Abstract
Aspirin therapy inhibits prostaglandin biosynthesis without directly acting on lipoxygenases, yet via acetylation of cyclooxygenase 2 (COX-2) it leads to bioactive lipoxins (LXs) epimeric at carbon 15 (15-epi-LX, also termed aspirin-triggered LX [ATL]). Here, we report that inflammatory exudates from mice treated with ω-3 polyunsaturated fatty acid and aspirin (ASA) generate a novel array of bioactive lipid signals. Human endothelial cells with upregulated COX-2 treated with ASA converted C20:5 ω-3 to 18R-hydroxyeicosapentaenoic acid (HEPE) and 15R-HEPE. Each was used by polymorphonuclear leukocytes to generate separate classes of novel trihydroxy-containing mediators, including 5-series 15R-LX5 and 5,12,18R-triHEPE. These new compounds proved to be potent inhibitors of human polymorphonuclear leukocyte transendothelial migration and infiltration in vivo (ATL analogue > 5,12,18R-triHEPE > 18R-HEPE). Acetaminophen and indomethacin also permitted 18R-HEPE and 15R-HEPE generation with recombinant COX-2 as well as ω-5 and ω-9 oxygenations of other fatty acids that act on hematologic cells. These findings establish new transcellular routes for producing arrays of bioactive lipid mediators via COX-2–nonsteroidal antiinflammatory drug–dependent oxygenations and cell–cell interactions that impact microinflammation. The generation of these and related compounds provides a novel mechanism(s) for the therapeutic benefits of ω-3 dietary supplementation, which may be important in inflammation, neoplasia, and vascular diseases.
Keywords: dietary PUFA, eicosanoids, leukocytes, cardiovascular disease
Introduction
Numerous reports of the past 25 years suggest that supplementation of dietary omega-3 polyunsaturated fatty acids (ω-3 PUFA) with linseed, canola, or fish oils has beneficial effects in human diseases and laboratory animals (for review see references 1 and 2). These have included lively discussions of potential antithrombotic, immunoregulatory, and antiinflammatory responses relevant in arteriosclerosis, arthritis, and asthma 1 as well as antitumor and antimetastatic effects 3. Their potential for preventative actions in cardiovascular diseases was recently bolstered with the finding that major dietary ω-3 PUFAs, eicosapentaenoic acid (C20:5 ω-3; EPA) and docosahexaenoic acid (C22:6 ω-3; DHA), have a dramatic effect on ischemia-induced ventricular fibrillation and can protect against sudden cardiac death in dogs 4. Emergence of such possible preventative and/or therapeutic actions of ω-3 PUFA supplementation in infant nutrition, cardiovascular diseases, and mental health has led to a call for recommended dietary intakes by an international workshop 5. Also, the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Prevenzione trial evaluated the effects of ω-3 PUFA supplementation with >11,300 patients surviving myocardial infarction taking ∼1 g of ω-3 PUFA daily (n = 2,836) along with recommended preventive treatments including aspirin, and reported a significant benefit with a decrease in cardiovascular death 6. However, the cellular and molecular mechanism(s) for dietary ω-3 protective actions in all of the studies to date remain largely unexplained.
It is believed that the actions of the major lipid of fish oil, C20:5, are based upon (a) preventing conversion of arachidonic acid (C20:4 ω-6; AA) to proinflammatory eicosanoids (i.e., prostaglandins [PGs] and leukotrienes [LTs]); (b) serving as an alternate substrate producing 5-series LTs that are less potent; and/or (c) conversion by cyclooxygenase (COX) to 3-series prostanoids (i.e., PGI3) with potencies equivalent to their 4-series PG counterparts to maintain antithrombotic actions 1 3 4. These and other explanations offered 1 2 3 4 5 have not been generally accepted because of the lack of molecular evidence in vivo and the high concentrations of ω-3 PUFA required to achieve putative “beneficial actions” in vitro.
