Abstract
We have established a method for real-time video analysis of the interaction of antigen-presenting cells (APCs) with T cells. Green fluorescent protein expression controlled by a nuclear factor of activated T cells (NFAT)-responsive promoter permits the visualization of productive antigen presentation in single T cells. The readout is rapid (within 2 h) and semiquantitative and allows analysis by video microscopy and flow cytometry. Using this approach, we demonstrate that macrophages have the capacity to simultaneously activate multiple T cells. In addition, the interaction of T cells with macrophages is extraordinarily dynamic: after initial stable contact, the T cells migrate continuously on the surface of the macrophage and from APC to APC during productive antigen presentation. Thus, T cells sum up signals from multiple interactions with macrophages during stimulation.
Keywords: nuclear factor of activated, T cells, hybridoma, green fluorescent protein, video microscopy
Macrophages comprise an important limb of the innate immune system; the phagocytes recognize infectious agents and kill them in phagocytic vacuoles 1. They then activate the adaptive immune system by degrading the organisms and presenting the pathogen-derived MHC class II–bound peptides to T cells 2. Recent observations suggest a central role for the actin cytoskeleton in mediating both the physical interaction of the APC with the T cell, and the subsequent signaling pathways leading to T cell activation 3. An appreciation of the cross-talk between the TCR and the cytoskeleton has given insight into how the TCR can recognize an MHC–peptide complex when the affinity of the TCR for the antigen is very low and the number of ligands is limited 4 5 6.
Initial contact is established between adhesion molecules like LFA-1 on the T cell and intracellular adhesion molecule 1 (ICAM-1) on the APC 7. These molecules then mediate a large, low affinity adhesion zone between the cells. Concomitantly, CD2 on the T cell binds its cognate ligand on the APC, leading to the establishment of a specialized membrane zone that is enriched in TCRs and MHC–peptide complexes 3 8 9. The high local densities of these molecules allow TCRs to sample a vast number of MHC–peptide complexes. Upon engagement of TCRs with cognate antigen complexes and the initiation of active TCR signaling, LFA-1 is converted from a low affinity state to a high affinity state, thereby stabilizing the cell–cell interaction 10. The stability of this interaction facilitates the stable establishment of a signaling zone that has been referred to both as a supramolecular activation cluster (SMAC) and as an “immunological synapse.” In this zone, T cell and APC membranes are in tight (15 nm) apposition, and T cell molecules such as TCR/CD3, CD4, and CD28 are clustered. In addition, this organized signaling zone recruits molecules important for TCR signaling such as Lck, Fyn, Zap70, and protein kinase C (PKC)-θ 3 7 11 12. Recent evidence suggests that cooperative signaling through the TCR and costimulatory CD28 triggers an actomyosin-based translocation of cholesterol-rich membrane lipid rafts, which carry signaling molecules to the contact zone 9. TCR stimulation also triggers changes in cell polarity and movement, both of which appear to have a role in T cell activation 13. Changes in T cell motility also have a role in T cell activation; once the cells engage both ICAM-1 and the MHC–peptide complex, they receive a stop signal that prevents further movement and facilitates the establishment of the organized signaling zone 14.
Although dendritic cells, macrophages, and B cells are all capable of MHC class II–dependent antigen presentation, most work on the morphological and cytoskeletal changes associated with APC–T cell interactions has been carried out using B cells. We have established a system that permits examination in real time of APC–T cell interactions during MHC class II antigen presentation. A T cell hybridoma, DO11.10, was stably transfected with a construct in which green fluorescent protein (GFP) expression is under the control of a nuclear factor of activated T cells (NFAT)-regulated promoter 15 16. Thus, upon productive interaction with an APC, the T cell becomes fluorescent. Our data demonstrate that macrophages can simultaneously activate a large number of T cells, and that the interaction of the T cells with the macrophages is extraordinarily dynamic. Although T cell–macrophage interactions need to be sustained for ∼2 h for productive activation, the T cells are seen to move from one area of the macrophage to another, as well as from one APC to the next. However, although the T cells move around, they do stop long enough to permit the recruitment of the requisite cytoskeletal elements and signaling molecules to the site of cell–cell contact. These data indicate that T cells can be stimulated by summing up signals received during multiple sequential interactions with an antigen-presenting macrophage.
