 39 kD that were readily labeled on the surfaces of erythrocytes containing parasites at the trophozoite/schizont stages, but not on the ring-infected or uninfected RBCs from the same blood sample (Fig. 1). The number, size, and degree of radiolabel incorporation of these polypeptides was variable and essentially unique for each isolate. Typically, the largest of these bands (39 kD) comigrated with the heavily radioiodinated monomer chains of erythrocytic glycophorin A and was therefore often indistinguishable from the host protein. One to three additional labeled polypeptides 33–37 kD in size formed a characteristic cluster of bands with isolate-specific patterns. We then proceeded to study the pRBC surfaces of 17 strains, lines, and clones of P. falciparum and found that radioiodinatable polypeptides in the range of 30–45 kD were commonly exported and surface expressed by a majority of these in vitro–propagated parasites (Fig. 2A–C). The number of bands and their relative radiolabel intensities was specific for each parasite. As determined by radioiodination experiments with highly synchronized cultures, the expression of the 30–45-kD polypeptide cluster on the pRBC surface occurred from 16 to 18 h after invasion until the end of the erythrocytic cycle (data not shown), matching the time course of expression of antigenic and adhesive phenotypes and the onset of PfEMP1 on the pRBC surface. These polypeptides were partially solubilized in Triton X-100, in contrast to the extreme insolubility of PfEMP1 in nonionic detergents. Additional parasite-specific radiolabeled bands were detected on pRBCs infected with some of the patient isolates or laboratory-cultured parasites (Fig. 1 and Fig. 2A–C). A 20-kD band was observed in isolate 199, whereas a polypeptide of 22 kD was radiolabeled in isolate 347 and the strain Dd2. Faint bands of 76–80 kD were visible in several of the in vitro–propagated parasites, and a polypeptide of 140 kD was detected in TM180, a strain also expressing several smaller (33–39 kD) polypeptides. Most prominent was the strongly radioiodinated band of 170 kD found in two of the clinical isolates (199 and 341) and strain F32, as well as strain TM284 and several of its clones (only TM284S2 is shown). None of these polypeptides were detected on pRBCs bearing trophozoite/schizont stages when the erythrocytes were surface 125I-labeled before parasite invasion (data not shown).
Figure 1.
Novel variable antigens expressed on the surfaces of erythrocytes harboring clinical isolates of P. falciparum. Frozen blood samples from malaria patients were thawed, maintained in culture until the development of trophozoite stages, and subsequently surface-radioiodinated as explained in Materials and Methods. Labeled erythrocytes bearing trophozoite and schizont stages were separated from ring-infected and noninfected cells (RBC) in Percoll/sorbitol gradients and detergent extracted, and the polypeptides were fractionated by SDS-PAGE. Molecular sizes are indicated (kD). Extracts from autologous RBCs were always run in adjoining lanes for identification of parasite-derived polypeptides, which are indicated by asterisks (<45 kD) or closed arrowheads (100–200 kD). PfEMP1 polypeptides are not indicated.
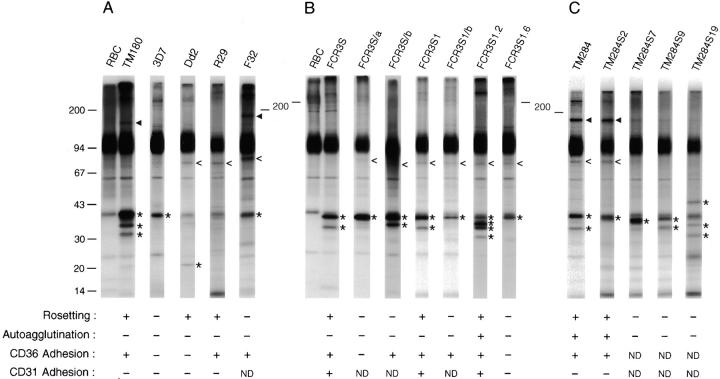
Figure 2.
Surface antigens on erythrocytes infected with parasites adapted to in vitro growth. (A) Cultures of P. falciparum strains and (B) parasite lines and clones derived from FCR3S or (C) from the TM284 strain were processed as described for Fig. 1. The adherence of pRBCs to uninfected erythrocytes (Rosetting), other pRBCs (Autoagglutination), or CHO cells transfected with the CD36 receptor was assessed as described elsewhere 18. Adhesion to PECAM1/CD31 was performed as described 39. Panels of R+ and R− clones were obtained from the TM284 strain by micromanipulation-assisted collection of trophozoite-bearing pRBC involved or not in rosettes. The parasite TM284S2 is one out of five clones displaying identical adhesive and surface-labeled polypeptide phenotypes. The parasite-derived polypeptides are indicated as in Fig. 1, with open arrowheads indicating bands of 45–100 kD.
The 30–45-kD Surface Antigens Are Clonally Variant but Stably Expressed In Vitro.
Upon examination of the panel of clones derived by micromanipulation from the TM284 strain (Fig. 2 C), we observed that all five rosetting (R+) clones (TM284S2, S3, S11, S12, and S20) had an identical pattern of surface radiolabeled polypeptides with bands detected at 39, 80 (faint), and 170 kD and a PfEMP1 polypeptide of 340 kD (TM284S2 is shown; gels optimized for PfEMP1 separation are not shown). In contrast, each of the three nonrosetting (R−) clones obtained (TM284S7, S9, and S19) showed unique combinations of bands in the range of 33–45 kD and no detectable PfEMP1 proteins in the gels. This result was consistent with the reported role of PfEMP1 as a rosetting ligand 14 15. Moreover, the data strongly suggested that the variation observed in the small surface antigens clustered in the range of 30–45 kD had its origin in a set of clonally variant genes. By comparing the patterns of surface-radiolabeled polypeptides expressed by the sibling parasites FCR3S1.2 (R+) and FCR3S1.6 (R−), sharing an isogenic background after their cloning by micromanipulation from the previously cloned FCR3S1 (R+), it was confirmed that the expression of the small surface antigens (31–39 kD) of this parasite is subjected to clonal genetic switching (Fig. 2 B).