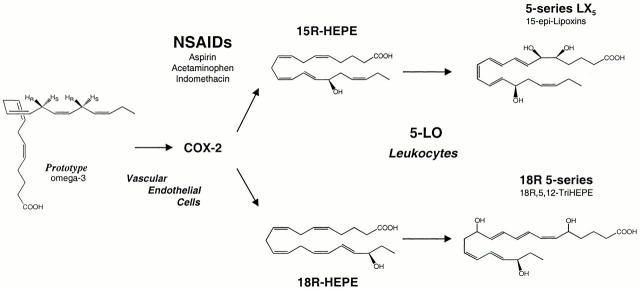
Although the proinflammatory roles of LT and PG are well appreciated 7 8, there is new evidence that other eicosanoids derived from arachidonate, namely lipoxins (LXs) and their endogenous analogues, the aspirin-triggered 15 epimer LXs (ATLs), are potent counterregulators of PMN-mediated injury and acute inflammation 9 10 11. At least two isoforms for COX, the classic site of action for nonsteroidal antiinflammatory drugs (NSAIDs), have been uncovered (COX-1 and 2) that appear to serve separate physiologic and pathophysiologic roles in humans 12. Each COX isoform carries dual enzymatic activities, a bisoxygenase and a peroxidase. Inhibition of COX-2 is the current focus of several pharmaceutical companies, as selective inhibition of COX–2 without blocking COX-1 could reduce unwanted side effects associated with traditional NSAIDs 13. In this regard, acetylation of COX-2 by the classic NSAID, aspirin (ASA), prevents the formation of prostanoids 12, but the acetylated enzyme remains active in situ to generate 15R-hydroxyeicosatetraenoic acid (15R-HETE) from C20:4 14 15 that is released and converted by activated inflammatory cells to the 15-epimeric LXs. Synthetic analogues of these natural local mediators with prolonged biological half-life display potent antiinflammatory properties 11 16, providing evidence that cell–cell interactions can be responsible for conversion of AA to mediators that possess antiinflammatory properties.
Oxidation of C20:4 via P450 in endothelial cells (ECs) may also lead to 11,12-epoxyeicosatetraenoic acid that appears to block EC activation 17, while nonenzymatic oxidation of EPA can downregulate EC adhesion molecules 18. As PMN–vessel interactions are pivotal to recruitment and PMN-dependent tissue injury, the local signals involved in their “cross-talk dialogue” are of interest. Our finding that aspirin-acetylated COX-2 remains active in vivo 14 to generate specific ATLs that can be effectors of well established antiinflammatory reactions offers a mechanism for beneficial effects of ASA that cannot be attributed to inhibition of prostanoids alone 8 12. New therapeutic applications for ASA and related NSAIDs continue to emerge. However, they usually require molecular definition to provide a rationale. This includes the reported prophylactic benefit of ASA in colorectal cancer and the lower risk of a second myocardial infarction (for review see reference 19). In view of the qualitatively overlapping beneficial profiles assigned to dietary ω-3 PUFA in human disease 1 2 3 4 5 6, we sought evidence for novel pathways for lipid-derived signals that could possibly provide a molecular basis and also serve as markers for these beneficial actions.
Materials and Methods
Zymosan, hematin, NADPH, and ASA were from Sigma-Aldrich. EPA (Cayman Chemical) and other synthetic standards, hydroxy fatty acids, and intermediates used for identification were purchased from Cascade Biochem Ltd. Bacillus megaterium was from American Type Culture Collection. Materials used in liquid chromatography tandem mass spectrometry (LC/MS/MS) analyses were from vendors given in reference 20.
Human PMNs were freshly isolated from venous blood of healthy volunteers (that declined taking medication for 2 wk before donation; Brigham and Women's Hospital protocol no. 88-02642) by Ficoll gradient and enumerated. Human umbilical vein or microvascular ECs (HUVECs or HMVECs, respectively) were cultured for transendothelial migration 10. HMVEC monolayers (one, two, or three passages) were seeded (∼2 × 105 cells/cm2) on polycarbonate permeable supports precoated with 0.1% gelatin for incubations with NSAIDs and PUFA.