Materials and Methods
Materials.
OVA peptide 323–339 was synthesized by Bio-Synthesis. IFN-γ was obtained from Genzyme Corp. All other reagents were obtained from Sigma Chemical Co. except where indicated.
Cell Culture.
The I-Ad–restricted OVA-specific murine T cell hybridoma DO11.10, the I-Ab–restricted Eα-specific hybridoma 1H3.1, the H-2b murine macrophage line BM12 (provided by Dr. K. Rock, University of Massachusetts Medical School, Worcester, MA), and the H-2d murine macrophage line RAW 264.7 (no. TIB-71; American Type Culture Collection) were grown in RPMI containing 10% fetal bovine serum, 1% l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained at 37°C in a humidified incubator with a 5% CO2 atmosphere.
Plasmid Construction.
The vector pNFATeGFP contains the Neor marker of pcDNA3 and drives enhanced GFP (eGFP) expression under control of an NFAT-induced promoter. The CMV promoter of pcDNA3 (Invitrogen) was replaced with the 0.2-kb ClaI-HindIII fragment of NFATZH (17; provided by Steve Fiering, Fred Hutchinson Cancer Research Center, Seattle, WA) containing a promoter consisting of three copies of the NFAT binding site linked to the IL-2 promoter (−72 to +47 of IL-2). The 0.7-kb NcoI-NotI fragment of peGFP (Clontech) containing the coding region for eGFP was then cloned downstream of the NFAT-induced promoter.
Transfection.
3 × 106 DO11.10 cells were placed in 0.5 ml sterile PBS in a 0.4-cm gap cuvette (Bio-Rad) together with 20 μg DNA. The sample was electroporated with a Gene Pulser (Bio-Rad) set at 250 μF and 300 V. The cells were grown for 24 h before adding G418 (GIBCO BRL) to 1 mg/ml final concentration. After 10 d selection, the surviving cells were cloned by limiting dilution. Clones were then screened for OVA-specific induction of GFP by APCs. One responding clone, 5.6, was designated DO11-GFP and used for the experiments reported here.
Antigen Presentation Assay.
For presentation by RAW 264.7 cells, the cells were plated with 20 U/ml IFN-γ at 3 × 104/well in 96-well plates or 2 × 105/well in 24-well plates and incubated 48 h before use. The cells were antigen loaded overnight with OVA added from a 100 mg/ml sterile stock, or for 2 h with OVA 323–339 peptide. The cells were then rinsed once with medium, DO11-GFP cells were added at 8 × 104/well in 96-well plates or 2 × 105 in 24-well plates, the plates were centrifuged at 450 g to initiate contact between the cells, and the cells were incubated together at 37°C in a CO2 incubator for the indicated times. Loose cells were transferred by vigorous pipetting into a fresh tube, and adherent cells were released from the dish with PBS containing 1 mM EDTA and added to the loose cells. The cells were analyzed by flow cytometry using a FACScan™ and CELLQuest™ software (Becton Dickinson). To rapidly identify the different cell populations so as to gate on just the APCs or on the T cells, RAW 264.7 cells were prelabeled at the beginning of the experiment with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) or the DO11-GFP cells were prelabeled with CellTracker™ Orange (CTO; Molecular Probes) as follows. For DiI labeling, RAW 264.7 cells were collected by centrifugation, washed one time with labeling buffer (300 mM glucose, 10 mM Hepes, pH 7.1), resuspended in labeling buffer containing 1 μg/ml DiI, and incubated for 15 min at 37°C. The cells were then washed twice with labeling buffer and once with medium before use. For CTO labeling, DO11 cells were collected by centrifugation and resuspended in medium containing 500 nM CTO, incubated 15 min at 37°C, washed twice with medium, and incubated for 30 min at 37°C before use. Separate experiments determined that neither of these treatments had any measurable effect on the data presented.
Time-Lapse Video Microscopy.