To investigate the stability of expression of the small surface antigens in parasite populations that were or were not exposed to selective pressures other than those inherent to in vitro growth, we 125I-labeled pRBCs periodically collected from year-long continuous cultures of FCR3S1, maintained with regular enrichment for the rosetting phenotype, and the Dd2 strain, cultured without selection but displaying rosetting rates consistently >65%. The results showed no change in the type of radioiodinated polypeptides of these two parasites grown during at least 150 generations, as assessed in at least 10 separate experiments of surface labeling of pRBCs (data not shown). Although similar stability in the small surface antigen types is a characteristic of other parasite strains undergoing long-term culture, a decrease in the expression of these radiolabeled bands has been sporadically observed in some cultures.
Molecular Heterogeneity of the Small Surface Polypeptides.
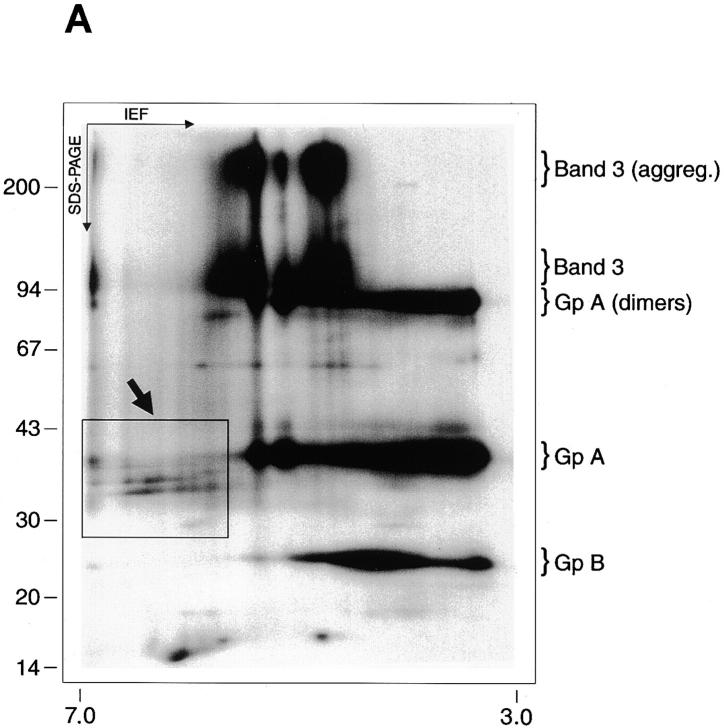
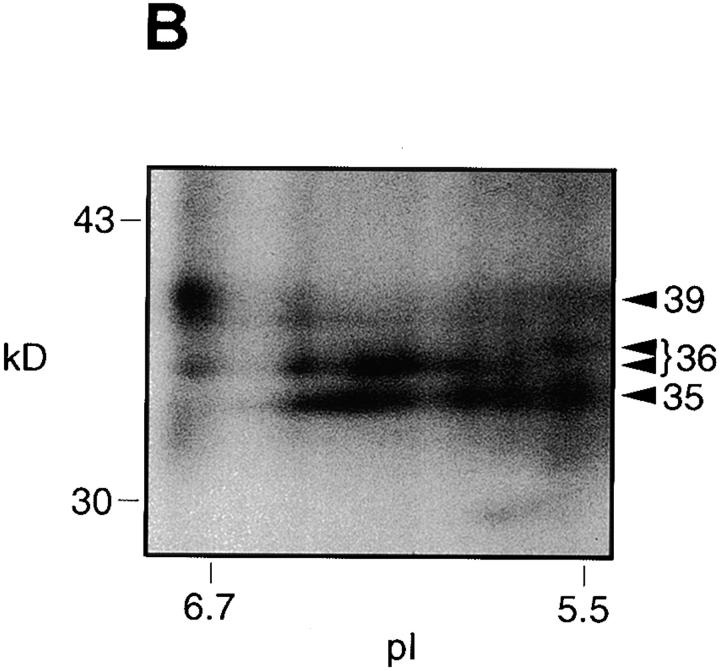
We noticed that some radiolabeled bands in the 30–45-kD cluster appeared characteristically dispersed (Fig. 2 B). This observation prompted us to investigate possible molecular heterogeneity not discerned in regular SDS-PAGE gels. A two-dimensional electrophoresis analysis of an SDS extract of FCR3S1.2 pRBCs (the one-dimensional SDS-PAGE pattern of this parasite is shown in Fig. 2 B) revealed the existence of charge and size microheterogeneity in the surface-radioiodinated polypeptides (Fig. 3a and Fig. b). The parasite-specific bands of 35 and 36 kD resolved into several molecular species or isoforms in the pI range of 5.5–6.5, displaying a charge composition distinct from the major proteins of the human erythrocyte surface, including the heavily glycosylated anion transporter Band 3 and the sialoglycoproteins, glycophorins A and B. Polyclonal sera to total erythrocytic antigens and mAbs to Band 3 and glycophorins A, B, and C were used in immunoprecipitation and Western blot for the identification of the normal RBC proteins undergoing radioiodination (results not shown). None of these antibodies recognized the parasite-specific surface-radioiodinatable antigens of FCR3S1.2 or any other strain/clone tested.
Figure 3.
Small antigens in the 30–45-kD cluster exhibit charge and size microheterogeneity. (A) FCR3S1.2 parasite cultures were surface radioiodinated, and the trophozoite-infected pRBCs were separated and processed as described in Materials and Methods. The isoelectric focusing of the Triton X-100/urea–reconstituted SDS extracts was carried out as described elsewhere 42. The focused polypeptides were size fractionated by SDS-PAGE in a 7.5–17.5% gradient gel and detected by PhosphorImagery. (B) Detail of the FCR3S1.2 clone–specific surface antigens. A set of pI markers (Bio-Rad Labs.) was used for the determination of the pH gradient after isoelectric focusing.
