Experimental allergic encephalomyelitis (EAE) is an animal model of multiple sclerosis that is induced by immunization with myelin antigens 1. The ability to generate EAE by activating myelin-specific T cells in the periphery of healthy animals demonstrates that central and peripheral mechanisms of tolerance are incomplete for these self-antigens. This lack of T cell tolerance toward myelin proteins was initially thought to be due to restricted expression of these antigens behind the blood–brain barrier. Recent data demonstrate that T cell epitopes from the two most abundant proteins in myelin, proteolipid protein (PLP) and myelin basic protein (MBP), are expressed not only within the central nervous system (CNS) and peripheral nervous system but within the thymus, spleen, and lymph nodes as well 2 3 4 5 6.
In this issue of The Journal of Experimental Medicine 7, as well as in a related paper in Nature Medicine 8, the question of whether PLP expression in the thymus shapes the PLP-specific TCR repertoire is explored. PLP exists in two isoforms: PLP is the full-length form, and DM20 is a shorter form generated by alternative splicing that lacks 35 amino acids 9. Although transcripts corresponding to both isoforms are detected in the thymus, Anderson et al. 7 and Klein et al. 8 report that the DM20 transcript is expressed intrathymically at much higher levels than the full-length PLP transcript. These findings suggest that determinants within DM20 may be much more effective at inducing central tolerance than determinants found only in the larger form of PLP.
Klein et al. demonstrate that T cells specific for several determinants that are shared between PLP and the DM20 do indeed undergo tolerance in wild-type mice. Using thymic grafts and bone marrow chimeras, they show that both thymic epithelial cells and bone marrow–derived cells mediate tolerance induction in PLP-specific T cells. Anderson et al. make the striking observation that T cells specific for a determinant that is unique to the larger form of PLP and missing from DM20 (PLP 139–151) are not tolerized in SJL mice. Both groups conclude that the level of thymic expression of DM20 is sufficient to mediate tolerance to epitopes in this isoform but that thymic expression of PLP is not sufficient to induce tolerance to the unique sequences in the PLP isoform. The ability to detect responses to PLP 139–151 in lymph node cells from SJL mice indicates that these T cells not only escape central tolerance but escape peripheral tolerance as well.
Interestingly, studies of tolerance to MBP provide a different perspective on central tolerance to CNS antigens. Like PLP transcripts, MBP transcripts have been detected in the thymus and other lymphoid organs 3 6 10. This occurs because the MBP gene complex contains two strong promoters, each of which initiates a family of alternatively spliced transcripts 2. One family encodes isoforms of classic MBP contained in myelin. The other family encodes isoforms called golli-MBP. These transcripts contain unique exons, as well as many (but not all) of the exons of classic MBP. Golli-MBP transcripts are expressed in the thymus and peripheral lymphoid tissues, whereas expression of classic MBP transcripts appears to be restricted to myelin-forming cells 10 11 12. This differential expression pattern suggests that epitopes in golli-MBP could induce central tolerance, but epitopes contained exclusively in classic MBP would not be available to induce tolerance in the thymus. This prediction was fulfilled in C3H mice 13 14, but, surprisingly, this correlation was not observed in B10.PL mice 15.
A comparison of the immune response to MBP in wild-type and MBP-deficient B10.PL mice revealed that T cells specific for two epitopes within MBP 121–150 undergo strong tolerance induction, whereas T cells specific for MBP 1–11 are not tolerized 15. MBP 1–11 is contained in the golli-MBP transcripts expressed in the thymus, whereas MBP 121–150 is not contained in these transcripts. Sequences corresponding to the MBP 121–150 region have not been detected by PCR in the thymus, and no cDNA clones generated from thymus RNA have been reported to contain MBP 121–150 sequences 10 16. Thus, tolerance to MBP epitopes is not correlated with the synthesis of these sequences in the thymus.
The differential tolerance to MBP epitopes in B10.PL mice correlates with the stability of the MBP peptide–MHC complexes. MBP 1–11 forms very unstable complexes with MHC molecules that do not appear to be effective in mediating central tolerance, whereas both epitopes in MBP 121–150 form extremely stable complexes 15 17 18. To study the mechanisms and site of tolerance induction to MBP, we have generated TCR-transgenic mice specific for MBP 121–150 epitopes. Unexpectedly, this model shows that extensive deletion of MBP 121–150-specific T cells occurs in the thymus (Huseby, E., and J. Goverman, manuscript in preparation). These data demonstrate that central tolerance to MBP epitopes can occur in the absence of synthesis of these epitopes in the thymus. It is possible that the very high stability of MBP 121–150–MHC complexes allows bone marrow–derived cells to transport sufficient amounts of these complexes to the thymus to induce central tolerance.