RAW 264.7 cells were plated 48 h before use on 35-mm glass-bottomed microwell dishes (MatTek Corp.) with 20 U/ml IFN-γ, and OVA was added to 10 mg/ml for the final 16 h. DO11-GFP cells were added to the dish at fivefold excess, centrifuged onto the RAW 264.7 cells to initiate contact, and the dish was immediately mounted on a Axiophot microscope (Carl Zeiss, Inc.) equipped with a cooled CCD camera (Princeton Instruments) and Metamorph™ digital imaging software system for image collection (Universal Imaging). Differential interference contrast (DIC) images were collected at 2-min intervals for the periods indicated in the text, and GFP images were collected before the first frame and after the last frame of the series.
Results
Characterization of Antigen-specific Induction of GFP in DO11 Cells.
To visualize antigen-specific interactions of macrophages with T cells by continuous video microscopy, we transfected T cells with a reporter construct that causes them to express GFP upon productive engagement of an APC. In this construct, GFP expression is regulated by a promoter, comprising three copies of the NFAT binding site linked to a minimal IL-2 promoter, that has been demonstrated to faithfully report NFAT activity in T cells when driving chloramphenicol acetyltransferase (CAT) or LacZ expression 15 16 17. We transfected the OVA-specific T cell hybridoma DO11.10 with this vector, selected stable clones, and found that >60% (12/19) of the clones showed GFP expression upon NFAT activation. In this paper, we report our results with a clone designated DO11-GFP. Identical results were obtained with additional clones.
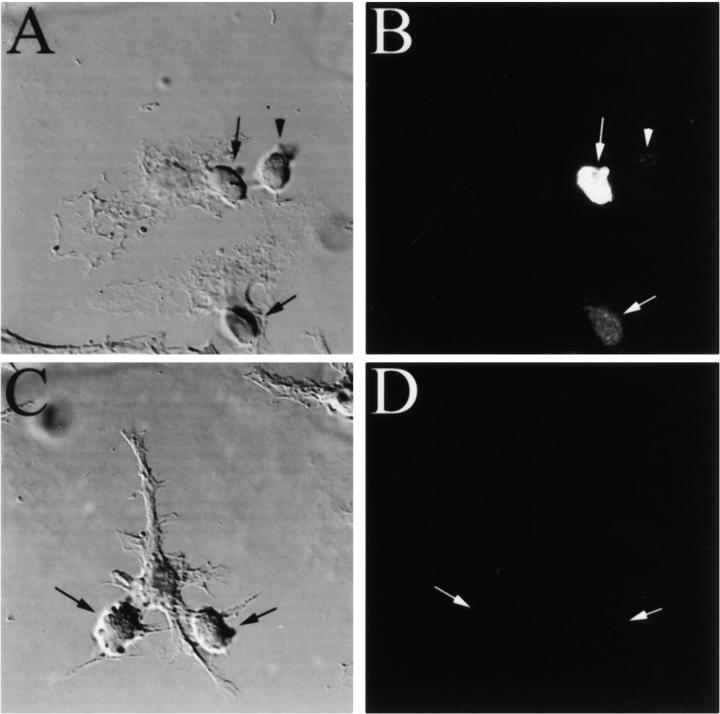
The mouse macrophage cell line RAW 264.7 was stimulated with IFN-γ to upregulate MHC class II expression and incubated overnight with OVA. When DO11-GFP cells were allowed to interact for 6 h with these macrophages, cells in direct contact with the macrophages expressed GFP (Fig. 1A and Fig. B, arrows), whereas those physically separated from the APC did not (Fig. 1A and Fig. B, arrowhead). By contrast, if the IFN-γ–stimulated RAW 264.7 cells were not first incubated with OVA, the DO11-GFP cells expressed no GFP, even when contacting the APC (Fig. 1C and Fig. D, arrows). Examination of >100 such pairs confirmed that cell–cell contact was required for T cell stimulation as reported by GFP expression; few GFP-positive DO11-GFP cells were observed not in contact with a macrophage.
Figure 1.
Productive antigen-specific interaction induces GFP expression in a T cell hybridoma. IFN-γ–stimulated RAW 264.7 cells were incubated for 2 h with (A and B) or without (C and D) 100 μg/ml of the OVA 323–339 peptide, and allowed to interact with a fivefold excess of DO11-GFP cells for 6 h. In the presence of peptide, T cells in contact with macrophages express GFP (B, arrows), whereas neighboring T cells not in direct contact with macrophages do not (B, arrowhead). In the absence of peptide, the DO11-GFP cells are not stimulated to express GFP (D, arrows) despite their adhesion to macrophages.