Small Surface Antigens in the 30–45-kD Cluster Are Identical to Predicted Proteins Encoded by the rif Family of P. falciparum Genes.
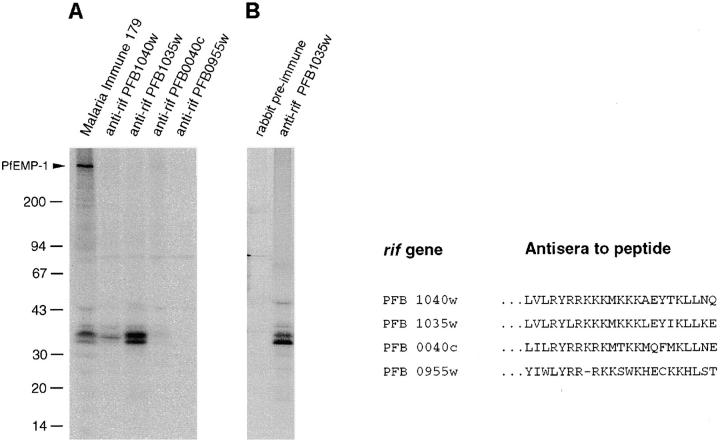
As part of the P. falciparum genome sequencing project, a number of intact transcriptional units sharing homology with the sequence originally called RIF (repetitive interspersed family; reference 23) were identified in the subtelomeric regions of chromosome 2 24. This large family of rif genes encoded potential transmembrane proteins of 30–40 kD termed rifins. We proceeded to investigate whether the variable 30–45-kD surface antigens characterized here were identical to predicted rif gene products. Rabbit antisera raised against the highly conserved 3′ end of the deduced amino acid sequence of rif genes PFB1040w, PFB1035w, PFB0040c, and PFB0955w were used to immunoprecipitate SDS extracts of surface- or metabolically labeled pRBCs of the FCR3S1.2 clone. Antibodies in three of the tested sera specifically recognized the small surface polypeptides of this parasite, in particular anti-rif PFB1035w, which immunoprecipitated bands of 35, 36 (doublet), and, faintly, 39 kD (Fig. 4). The results proved that the small variant antigens expressed on the pRBC surface are products of the rif loci.
Figure 4.
Recognition of the small surface antigens in the 30–45-kD cluster by antibodies to rif gene–predicted peptides. (A) Immunoprecipitation of [125I]Na surface-labeled SDS extracts of FCR3S1.2 pRBCs with either human immune sera (no. 179) or rabbit antisera raised to the 3′ conserved end domain of the indicated rif genes mapping to chromosome 2 of P. falciparum strain 3D7 24. (B) Immunoprecipitation of [35S]cysteine metabolically labeled SDS extracts of FCR3S1.2 pRBCs with rabbit serum anti-rif–predicted peptide.
Surface-expressed Rifins, Rosetting, and Other Binding Phenotypes.
We next investigated whether the expression of rifin proteins on the pRBC surface was associated with rosetting binding to uninfected erythrocytes. For this purpose, we analyzed surface radiolabeled pRBCs in R+ and R− parasites cloned by limiting dilution and subcloned by micromanipulation. The rosetting clone FCR3S1, derived from the strain FCR3S by limiting dilution, consistently showed a radioiodinated polypeptide of 35 kD. The highly rosetting, autoagglutinating, and cytoadherent subclone FCR3S1.2, obtained from FCR3S1 by micromanipulation, expressed radiolabeled bands of 31, 35, and 36 kD. In contrast, the nonrosetting, noncytoadherent sibling subclone FCR3S1.6 only exhibited the 39-kD band, a polypeptide always found on pRBCs infected with parasites of the FCR3S lineage (Fig. 2 B). These preliminary results were suggestive of an association between the rosetting capacity of the parasite and the expression of particular rifin variants on the host cell surface. Such an association was not observed when a similar clonal analysis was performed in another P. falciparum strain. Parasite clones with phenotype R+ derived from the rosetting strain TM284 (only TM284S2 is shown here) did not display radioiodinatable polypeptides of low molecular mass other than the 39 kD polypeptide, whereas nonrosetting clones (TM284S7, TM284S9, and TM284S19) showed multiple radioiodinated polypeptides in the range of 33–45 kD (Fig. 2 C). However, polypeptides outside the cluster of small variant antigens were associated with rosetting binding in TM284 parasites. A prominent surface-labeled band corresponding to a polypeptide of 170 kD and an additional 82-kD polypeptide were detected in TM284 and all of its R+ clones but were not or were very faintly detected in R− clones (Fig. 2 C). In FCR3S and TM284 parasites, changes in binding phenotype always concurred with changes in the expression of PfEMP1 polypeptides (data not shown).
Controlled proteolytic digestion of intact radioiodinated pRBCs was used to further explore possible associations between the expression of surface antigens and binding phenotypes. Upon incubation of surface-radioiodinated, trophozoite-infected erythrocytes with trypsin, complete deletion of the 35-kD rifin band of FCR3S1 occurred at concentrations of the protease of 100 μg/ml or higher (Fig. 5). The 39-kD polypeptide of this parasite showed a reduced sensitivity to the protease. In comparison, the 285-kD PfEMP1 polypeptide expressed by FCR3S1 was cleaved at concentrations of trypsin <1 μg/ml, in agreement with our and others' data on the high trypsin sensitivity of most PfEMP1 polypeptides. The proteolytic treatment left the bulk of Triton X-100– and SDS-soluble polypeptides unaltered, with the exception of the glycophorins. The sensitivity of the rosetting phenotype to trypsin digestion of intact pRBCs closely matched that of the PfEMP1 protein, but not the removal of the 35- or 39-kD rifins from the pRBC surface. Similarly, the trypsin sensitivity of other adhesive phenotypes of erythrocytes bearing FCR3S1 or its clone FCR3S1.2, such as autoagglutination of infected RBCs, binding to the blood group A trisaccharide, adherence to the CD36 receptor, and binding of normal human Igs paralleled the cleavage of PfEMP1 (data not shown). In contrast, an association was found between the deletion of the 35-kD rifin polypeptide and the abrogation of pRBC binding to PECAM1/CD31, expressed constitutively on HUVECs or L cells transfected with the corresponding gene (Fig. 5).