The observation that protein synthesis within the thymus is not a requirement for central tolerance to high-affinity MBP epitopes raises the question of why PLP 139–151-specific T cells are not tolerized in a fashion similar to MBP 121–150-specific T cells. One possibility is that the affinity of the PLP 139–151-specific TCRs is lower for their ligand than the affinity of MBP 121–150-specific TCRs. The affinities of the TCRs have not been measured in either system. However, the MBP 1–11- and MBP 121–150-specific T cells differ only 10-fold in the amount of antigen required for half-maximal stimulation, suggesting that MBP 121–150-specific TCRs do not have unusually high affinity. A more likely explanation is that the high stability of the MBP 121–150–I-Au complexes allows APCs that have internalized MBP to retain these complexes on the cell surface while migrating to sites of tolerance induction. The PLP 139–151 epitope is also reported to have high affinity for I-As 19; however, it may not be comparable to the affinity of MBP 121–150 for I-Au. A third explanation, for which no data are yet available, is that MBP may be preferentially internalized and processed by APCs in the periphery relative to PLP.
The studies by Anderson et al. 7 of PLP-specific T cells yielded another surprising finding. In SJL and Balb.S mice, which are both very susceptible to induction of EAE, Anderson et al. find a very high precursor frequency (∼1/40,000) of peripheral PLP 139–151-specific T cells. B10.S mice that are more resistant to EAE exhibit a somewhat lower precursor frequency of PLP 139–151-specific T cells. The authors observe that PLP-specific T cells were predominantly found in the CD44hi subset of peripheral T cells in SJL mice and suggest that these cells undergo expansion in the periphery. The high frequency of peripheral PLP 139–151-specific T cells was also found in PLP-deficient and germ-free mice, suggesting that these cells are expanded in the periphery by interactions with a cross-reactive self-antigen. This interaction did not appear to trigger the T cells to differentiate, however, because they produced cytokines consistent with a Th0 phenotype when stimulated with PLP 139–151 in vitro. The preliminary phenotype described for these PLP-specific T cells is consistent with the “central memory” T cell subset described by Sallusto et al. 20.
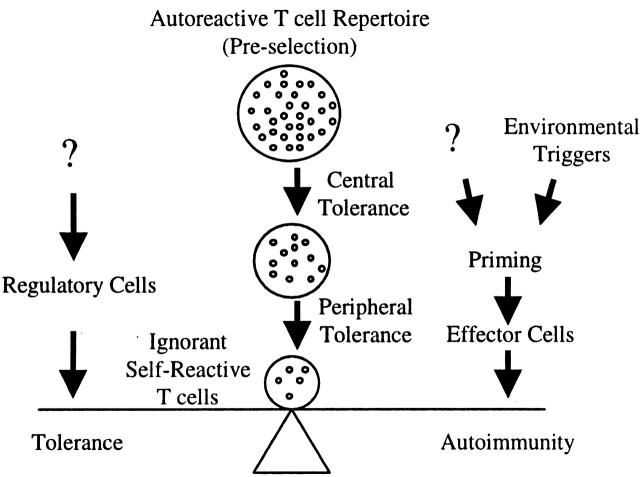
The studies of Anderson et al. raise a key question: why don't mice that have such a remarkably high precursor frequency of nontolerized T cells specific for a myelin antigen develop spontaneous EAE? To understand why self-reactive T cells fail to cause autoimmunity, it is useful to think about the steps that are required for the pathogenesis of autoimmune disease. First, autoreactive T cells must be positively selected in the thymus and migrate to the periphery. Opposing this step is induction of tolerance during T cell maturation in the thymus 21 22. Next, autoreactive T cells that escape central tolerance are primed by antigen in the periphery such that they differentiate into effector T cells. If the expression of the self-antigen in the periphery is too low to initiate interactions with T cells, the T cells will not proceed further along the pathway and remain in a state of ignorance 23. If the T cells do recognize self-antigen in the periphery, their differentiation into effector cells should be prevented by numerous mechanisms of peripheral tolerance that operate when self-antigens are not presented in an appropriate immunogenic context 24 25. A breakdown in peripheral tolerance can occur when environmental factors such as infectious agents alter presentation of self-antigens or stimulate self-reactive T cells through molecular mimicry 26. This leads to the final step in which differentiated, autoreactive T cells enter peripheral tissues and respond to antigen presented in situ. Opposing this step are regulatory T cells that appear to play a critical role in preventing autoimmune disease (these cells may act during the priming step as well) 27.