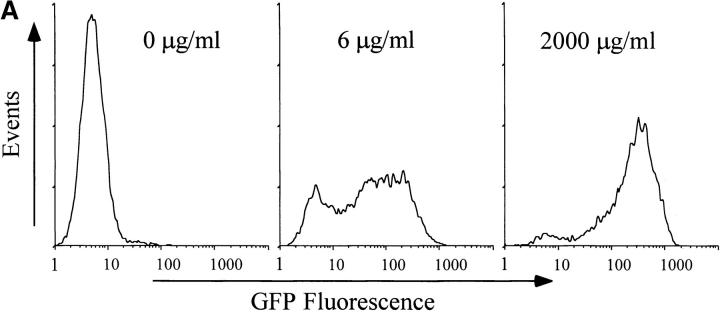
We next examined the antigen concentration dependence of the response of DO11-GFP cells to RAW 264.7 cells. IFN-γ–stimulated RAW 264.7 cells were loaded with OVA 323–339 peptide at the indicated concentrations for 2 h before DO11-GFP cells were added. After 6 h of interaction, the cells were harvested and analyzed by flow cytometry for GFP expression. As the peptide dose increased, both the number of T cells expressing GFP and the relative fluorescence intensity of the GFP-positive cells increased (Fig. 2 A). At intermediate antigen concentrations, a bimodal distribution of cells was observed, with some cells responding to stimulation while others did not respond. At higher antigen concentrations, the cells became more uniformly GFP-positive. Translated into a dose–response curve (Fig. 2 B), the data show that the stimulation is dose dependent and saturable. Interestingly, the antigen dose that resulted in the maximum number of cells becoming GFP-positive was an order of magnitude lower than the dose required to stimulate maximal GFP expression. This suggests that even under conditions when all of the DO11-GFP cells were productively interacting with antigen-presenting macrophages, more antigen still resulted in a stronger NFAT-mediated response in the hybridoma. This result was also obtained if OVA was used as the antigen instead of synthetic peptide, or if primary peritoneal macrophages were used as APCs (data not shown). Similar data were obtained by transfecting the NFAT-GFP reporter construct into the T cell hybridoma 1H3.1 18, which recognizes a peptide derived from the I-E α chain, and then stimulating the cells with the I-Ab macrophage cell line BM12 or by primary macrophages (data not shown). Immunoblot analysis demonstrated that GFP fluorescence accurately reflected the level of GFP expression in the T cell hybridoma (data not shown). These data are at variance with previous observations using the same promoter driving a β-galactosidase reporter, which showed that T cell activation occurs by a binary mechanism in which the cells are either on or off 19 20. A possible explanation for this discrepancy might be the differences in stability of GFP (long-lived) and β-galactosidase (short-lived).
Figure 2.
Analysis of antigen-induced GFP expression by flow cytometry. (A) RAW 264.7 cells were labeled with the fluorescent lipid DiI, stimulated for 48 h with IFN-γ, and loaded for 2 h with the indicated concentration of OVA 323–339 peptide. DO11-GFP cells were added at a 1:1 ratio, and the cells were allowed to interact for 6 h. GFP expression in the DO11-GFP cells was analyzed by flow cytometry. (B) IFN-γ–stimulated RAW 264.7 cells were preloaded for 2 h with the indicated dose of antigenic peptide, mixed 1:1 with CTO-labeled DO11-GFP cells, and allowed to interact for 6 h. The cells were harvested, and GFP expression in the T cell hybridoma was analyzed by flow cytometry. The percentage of T cells expressing GFP increased as a function of peptide concentration (○), as did the average fluorescence of the GFP-positive cells (□).