The lack of association between the expression of non-PfEMP1 antigens on the pRBC surface and adherent phenotypes of the parasite, with the exception of binding to PECAM1/CD31, was further evidenced when we panned the rosetting FCR3S and FCR3S1 parasites on C32 melanoma cells expressing the CD36 receptor. The resulting highly CD36-cytoadherent, nonrosetting parasite lines expressed different patterns of surface-radioiodinatable polypeptides, 36 and 39 kD in FCR3S/b and 39 kD in FCR3S1/b. Polypeptides of 39 kD were also detected in noncytoadherent, nonrosetting parasites (Fig. 2 B).
Immune Recognition of Variant Rifin Antigens.
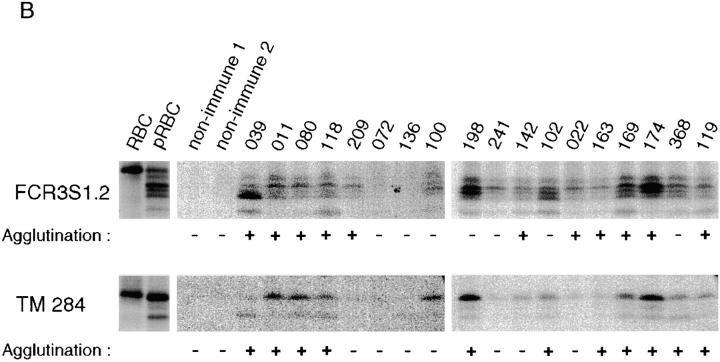
To assess the natural antigenicity of rifin polypeptides, we initially tested two human hyperimmune sera in immunoprecipitation of SDS extracts of surface 125I-labeled pRBCs of the FCR3S1.2 clone. Antibodies in these two sera recognized radioiodinated rifins of 35, 36 (doublet), and 39 kD in addition to PfEMP1 (Fig. 6 A). Rabbit antisera raised against GST fusion proteins comprising the semiconserved DBL1 and the highly conserved ATS domains of the var/PfEMP1 expressed by FCR3S1.2 immunoprecipitated PfEMP1 polypeptides but did not react with rif gene products. Having obtained the first evidence that rifin polypeptides were naturally immunogenic, we next carried out an expanded analysis of the antigenicity of these parasite products in natural infections. For this purpose, we used a panel of 18 sera from malaria-experienced individuals living in different geographical regions of Africa (Kenya, Liberia, and The Gambia) in assays of immunoprecipitation and agglutination of FCR3S1.2 and TM284 parasites (Fig. 6 B). Most of the sera immunoprecipitated one or more radioiodinated rifin polypeptides in the range of 31–39 kD, both in FCR3S1.2 and TM284 parasites. Qualitative variation was observed in the antigenic pattern recognized by different sera in each parasite, a result consistent with the extent of the rif gene repertoire and the switching in expressed rifin types. At the same time, sharing of epitopes between parasite-specific variant forms of rifins was suggested by the corresponding reactivities of a majority of the individual sera to the 36-kD doublet of the FCR3S1.2 parasite and the 39-kD band of the TM284 strain, a result anticipated if conservation at the 5′ and 3′ ends is a widespread feature of rif products. The major radioiodinated 170-kD polypeptide of TM284 was not immunoprecipitated by immune sera. The PfEMP1 polypeptides of the two parasites studied were recognized by all of the sera except 72, 100, and 136 (data not shown). The agglutination of trophozoite-bearing pRBCs by the immune sera was generally, but not strictly, correlated with their capacity to immunoprecipitate the small surface antigens.
Figure 6.
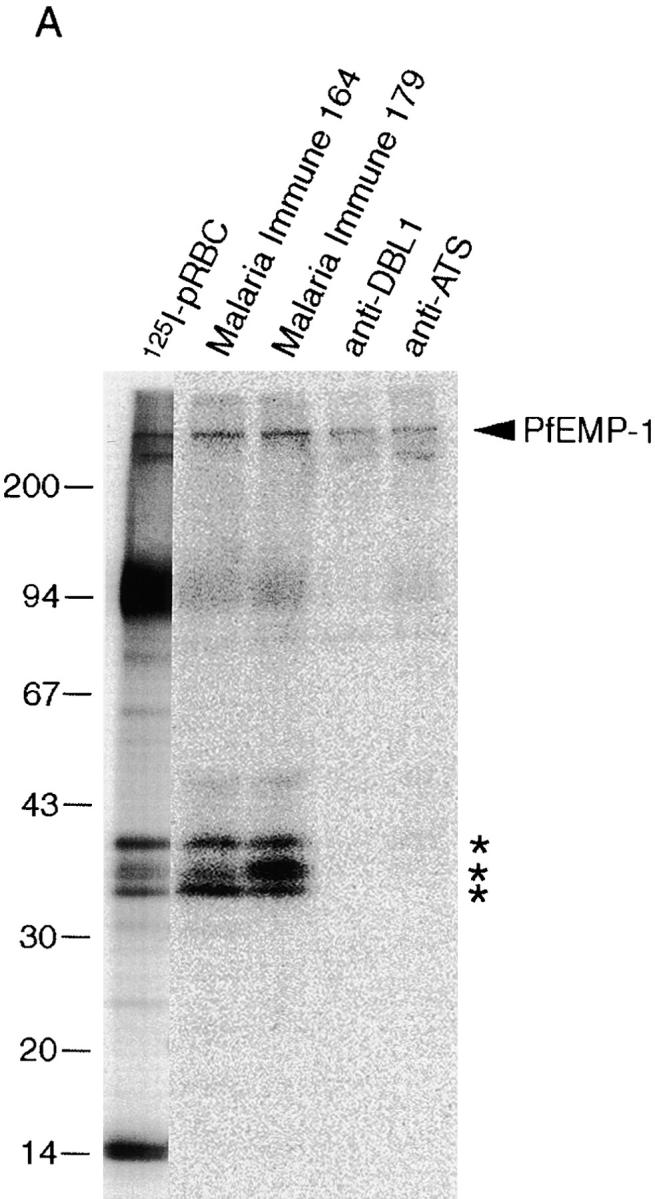
Natural antigenicity and variable immunorecognition of rifins. (A) SDS extracts of surface-labeled FCR3S1.2 pRBCs were immunoprecipitated with either human immune sera (nos. 164 and 179) or rabbit antisera specific to the semiconserved DBL1 and the conserved ATS domains of the var1 PfEMP1 protein of FCR3S1.2 15. Immunoprecipitation of surface-labeled antigens and assessment of agglutinating capacity by sera from individuals living in malaria-endemic areas of Kenya (nos. 39, 11, 80, 118, and 209), The Gambia (nos. 72, 136, and 100), and Liberia (nos. 198, 241, 142, 102, 22, 163, 169, 174, 368, and 119). The complete SDS-PAGE patterns of surface-radiolabeled proteins of parasites FCR3S1.2 and TM284 are shown in Fig. 2B and Fig. C, respectively.