Given this perspective on the development of autoimmune disease, what are the predicted consequences of having a large pool of nontolerant, PLP-specific T cells in the periphery? Anderson et al. suggest that the increased number of peripheral PLP-specific T cells enhances disease susceptibility in SJL and Balb.S mice, presumably by increasing the chances of priming PLP-specific T cells. Although a high precursor frequency may increase susceptibility to EAE, it is clearly not the only factor. There are enough PLP-specific T cells in the lymph nodes of B10.S mice to be able to detect a proliferative response to PLP in vitro without prior immunization, yet this strain is fairly resistant to EAE induction. The high precursor frequency of PLP 139–151-specific T cells in SJL mice is also not sufficient to cause spontaneous disease. It is important to remember, however, that laboratory mice live in a controlled environment with significantly less exposure to environmental stimuli than they would encounter if they lived in the wild. Therefore, if a similarly large increase in peripheral PLP-specific T cells occurred in a subset of people, this factor combined with environmental influences may be sufficient to trigger multiple sclerosis (MS). If the analogy between SJL mice and humans holds, it would suggest that HLA molecules associated with susceptibility to MS would bind with low or moderate affinity to epitopes of PLP or MBP that are not represented in the isoforms of these proteins expressed at higher levels in the thymus.
In contrast to wild-type SJL mice, a very high incidence of spontaneous EAE is observed in PLP 139–151-specific TCR–transgenic mice on the SJL background 28. One explanation for the spontaneous EAE that occurs in the TCR-transgenic mice is that many more PLP-specific T cells are available to encounter environmental triggers and differentiate into effector T cells. As tissue destruction mediated by a small number of T cells begins, the amount of self-antigen released into the bloodstream is increased. An increase in the concentration of self-antigen may fuel priming of more PLP-specific T cells in the periphery until continuing along the path toward autoimmunity becomes inevitable.
Spontaneous EAE is also observed in MBP 1–11 TCR–transgenic mice. Different MBP 1–11 TCR–transgenic models exhibit different incidences of disease, even though the antigen specificity of the TCRs used in these models is the same. Mice described by Goverman et al. exhibit a higher incidence of spontaneous EAE than mice described by Lafaille et al. when both types of transgenic mice are backcrossed onto B10.PL and cohoused in the same environment 29 30 31. MBP 1–11-specific TCR–transgenic mice described by Liu et al. 32 do not exhibit any spontaneous EAE. It is possible that different degrees of allelic exclusion or differences in copy number of the transgenes in these transgenic models affect the precursor frequency of MBP 1–11-specific T cells in the periphery.
Although changes in precursor frequency of self-reactive T cells no doubt contribute to susceptibility to autoimmune disease, the abundance of regulatory T cells may be the critical factor in determining whether the pathway toward autoimmune disease is completed. Regulatory cells play a key role in preventing autoimmune disease in MBP 1–11 TCR–transgenic mice. The incidence of EAE increases dramatically when MBP-specific TCR–transgenic mice are bred onto backgrounds in which regulatory T cells are eliminated by preventing expression of endogenous TCRs 30 33 34. Thus, the differences in susceptibility of the various MBP 1–11 TCR–transgenic models to spontaneous EAE could be explained by a larger or more effective pool of regulatory cells in some models versus others. Similarly, the spontaneous EAE observed in PLP 139–150 TCR–transgenic mice may not be caused by an increase in PLP-specific T cells above a critical threshold, but rather because the restricted TCR repertoire in the transgenic mice limits the number of regulatory T cells available to suppress the autoreactive responses.
The picture that emerges from these studies is that autoimmune disease susceptibility reflects in part the ability of particular MHC molecules to present selected epitopes of self-antigens that are not synthesized in the thymus. Self-antigen epitopes that contribute to autoimmune disease also must not form such stable peptide–MHC complexes that they either induce peripheral tolerance or are retained on APCs migrating into the thymus from the periphery. These self-reactive, nontolerant T cells that escape central tolerance are balanced in the periphery between a state of ignorance and autoimmunity. The factors that tip this balance toward autoimmunity are most likely environmental factors that trigger priming of the T cells in the periphery. The effectiveness of regulatory cells pushes the balance back toward a state of ignorance. This balance becomes more difficult to maintain as the pool of autoreactive T cells increases. A large number of autoreactive T cells increases the likelihood that some of these T cells will be affected by environmental influences such that more regulatory T cells are required to inhibit these responses. Circumstances that either increase the number of nontolerant, self-reactive T cells and/or decrease the number of regulatory cells will lower the threshold required for induction of autoimmunity (Fig. 1). How the regulatory T cells that prevent autoimmunity are themselves regulated is one of the most important areas of future research in autoimmunity.