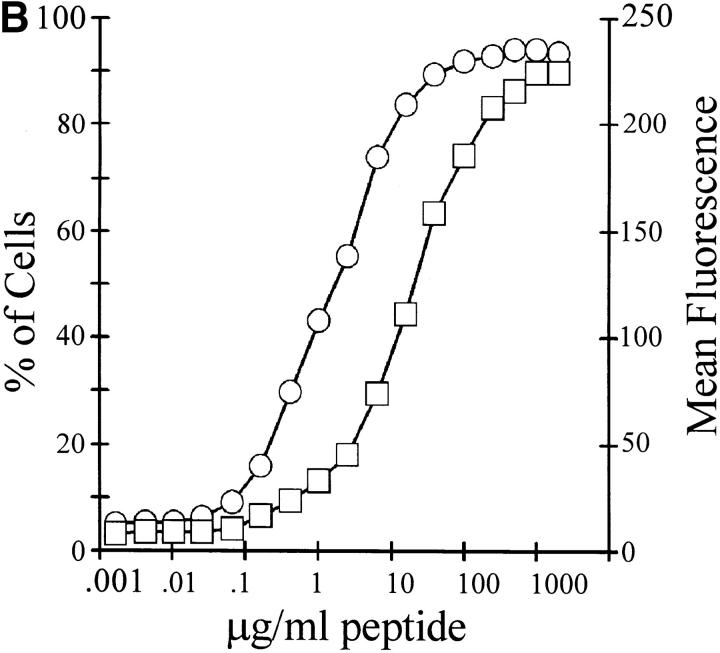
We next examined the temporal induction of GFP expression during antigen presentation. IFN-γ–stimulated RAW 264.7 cells were loaded with the indicated dose of OVA, and DO11-GFP cells were allowed to interact with them for the indicated times. The T cells were subsequently harvested, and the average fluorescence of the GFP-positive cells was measured (Fig. 3). GFP expression was detectable after 2 h and leveled off after ∼12 h. The rate and extent of GFP accumulation differed for different doses of OVA; higher doses of antigen resulted in more rapid accumulation of GFP to higher final levels in individual stimulated T cells.
Figure 3.
Kinetics of antigen-dependent stimulation of GFP expression in DO11-GFP cells. IFN-γ–stimulated RAW 264.7 cells were loaded overnight with OVA at a final concentration of 25 mg/ml (○), 2 mg/ml (□), or 0.5 mg/ml (▵). Control cells were also IFN-γ stimulated but were not incubated with antigen (▪). CTO-labeled DO11-GFP cells were added at a 1:1 ratio and allowed to interact with the RAW 264.7 cells for the indicated periods. The cells were harvested, and GFP expression in the T cell hybridoma was analyzed by flow cytometry.
Productive Macrophage–T Cell Interactions Are Dynamic.
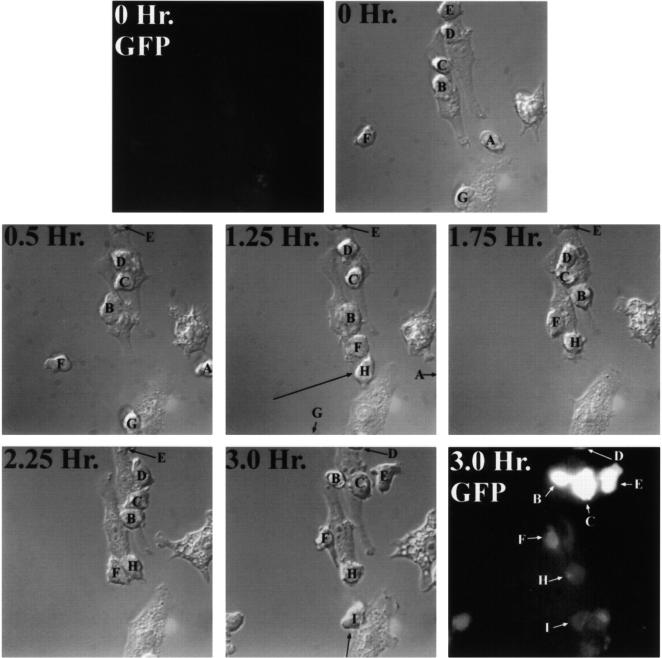
The rapid, antigen-specific expression of GFP in DO11-GFP cells allowed real-time visualization of productive APC–T cell interactions by video microscopy: we observed that macrophage–DO11-GFP cell interaction is dynamic. IFN-γ–stimulated RAW 264.7 cells were preloaded overnight with OVA, DO11-GFP cells were added, and the cells were mounted on a temperature-controlled microscope stage and observed for 3 h. At the beginning of the experiment, no cells were fluorescent, as shown in Fig. 4. After 3 h, many T cells in the field were expressing GFP.