To study the relative contribution of surface-exposed epitopes on rifin and PfEMP1 antigens to the agglutination reaction, we took advantage of the differential sensitivity that members of these two protein families display to controlled trypsin proteolysis of intact pRBCs. Rifin polypeptides of the clone FCR3S1 (35 and 39 kD) are at least 100-fold less sensitive to the protease than the radioiodinated PfEMP1 of this parasite, which is no longer detected after digestion with 1 μg/ml trypsin. Antibodies in immune sera agglutinated trophozoite-bearing pRBCs digested with 1, 10, or 100 μg/ml trypsin before incubation with antibody (Table ), suggesting the presence of epitopes in rifins targeted by surface-reactive, agglutinating antibodies raised during P. falciparum infections.
Discussion
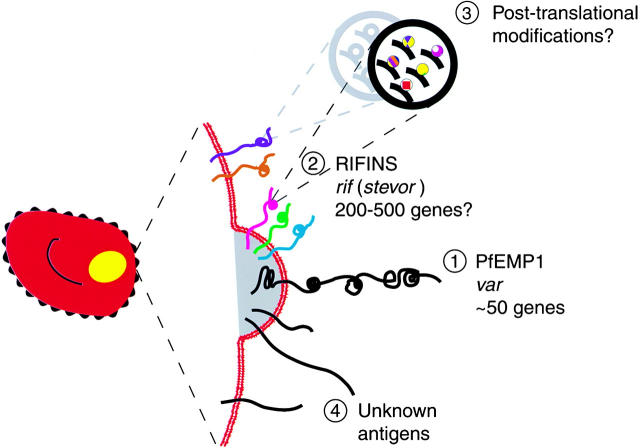
In this study, we show that the vast structural and antigenic variability created by P. falciparum on the surface of the host erythrocyte has its molecular basis not only in the PfEMP1 polypeptides, encoded by the var family of genes, but also in multiple additional parasite-derived products, including a prominent group of relatively small antigens encoded by the rif family of genes. These polypeptides are clonally variant and may undergo posttranslational modifications resulting in molecular microheterogeneity, a conceptually new source of antigenic diversity on the pRBC surface (Fig. 7).
Figure 7.
Antigenic diversity on the infected erythrocyte surface. Sources of the structural variation created by P. falciparum on its host cell can be: 1 the large variant antigen PfEMP1, encoded by the gene family var, with ∼50 genes per genome; 2 the small antigens rifins encoded by the multigene family rif, also termed stevor (200–500 genes) (the location of these products at knobs is not presently established); 3 modifications (not yet defined) of the translated polypeptide resulting in further layers of molecular microheterogeneity; or 4 additional parasite antigens transported to the pRBC surface.
Besides the variant adhesin PfEMP1, several undefined polypeptides of 20–55 kD have reportedly been detected on the surfaces of erythrocytes infected with mature stages of P. falciparum 16 17. In each case, the detection of these polypeptides was performed after lactoperoxidase-catalyzed iodination of intact infected erythrocytes, a procedure used for the radiolabeling of exofacially exposed proteins on cell plasma membranes 25. In our analysis of parasite-derived molecules exported to the host cell surface, with a focus on products different from var/PfEMP1, we have studied 23 clinical isolates, laboratory-adapted strains, and lines or clones selected for binding phenotypes, including rosetting and adhesion to the CD36 receptor. At least 12 polypeptides, ranging in size from 20 to 170 kD, were identified by mild radioiodination of intact trophozoite-bearing erythrocytes. The most common and prominent of these products, both in fresh clinical isolates and long-term–cultured parasites, were detected as a distinct group or cluster of bands in the range of 30–45 kD. The large family of rif genes and its purported subfamily stevor, encoding potential transmembrane proteins (rifins) with a predicted size of 30–40 kD, has been recently identified in P. falciparum 24 26. The rif genes have a two-exon structure, with the first exon coding for a predicted signal peptide and the second for polypeptides containing an extracellular domain with conserved cysteine residues and a highly variable region, a transmembrane segment, and a short cytoplasmic tail that is highly conserved. According to preliminary estimations, there may be 200–500 rif genes in the P. falciparum genome. At least some of the rif genes are transcribed in blood stages 24. Our finding that rabbit antibodies raised to the conserved basic COOH terminus of rifin-deduced amino acid sequences specifically immunoprecipitate surface- or metabolically labeled polypeptides of 30–45 kD indicates that these antigens are the products of the presumably most abundant gene family in P. falciparum.