Figure 1.
Autoreactive T cells that are not eliminated or rendered unresponsive by tolerance mechanisms normally remain quiescent in the periphery. Priming events that trigger their differentiation into effector T cells can initiate autoimmune responses. The function of regulatory T cells is to inhibit these responses and prevent autoimmunity.
Acknowledgments
The authors would like to thank Drs. Thea Brabb and Claes Ohlen as well as Audrey Seamons and Antoine Perchellet for helpful comments on the manuscript.
J. Goverman is supported in part by a Harry Weaver Junior Faculty Award (2080-A-2) from the National Multiple Sclerosis Society. E.S. Huseby is supported by the Samuel and Althea Stroum Endowed Diabetes Fellowship.
References
- Martin R., McFarland H.F. Immunology of multiple sclerosis and experimental allergic encephalomyelitis. In: Raine C.S., McFarland H.F., Tourtellotte W.W., editors. Multiple SclerosisClinical and Pathogenic Basis. Chapmand and Hall; London: 1997. pp. 221–242. [Google Scholar]
- Campagnoni A.T., Pribyl T.M., Campagnoni C.W., Kampf K., Amur Umarjee S., Landry C.F., Handley V.W., Newman S.L., Garbay B., Kitamura K. Structure and developmental regulation of Golli-mbp, a 105-kilobase gene that encompasses the myelin basic protein gene and is expressed in cells in the oligodendrocyte lineage in the brain. J. Biol. Chem. 1993;268:4930–4938. [PubMed] [Google Scholar]
- Grima B., Zelenika D., Pessac B. A novel transcript overlapping the myelin basic protein gene. J. Neurochem. 1992;59:2318–2323. doi: 10.1111/j.1471-4159.1992.tb10126.x. [DOI] [PubMed] [Google Scholar]
- Voskuhl R.R. Myelin protein expression in lymphoid tissuesimplications for peripheral tolerance. Immunol. Rev. 1998;164:81–92. doi: 10.1111/j.1600-065x.1998.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Fritz R.B., Zhao M.L. Thymic expression of myelin basic protein (MBP). Activation of MBP-specific T cells by thymic cells in the absence of exogenous MBP. J. Immunol. 1996;157:5249–5253. [PubMed] [Google Scholar]
- Mathisen P.M., Pease S., Garvey J., Hood L., Readhead C. Identification of an embryonic isoform of myelin basic protein that is expressed widely in the mouse embryo. Proc. Natl. Acad. Sci. USA. 1993;90:10125–10129. doi: 10.1073/pnas.90.21.10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A.C., Nicholson L.B., Legge K.L., Turchin V., Zaghouani H., Kuchroo V.K. High frequency of autoreactive myelin proteolipid protein–specific T cells in the periphery of naive micemechanisms of selection of the self-reactive repertoire. J. Exp. Med. 2000;191:761–770. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein L., Klugmann M., Nave K.A., Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nat. Med. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- Nave K.A., Lai C., Bloom F.E., Milner R.J. Splice site selection in the proteolipid protein (PLP) gene transcript and primary structure of the DM-20 protein of central nervous system myelin. Proc. Natl. Acad. Sci. USA. 1987;84:5665–5669. doi: 10.1073/pnas.84.16.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pribyl T.M., Campagnoni C.W., Kampf K., Kashima T., Handley V.W., McMahon J., Campagnoni A.T. The human myelin basic protein gene is included within a 179-kilobase transcription unitexpression in the immune and central nervous systems. Proc. Natl. Acad. Sci. USA. 1993;90:10695–10699. doi: 10.1073/pnas.90.22.10695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity A.N., Campagnoni A.T. Regional expression of myelin protein genes in the developing mouse brainin situ hybridization studies. J. Neurosci. Res. 1988;21:238–248. doi: 10.1002/jnr.490210216. [DOI] [PubMed] [Google Scholar]
- Trapp B.D., Moench T., Pulley M., Barbosa E., Tennekoon G., Griffin J. Spatial segregation of mRNA encoding myelin-specific proteins. Proc. Natl. Acad. Sci. USA. 1987;84:7773–7777. doi: 10.1073/pnas.84.21.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huseby E.S., Ohlen C., Goverman J. Cutting edgemyelin basic protein-specific cytotoxic T cell tolerance is maintained in vivo by a single dominant epitope in H-2k mice. J. Immunol. 1999;163:1115–1118. [PubMed] [Google Scholar]