Figure 4.
Time-lapse imaging of macrophage–T cell interactions. IFN-γ–stimulated RAW 264.7 cells were loaded overnight with 10 mg/ml OVA. DO11-GFP cells were added at a 5:1 ratio to macrophages, the cells were mounted on a temperature-controlled microscope stage, and cell–cell interactions were continuously observed by video microscopy for 3 h. The panels show selected video images collected at the indicated time intervals after addition of DO11-GFP cells. At the beginning of the experiment (0 Hr.), no cells expressed GFP (top left). After 3 h, many T cells in the field were expressing GFP (bottom right). Individual T cells are labeled alphabetically.
Fig. 4 illustrates several points about the dynamic interaction between the RAW 264.7 and DO11-GFP cells. First, T cells that were bound to macrophages for the entire 3 h (cells B, C, and E) expressed more GFP than cells that bound to macrophages later in the experiment (cells F, H, and I), consistent with the dose and time dependence illustrated above. Cells A, D, and G moved out of the field of vision during the experiment and could not be followed. Generally, 1.5–2 h of interaction was required for detection of GFP. In separate experiments using populations of cells analyzed by flow cytometry, we confirmed that separating macrophages from T cells within 1.5 h of initiating the interaction prevented the induction of GFP in T cells (data not shown). Second, when rapidly moving T cells encountered antigen-presenting macrophages, the T cells became attached to the surface of the macrophage, but not restricted to one region of the presenting cell. For example, cell F moved rapidly between 0.5 and 1.25 h across the field of view and attached to a macrophage. It then wandered up and down one side of the macrophage, being activated to the same extent as cells that interacted with the macrophage for the same amount of time (cell H). Analysis of a large number of T cells (n = 50) demonstrated that 90% of them moved around on the surface of macrophages during antigen presentation. Third, macrophages are clearly capable of interacting simultaneously with multiple T cells. Cells B, C, D, and E all bound to the macrophage in the top center of the field at some point in the series, whereas cells B, C, F, and H all interacted with the macrophage in the center of the field. In other experiments, as many as 10–12 T cells could be observed interacting simultaneously with a single macrophage (data not shown). Fourth, nearly 50% of T cells (n = 50) that were bound to one macrophage migrated to neighboring macrophages during the 3-h observation period. Thus, cells B and C began the series on one macrophage and ended on another. The fact that GFP expression was very high in cells B and C after 3 h indicated that the interactions with both macrophages were productive: cell C spent 0.5 h on the first macrophage and 2.5 h on the second macrophage, whereas cell B spent 1.25 h on the first macrophage and 1.75 h on the second macrophage. Comparison of the high GFP expression levels of cells B and C to the low expression level of cell F (which only interacted with a macrophage for 1.75 h) further supported the notion that the GFP expression levels in cells B and C were the result of the cumulative interactions of these cells with both macrophages. The data also indicate that the time of interaction between the macrophage and T cell is the predominant determinant of GFP expression, rather than one macrophage being a better APC than another macrophage. Similarly dynamic interactions were observed between the NFAT-GFP–transfected 1H3.1 hybridoma and macrophages (data not shown).