A salient feature of rifin polypeptides is their variability, in size as well as in the number of distinct components expressed in different parasites. The capacity of P. falciparum to undergo clonal antigenic variation has been established 27 28, and the switching in the expression of different var genes, or their product PfEMP1, is correlated with clonal changes in surface antigenic determinants of the pRBC 3. Rifin antigens are clonally variant, as the analysis of clones and subclones of TM284 and FCR3S1.2 parasites demonstrate. These variable products, which are partially solubilized by neutral detergents (e.g., Triton X-100) but require treatment with 1–2% SDS, conditions that disrupt the host erythrocyte cytoskeleton for complete dissolution, are transported to the plasma membrane of the infected RBC and exposed on the surface according to several criteria. They are (a) readily and consistently labeled by the lactoperoxidase/Na125I/H2O2 method under conditions that fail to label hemoglobin and (b) cleaved by trypsin treatment of intact infected erythrocytes under conditions that do not cleave any other RBC or malarial proteins, except glycophorin polypeptides and the PfEMP1 antigen.
It is unknown how rif gene expression is regulated. The clusters with varying numbers of radiolabeled bands detected in freshly isolated as well as in recently cloned parasites may reflect, besides several other possible explanations, (a) the nonclonal composition of the parasites 29 30, (b) posttranscriptional processing of the product of a single gene, or (c) the simultaneous expression of several rif genes. Implicit in this last alternative would be a partial lack of allelic restrictive processes (e.g., allelic exclusion) controlling the expression of products from the rif loci. This is in contrast to the “one parasite–one gene” modality of surface protein expression in the var/PfEMP1 multigene family 31. The finding of microheterogeneity in the rifins could be explained by the concurrent expression of several rif genes encoding polypeptides of similar molecular mass. Alternatively, superimposed to the variation created at the pRBC surface by the display of proteins comprising semivariable and hypervariable regions, e.g., var/PfEMP1, additional layers of antigenic variation could be generated in asexual stages of P. falciparum by the addition of carbohydrates, phosphate groups, or perhaps unusual modifications of polypeptides targeted to the erythrocyte surface. Examples of posttranslational modifications modulating the surface architecture, and likely the survival/virulence of pathogenic microorganisms, are the glycosylation and glycerophosphorylation of antigenically variable pilins in the prokaryote Neisseria species 32 33 or the glycosylation and palmitoylation of the variant surface proteins of the eukaryotic parasite Giardia species 34. Analysis of the rif gene amino acid sequences available to date reveals numerous and relatively conserved potential sites for O- and N-glycosylation.
Allowing for the limited number of clinical isolates included in this study, it is interesting to note that rif gene expression is detected as prominent bands in each of the wild parasites examined but absent or faint in some of the strains or parasite lines that were long-term cultured in the laboratory. Chromosomal truncations, resulting in gene deletions as well as gametocytogenesis and cytoadherence phenotype losses, commonly occur in P. falciparum propagated mitotically in vitro 35 36 37 38. The function(s) of the variant rifin antigens being unknown, it is likely to be essential for survival of the parasite confronted with the host's environment and defence mechanisms but not indispensable for in vitro growth. The variant nature of the rifin antigens further suggests that presence at the pRBC surface is a compelling need.
Our data do not support a direct role of rif products in rosetting binding. An accessory function in conjunction with the rosetting ligand PfEMP1 14 15 cannot be, however, completely disregarded. We have previously shown that the trypsin sensitivity of other binding phenotypes of multiadhesive parasites, i.e., binding to blood group A, autoagglutination of infected erythrocytes, binding of normal Igs, and adhesion to the CD36 receptor correlate with the enzymatic digestion of PfEMP1 18. In contrast, here we show that cytoadherence to the endothelial receptor PECAM1/CD31 is a phenotype associated with the presence on the pRBC surface of at least one radioiodinatable polypeptide, the 35-kD rifin in FCR3S1, but apparently not to the radioiodinated domain(s) of the PfEMP1 in this parasite. It remains a possibility that a trypsin-resistant domain of PfEMP1 contains the CD31 binding specificity. The 35-kD rifin may have, in conjunction with PfEMP1 or not, either a direct or an accessory role in the binding of pRBCs to CD31 and other receptors. Although binding to PECAM1/CD31 is a feature of only some laboratory-adapted strains 39, the extent of this binding trait in natural P. falciparum populations and its relationship to severe forms of malaria remains to be investigated. The prominent labeling of a 170-kD polypeptide in some isolates and laboratory strains and its paucity in nonbinding clones has not escaped our attention. Its labeling pattern may indicate molecular abundance at the pRBC surface. In immunoprecipitation and Western blot assays, this band is neither recognized by antibodies to the highly conserved COOH-terminal segment of PfEMP1 nor by immune sera that immunoprecipitate PfEMP1 and components of the 30–45-kD rifin cluster from the same parasite. It remains to be determined whether the 170-kD polypeptide is too antigenically diverse to be detected by heterologous antibodies or is not naturally immunogenic. Regardless of the function of rifins as well as the origin and function of the other novel surface-exposed polypeptides described here, their diversity, stability of expression, and multiplicity of combinatorial patterns (19 distinct types in 23 different parasites) suggests a potential for use as an isolate typing tool, conceivably in assays where the expressed PfEMP1 variant is simultaneously assessed.
Antigens exposed on the pRBC surface undergo clonal variation at rates of up to 1–2% per generation 28. The variant adhesin PfEMP1, which mediates binding of infected erythrocytes to vascular endothelial cells and to uninfected erythrocytes, plays a major role in this antigenic variation 3 27 31. Adults living in areas of high malaria endemicity have antibodies in their sera capable of reacting with antigenic determinants of many P. falciparum isolates from distinct geographic regions 8 19. These antibodies recognize conserved and polymorphic parasite antigens, including PfEMP1 9 40. Our data indicate that rifin proteins are located on the surface of the pRBC and, moreover, that these parasite-derived components are antigenic in the course of a P. falciparum infection, eliciting a substantial humoral immune response. Furthermore, we show here that human immune sera agglutinate infected erythrocytes under conditions such that most, if not all, PfEMP1 products have been removed from the pRBC surface. The presence in sera of agglutinating or opsonizing antibodies reactive with the infected erythrocyte surface has been shown to correlate with protection from lethal disease 8 41. This preliminary data opens a new avenue for studies conducive to the eventual elucidation of targets for immune protection against P. falciparum malaria. The relative contribution of the rifins, PfEMP1, and other surface antigens on the pRBC to the triggering of the antibody response, Ig classes, and immunity buildup have yet to be determined.