- Targoni O.S., Lehmann P.V. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J. Exp. Med. 1998;187:2055–2063. doi: 10.1084/jem.187.12.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington C.J., Paez A., Hunkapiller T., Mannikko V., Brabb T., Ahearn M., Beeson C., Goverman J. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. 1998;8:571–580. doi: 10.1016/s1074-7613(00)80562-2. [DOI] [PubMed] [Google Scholar]
- Fritz R.B., Kalvakolanu I. Thymic expression of the golli-myelin basic protein gene in the SJL/J mouse. J. Neuroimmunol. 1995;57:93–99. doi: 10.1016/0165-5728(94)00167-m. [DOI] [PubMed] [Google Scholar]
- Fairchild P.J., Wildgoose R., Atherton E., Webb S., Wraith D.C. An autoantigenic T cell epitope forms unstable complexes with class II MHCa novel route for escape from tolerance induction. Int. Immunol. 1993;5:1151–1158. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]
- Mason K., Denney D.W., Jr., McConnell H.M. Kinetics of the reaction of a myelin basic protein peptide with soluble IAu. Biochemistry. 1995;34:14874–14878. doi: 10.1021/bi00045a031. [DOI] [PubMed] [Google Scholar]
- Greer J.M., Sobel R.A., Sette A., Southwood S., Lees M.B., Kuchroo V.K. Immunogenic and encephalitogenic epitope clusters of myelin proteolipid protein. J. Immunol. 1996;156:371–379. [PubMed] [Google Scholar]
- Sallusto F., Lenig D., Forster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- Jameson S.C., Hogquist K.A., Bevan M.J. Positive selection of thymocytes. Annu. Rev. Immunol. 1995;13:93–126. doi: 10.1146/annurev.iy.13.040195.000521. [DOI] [PubMed] [Google Scholar]
- Anderson G., Moore N.C., Owen J.J., Jenkinson E.J. Cellular interactions in thymocyte development. Annu. Rev. Immunol. 1996;14:73–99. doi: 10.1146/annurev.immunol.14.1.73. [DOI] [PubMed] [Google Scholar]
- Miller J.F., Heath W.R. Self-ignorance in the peripheral T-cell pool. Immunol. Rev. 1993;133:131–150. doi: 10.1111/j.1600-065x.1993.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Miller J.F., Kurts C., Allison J., Kosaka H., Carbone F., Heath W.R. Induction of peripheral CD8+ T-cell tolerance by cross-presentation of self antigens. Immunol. Rev. 1998;165:267–277. doi: 10.1111/j.1600-065x.1998.tb01244.x. [DOI] [PubMed] [Google Scholar]
- Van Parijs L., Abbas A.K. Homeostasis and self-tolerance in the immune systemturning lymphocytes off. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- Albert L.J., Inman R.D. Molecular mimicry and autoimmunity. N. Engl. J. Med. 1999;341:2068–2074. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- Mason D., Powrie F. Control of immune pathology by regulatory T cells. Curr. Opin. Immunol. 1998;10:649–655. doi: 10.1016/s0952-7915(98)80084-8. [DOI] [PubMed] [Google Scholar]
- Waldner H., Whitters M.J., Sobel R.A., Collins M., Kuchroo V.K. Fulminant spontaneous autoimmunity of the central nervous system in myelin proteolipid protein specific T cell receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 2000;In press doi: 10.1073/pnas.97.7.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goverman J., Woods A., Larson L., Weiner L.P., Hood L., Zaller D.M. Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- Lafaille J.J., Nagashima K., Katsuki M., Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Goverman J. Tolerance and autoimmunity in TCR transgenic mice specific for myelin basic protein. Immunol. Rev. 1999;169:147–159. doi: 10.1111/j.1600-065X.1999.tb01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G.Y., Fairchild P.J., Smith R.M., Prowle J.R., Kioussis D., Wraith D.C. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- Olivares-Villagomez D., Wang Y., Lafaille J.J. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein–specific transgenic mice from spontaneous autoimmune encephalomyelitis. J. Exp. Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Keere F., Tonegawa S. CD4(+) T cells prevent spontaneous experimental autoimmune encephalomyelitis in anti–myelin basic protein T cell receptor transgenic mice. J. Exp. Med. 1998;188:1875–1882. doi: 10.1084/jem.188.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]