Discussion
We have used a novel system for examining the interaction of macrophages with T cell hybridomas by video and immunofluorescence microscopy and FACS® analysis. Due to the difficulty in transfecting primary T cells and T cell lines, our studies were restricted to T cell hybridomas, which may behave differently. Although the stimulation of the NFAT pathway only reflects one aspect of T cell activation, the rapidity of the response allows real-time analysis of productive APC–T cell interactions. It has been known for a long time that macrophages and dendritic cells can bind many T cells at the same time; our system has permitted us to directly observe these interactions and to conclude that a single macrophage is capable of simultaneously activating multiple T cells. Most interestingly, these interactions are dynamic, and the T cells move both from contact site to contact site on individual macrophages and from APC to APC while being activated. We were surprised to observe that the T cell–macrophage interactions were so dynamic, because the observations of Dustin and colleagues demonstrated that T cells stop migrating upon interaction with ICAM-1 and MHC class II–peptide complex embedded in a lipid bilayer 14 21. It is possible that this inhibition of migration reflects an initial event that permits the formation of a productive contact zone, and that subsequent movement of T cells could not be detected because the full repertoire of signals required for T cell activation was not present. This view is supported by the fact that the T cells do stop for some minutes at particular sites on the macrophage, perhaps allowing the establishment of signaling junctions. Indeed, when individual macrophage–T cell pairs were observed by laser scanning confocal microscopy, we noticed the recruitment of cytoskeletal and signaling molecules such as actin, talin, and tyrosine-phosphorylated proteins to the site of cell–cell contact (data not shown), as has been described in other studies of APC–T cell interactions 3 22 23. Interestingly, T cells immobilized on a lipid bilayer containing ICAM-1 and the MHC class II–peptide complex can be stimulated to move upon activation of PKC by phorbol esters 14. It is well known that PKC is activated when T cells interact productively with APCs, and PKC-θ translocates to the sites of APC–T cell interaction 11. Thus, it is possible that the stop signal is reversed by PKC upon T cell activation. The data also imply that the contact zone need not be stable for hours to mediate T cell activation, although T cells have to interact continuously with macrophages for ∼2 h to be activated. This notion is further supported by the observation that T cells that interacted continuously with antigen-presenting macrophages for 3 h, albeit dynamically, were considerably brighter than cells that interacted with the APCs for shorter periods of time. In contrast to macrophages, previous studies have demonstrated that B cells make stable 1:1 pairs with T cells during antigen presentation 24 25, and we also observed that B cells invariably interact with DO11-GFP cells in a one to one fashion (data not shown). These observations make teleological sense, since macrophages need to activate many T cells to establish a concerted Th1 response, whereas a single B cell clone has more chance of being expanded by the focused and sustained delivery of cytokines.
T cells need to interact with APCs for a sufficient length of time to allow the TCR to sample the large array of MHC class II–peptide complexes present on the surface of the APC. This requires that the motile T cell rearranges its cytoskeleton and becomes static. Our data demonstrate that this stasis is transient, and that the T cell–APC interaction is quite dynamic during antigen presentation. Our data indicate that T cells can be stimulated by summing up signals received during multiple sequential interactions with an antigen-presenting macrophage.
Acknowledgments
This work was supported by grants AI25032 and AI31972 from the National Institutes of Health. D.M. Underhill is an Irvington Institute Postdoctoral Fellow.
References
- Aderem A., Underhill D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Davis M.M., Boniface J.J., Reich Z., Lyons D., Hampl J., Arden B., Chien Y. Ligand recognition by alpha beta T cell receptors. Annu. Rev. Immunol. 1998;16:523–544. doi: 10.1146/annurev.immunol.16.1.523. [DOI] [PubMed] [Google Scholar]
- Monks C.R., Freiberg B.A., Kupfer H., Sciaky N., Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A., Lezzi G., Viola A. From TCR engagement to T cell activationa kinetic view of T cell behavior. Cell. 1999;96:1–4. doi: 10.1016/s0092-8674(00)80952-6. [DOI] [PubMed] [Google Scholar]