This represents the first report of natural immune responses to surface molecules of intraerythrocytic P. falciparum distinct from PfEMP1. The results indicate that more than one family of genes encoding variable surface-targeted proteins, perhaps with additional epigenetic generation of submolecular variation, accounts for the remarkable diversity generated by the parasite in its host cell.
Acknowledgments
We thank K. Berzins and A. Barragan for the gift of sera. We are also indebted to A. Barragan, M. Schlichtherle, and S. Svärd for helpful discussions and critical reading of the manuscript.
These studies were supported by grants from the United Nations Developing Program/World Bank/World Health Organization Special Program for Research and Training in Tropical Diseases (TDR) and the Swedish Medical Research Council. V. Fernandez was supported in part by the Karolinska Institutet and the Swedish Society for Medical Research.
Footnotes
1used in this paper: CHO, chinese hamster ovary; HUVECs, human umbilical vein endothelial cells; PECAM1, platelet/endothelial cell adhesion molecule 1; PfEMP1, P. falciparum erythrocyte membrane protein 1
References
- Miller L.H., Good M.F., Milon G. Malaria pathogenesis. Science. 1994;264:1878–1883. doi: 10.1126/science.8009217. [DOI] [PubMed] [Google Scholar]
- Baruch D.I., Pasloske B.L., Singh H.B., Bi X., Ma X.C., Feldman M., Taraschi T.F., Howard R.J. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- Smith J.D., Chitnis C.E., Craig A.G., Roberts D.J., Hudson-Taylor D.E., Peterson D.S., Pinches R., Newbold C.I., Miller L.H. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., Heatwole V.M., Werttheimer S.P., Guinet F., Herrfeldt J.A., Peterson D.S., Ravetch J.A., Wellems T.E. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Howard R.J. Malarial proteins at the membrane of Plasmodium falciparum-infected erythrocytes and their involvement in cytoadherence to endothelial cells. Prog. Allergy. 1988;41:98–147. doi: 10.1159/000415221. [DOI] [PubMed] [Google Scholar]
- Forsyth K.P., Philip G., Smith T., Kum E., Southwell B., Brown G.V. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparum parasites in Papua New Guinea. Am. J. Trop. Med. Hyg. 1989;41:259–265. [PubMed] [Google Scholar]
- Iqbal J., Perlmann P., Berzins K. Serological diversity of antigens expressed on the surface of erythrocytes infected with Plasmodium falciparum . Trans. R. Soc. Trop. Med. Hyg. 1993;87:583–588. doi: 10.1016/0035-9203(93)90097-a. [DOI] [PubMed] [Google Scholar]
- Barragan A., Kremsner P.G., Weiss W., Wahlgren M., Carlson J. Age-related buildup of humoral immunity against epitopes for rosette formation and agglutination in african areas of malaria endemicity. Infect. Immun. 1998;66:4783–4787. doi: 10.1128/iai.66.10.4783-4787.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K., Howard R.J. Antigens induced on erythrocytes by P. falciparumexpression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- Newbold C.I., Pinches R., Roberts D.J., Marsh K. Plasmodium falciparumthe human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp. Parasitol. 1992;75:281–292. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- Bull P.C., Lowe B.S., Kortok M., Molyneux C.S., Newbold C.I., Marsh K. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat. Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull P.C., Lowe B.S., Kortok M., Marsh K. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenyaevidence for rare and prevalent variants. Infect. Immun. 1999;67:733–739. doi: 10.1128/iai.67.2.733-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch D.I., Gormley J.A., Ma C., Howard R.J., Pasloske B.L. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe A., Moulds J.M., Newbold C.I., Miller L.H. P. falciparum rosetting mediated by a parasite-variant erythrocyte membrane protein and complement-receptor 1. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- Chen Q., Barragan A., Fernandez V., Sundström A., Schlichtherle M., Sahlen A., Carlson J., Datta S., Wahlgren M. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum . J. Exp. Med. 1998;187:15–23. doi: 10.1084/jem.187.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmby H., Cavelier L., Petersson U., Wahlgren M. Rosetting Plasmodium falciparum-infected erythrocytes express unique strain-specific antigens on their surface. Infect. Immun. 1993;61:284–288. doi: 10.1128/iai.61.1.284-288.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley H.A., Reese R.T. Plasmodium falciparum polypeptides associated with the infected erythrocyte plasma membrane. Proc. Natl. Acad. Sci. USA. 1986;83:6093–6097. doi: 10.1073/pnas.83.16.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez V., Treutiger C.J., Nash G.B., Wahlgren M. Multiple adhesive phenotypes linked to rosetting-binding of erythrocytes in Plasmodium falciparum malaria. Infect. Immun. 1998;66:2969–2975. doi: 10.1128/iai.66.6.2969-2975.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguiar J.C., Albrecht G.R., Cegielski P., Greenwood B.M., Jensen J.B., Lallinger G., Martinez A., McGregor I.A., Minjas J.N., Neequaye J. Agglutination of Plasmodium falciparum-infected erythrocytes from east and west African isolates by human sera from distant geographic regions. Am. J. Trop. Med. Hyg. 1992;47:621–632. doi: 10.4269/ajtmh.1992.47.621. [DOI] [PubMed] [Google Scholar]
- Carlson J., Holmquist G., Taylor D.W., Perlmann P., Wahlgren M. Antibodies to a histidine-rich protein (PfHRP1) disrupt spontaneously formed Plasmodium falciparum erythrocyte rosettes. Proc. Natl. Acad. Sci. USA. 1990;87:2511–2515. doi: 10.1073/pnas.87.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magowan C., Wollish W., Anderson L., Leech J. Cytoadherence by Plasmodium falciparum–infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J. Exp. Med. 1988;168:1307–1320. doi: 10.1084/jem.168.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholander C., Treutiger C.J., Hultenby K., Wahlgren M. Novel fibrillar structure confers adhesive property to malaria-infected erythrocytes. Nat. Med. 1996;2:204–208. doi: 10.1038/nm0296-204. [DOI] [PubMed] [Google Scholar]
- Weber J.L. Interspersed repetitive DNA from Plasmodium falciparum . Mol. Biochem. Parasitol. 1988;29:117–124. doi: 10.1016/0166-6851(88)90066-7. [DOI] [PubMed] [Google Scholar]
- Gardner M.J., Tettelin H., Carucci D.J., Cummings L.M., Aravind L., Koonin E.V., Shallom S., Mason T., Yu K., Fujii C. Chromosome 2 sequence of the human malaria parasite Plasmodium falciparum . Science. 1998;282:1126–1132. doi: 10.1126/science.282.5391.1126. [DOI] [PubMed] [Google Scholar]
- Howard R.J., Barnwell J.W., Kao V., Daniel W.A., Aley S.B. Radioiodination of new protein antigens on the surface of Plasmodium knowlesi schizont-infected erythrocytes. Mol. Biochem. Parasitol. 1982;6:343–367. doi: 10.1016/0166-6851(82)90024-x. [DOI] [PubMed] [Google Scholar]
- Cheng Q., Cloonan N., Fischer K., Thompson J., Wayne G., Lanzer M., Saul A. stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol. Biochem. Parasitol. 1998;97:161–176. doi: 10.1016/s0166-6851(98)00144-3. [DOI] [PubMed] [Google Scholar]
- Biggs B.-A., Gooze L., Wycherley K., Wollish W., Southwell B., Leech J.H., Brown G.V. Antigenic variation in Plasmodium falciparum . Proc. Natl. Acad. Sci. USA. 1991;88:9171–9174. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D.J., Craig A.G., Berendt A.R., Pinches R., Nash G., Marsh K., Newbold C.I. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaithong S., Beale G.H., Fenton B., McBride J., Rosario V., Walker A., Walliker D. Clonal diversity in a single isolate of the malaria parasite Plasmodium falciparum . Trans. R. Soc. Trop. Med. Hyg. 1984;78:242–245. doi: 10.1016/0035-9203(84)90287-6. [DOI] [PubMed] [Google Scholar]
- Conway D.J., Greenwood B.M., McBride J.S. The epidemiology of multiple-clone Plasmodium falciparum infections in Gambian patients. Parasitology. 1991;103:1–6. doi: 10.1017/s0031182000059217. [DOI] [PubMed] [Google Scholar]
- Chen Q., Fernandez V., Sundström A., Schlichtherle M., Data S., Hagblom P., Wahlgren M. Developmental selection of var gene expression in Plasmodium falciparum . Nature. 1998;394:392–395. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- Stimson E., Virji M., Makepeace K., Dell A., Morris H.R., Payne G., Saunders J.R., Jennings M.P., Barker S., Panico M. Meningococcal pilina protein substituted with digalactosyl 2,4-diacetoamido-2,4,6-trideoxyhexose. Mol. Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- Stimson E., Virji M., Barker S., Panico M., Blench I., Saunders J.R., Payne G., Moxon E.R., Dell A., Morris H.R. Discovery of a novel protein modificationalpha-glycerophosphate is a substitute of meningococcal pilin. Biochem. J. 1996;316:29–33. doi: 10.1042/bj3160029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanastasiou P., McConville M.J., Ralton J., Kohler P. The variant-specific surface protein of Giardia, VSP4A1, is a glycosylated and palmitoylated protein. Biochem. J. 1997;322:49–56. doi: 10.1042/bj3220049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langreth S.G., Peterson E. Pathogenicity, stability, and immunogenicity of a knobless clone of Plasmodium falciparum in Colombian owl monkeys. Infect. Immun. 1985;47:760–766. doi: 10.1128/iai.47.3.760-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley M.W., Biggs B.-A., Forsyth K.P., Brown H.J., Thompson J.K., Brown G.V., Kemp D.J. Chromosome 9 from independent clones and isolates of Plasmodium falciparum undergoes subtelomeric deletions with similar breakpoints in vitro. Mol. Biochem. Parasitol. 1990;40:137–145. doi: 10.1016/0166-6851(90)90087-3. [DOI] [PubMed] [Google Scholar]
- Scherf A., Carter R., Petersen C., Alano P., Nelson R., Aikawa M., Mattei D., Pereira da Silva L., Leech J. Gene inactivation of Pf11-1 of Plasmodium falciparum by chromosome breakage and healingidentification of a gametocyte-specific protein with a potential role in gametocytogenesis. EMBO (Eur. Mol. Biol. Organ.) J. 1992;11:2293–2301. doi: 10.1002/j.1460-2075.1992.tb05288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day K.P., Karamalis F., Thompson J., Barnes D.A., Peterson C., Brown H., Brown G.V., Kemp D.J. Genes necessary for expression of a virulence determinant and for transmission of Plasmodium falciparum are located on a 0.3-megabase region of chromosome 9. Proc. Natl. Acad. Sci. USA. 1993;90:8292–8296. doi: 10.1073/pnas.90.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treutiger C.J., Heddini A., Fernandez V., Muller W.A., Wahlgren M. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat. Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- van Schravendijk M.R., Rock E.P., Marsh K., Ito Y., Aikawa M., Neequaye J., Ofori-Adjei D., Rodriguez R., Patarroyo M.E., Howard R.J. Characterization and localization of Plasmodium falciparum surface antigens on infected erythrocytes from west African patients. Blood. 1991;78:226–236. [PubMed] [Google Scholar]
- Marsh K., Otoo L., Hayes R.J., Carson D.C., Greenwood B.M. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans. R. Soc. Trop. Med. Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P.H. High resolution two dimensional electrophoresis of proteins. J. Biol. Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]