- Wulfing C., Davis M.M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science. 1998;282:2266–2269. doi: 10.1126/science.282.5397.2266. [DOI] [PubMed] [Google Scholar]
- Penninger J.M., Crabtree G.R. The actin cytoskeleton and lymphocyte activation. Cell. 1999;96:9–12. doi: 10.1016/s0092-8674(00)80954-x. [DOI] [PubMed] [Google Scholar]
- Shaw A.S., Dustin M.L. Making the T cell receptor go the distancea topological view of T cell activation. Immunity. 1997;6:361–369. doi: 10.1016/s1074-7613(00)80279-4. [DOI] [PubMed] [Google Scholar]
- Dustin M.L., Shaw A.S. Costimulationbuilding an immunological synapse. Science. 1999;283:649–650. doi: 10.1126/science.283.5402.649. [DOI] [PubMed] [Google Scholar]
- Viola A., Schroeder S., Sakakibara Y., Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- Dustin M.L., Olszowy M.W., Holdorf A.D., Li J., Bromley S., Desai N., Widder P., Rosenberger F., van der Merwe P.-A., Allen P.M., Shaw A.S. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- Monks C.R., Kupfer H., Tamir I., Barlow A., Kupfer A. Selective modulation of protein kinase C-theta during T-cell activation. Nature. 1997;385:83–86. doi: 10.1038/385083a0. [DOI] [PubMed] [Google Scholar]
- van der Merwe P.A., McNamee P.N., Davies E.A., Barclay A.N., Davis S.J. Topology of the CD2-CD48 cell-adhesion molecule compleximplications for antigen recognition by T cells. Curr. Biol. 1995;5:74–84. doi: 10.1016/s0960-9822(95)00019-4. [DOI] [PubMed] [Google Scholar]
- Kupfer A., Singer S.J. Cell biology of cytotoxic and helper T cell functionsimmunofluorescence microscopic studies of single cells and cell couples. Annu. Rev. Immunol. 1989;7:309–337. doi: 10.1146/annurev.iy.07.040189.001521. [DOI] [PubMed] [Google Scholar]
- Dustin M.L., Bromley S.K., Kan Z., Peterson D.A., Unanue E.R. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc. Natl. Acad. Sci. USA. 1997;94:3909–3913. doi: 10.1073/pnas.94.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D.B., Shaw J.P., Bush M.R., Replogle R.E., Belagaje R., Crabtree G.R. Characterization of antigen receptor response elements within the interleukin-2 enhancer. Mol. Cell. Biol. 1988;8:1715–1724. doi: 10.1128/mcb.8.4.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C.L., Guidos C., Crabtree G.R. Cell type specificity and activation requirements for NFAT-1 (nuclear factor of activated T-cells) transcriptional activity determined by a new method using transgenic mice to assay transcriptional activity of an individual nuclear factor. J. Biol. Chem. 1990;265:15788–15795. [PubMed] [Google Scholar]
- Mattila P.S., Ullman K.S., Fiering S., Emmel E.A., McCutcheon M., Crabtree G.R., Herzenberg L.A. The actions of cyclosporin A and FK506 suggest a novel step in the activation of T lymphocytes. EMBO (Eur. Mol. Biol. Organ.) J. 1990;9:4425–4433. doi: 10.1002/j.1460-2075.1990.tb07893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudensky A.Y., Rath S., Preston-Hurlburt P., Murphy D.B., Janeway C.A. On the complexity of self. Nature. 1991;353:660–662. doi: 10.1038/353660a0. [DOI] [PubMed] [Google Scholar]
- Fiering S., Northrop J.P., Nolan G.P., Mattila P.S., Crabtree G.R., Herzenberg L.A. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T-cell antigen receptor. Genes Dev. 1990;4:1823–1834. doi: 10.1101/gad.4.10.1823. [DOI] [PubMed] [Google Scholar]
- Karttunen J., Shastri N. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc. Natl. Acad. Sci. USA. 1991;88:3972–3976. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., Bromley S.K., Sumen C., Davis M.M., Shaw A.S., Allen P.M., Dustin M.L. The immunological synapsea molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Burn P., Kupfer A., Singer S.J. Dynamic membrane-cytoskeletal interactionsspecific association of integrin and talin arises in vivo after phorbol ester treatment of peripheral blood lymphocytes. Proc. Natl. Acad. Sci. USA. 1988;85:497–501. doi: 10.1073/pnas.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Singer S.J. The specific interaction of helper T cells and antigen-presenting B cells. IV. Membrane and cytoskeletal reorganizations in the bound T cell as a function of antigen dose. J. Exp. Med. 1989;170:1697–1713. doi: 10.1084/jem.170.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Swain S.L., Janeway C.A., Jr., Singer S.J. The specific direct interaction of helper T cells and antigen-presenting B cells. Proc. Natl. Acad. Sci. USA. 1986;83:6080–6083. doi: 10.1073/pnas.83.16.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Swain S.L., Singer S.J. The specific direct interaction of helper T cells and antigen-presenting B cells. II. Reorientation of the microtubule organizing center and reorganization of the membrane-associated cytoskeleton inside the bound helper T cells. J. Exp. Med. 1987;165:1565–1580. doi: 10.1084/jem.165.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]