Inflammatory exudates were initiated with intrapouch injection of TNF-α (R&D Systems) into 6 d dorsal air pouches 16 with 6–8-wk-old male FVB mice (fed standard rodent diet 5001 containing 0.26% n-3 fatty acids) followed by ASA (500 μg) at 3.5 h and 300 μg C20:5/pouch at 4 h. At 6 h, pouches were lavaged (3 ml saline), and exudate cells were enumerated and activated (4 μM A23187, 37°C, 20 min). Inhibition of TNF-α–stimulated (100 ng/pouch, FVB strain) PMN infiltration with intravenous tail injection of either 18R-hydroxyeicosapentaenoic acid (HEPE), 5,12,18R-HEPE, or 15-epi-LXA4 analogue was determined 16 with pouch lavages taken at 4 h.
Specific [3H]LTB4 (NEN Life Science Products) binding was performed with human embryonic kidney (HEK)293 cells stably transfected with human LTB4 receptor 11. Human recombinant COX-2 (a gift from Dr. R.A. Copeland, DuPont Merck, Wilmington, DE) was overexpressed in Sf9 insect cells (American Type Culture Collection) with microsomal fractions (∼8 μl) suspended in Tris (100 mM, pH 8.0) as in reference 21. NSAIDs were incubated (i.e., ASA, ∼1 mM) at 37°C for 30 min before addition of PUFA (20 μM), and conversions were also monitored using 1-14C–labeled C20:4 or C20:5 (NEN Life Science Products). B. megaterium was grown in Bacto Nutrient Broth (Fisher Scientific) at 30°C with shaking. To prepare standards for 18R-HEPE, a biogenic synthesis was used with B. megaterium sonicates incubated with NADPH (2 mM) and C20:5 (330 μM) in 2 M Tris buffer, pH 8.1. Similar conditions were employed to convert LTB5 (15 μM) to novel products; see Results. Incubations were extracted 9 with deuterium-labeled internal standards (15-HETE and C20:4) for LC/MS/MS analysis 14 16 using a Finnigan LCQ equipped with a LUNA C18-2 (150 × 2 mm; 5 μM) column and a rapid spectra scanning UV/Vis detector. Also, a Chiralcel OB-H column (J.T. Baker) was used to determine R and S alcohol configurations of monohydroxy-PUFA using isocratic (hexane/isopropanol 96:4 vol/vol). Detailed procedures for isolation, quantitation, and structural determination of lipid-derived mediators were recently reported 20 and used here essentially as described for the elucidation of the novel products.
Results and Discussion
Because ASA triggers formation of epimeric forms of naturally occurring bioactive eicosanoids 9, we tested the concept that NSAIDs might promote the formation of novel mediators from ω-3 PUFAs. Inflammatory exudates formed in murine air pouches via intrapouch injections of TNF-α with ω-3 and ASA on board (2 h) generated several novel compounds (Fig. 1). These mice were fed a standard rodent diet containing 0.26% ω-3 PUFA. LC/MS/MS analyses of the exudate-derived materials demonstrated monohydroxy acids, depicted in selected ion chromatograms from acquired results recalled at m/z 317 (Fig. 1 A), i.e., 18-hydroxy-EPA (18-HEPE) and 5-HEPE, which coeluted with synthetic 5S-HEPE as well as novel trihydroxy-containing compounds derived from C20:5. LC retention times and MS/MS spectra (Fig. 1B and Fig. C) gave product ions consistent with structures shown in the respective insets, namely m/z 317 = [M-H]−, 299 = [M-H]−–H2O, 273 = [M-H]−–CO2, 255 = [M-H]−–H2O,–CO2. Diagnostic ions consistent with 18-HEPE identification were present at m/z 259 (Fig. 1 B) and 5-HEPE at m/z 115 (Fig. 1 C). These criteria were used throughout for identification. The stereochemistry of the alcohol at carbon 18 was established for exudate-derived 18-HEPE using a chiral column, and a reference 18R-HEPE was prepared via biogenic synthesis using B. megaterium (see Materials and Methods). This microbe monooxygenates fatty acids and, for example, converts C20:4 to 18R-HETE 22 23. The alcohol configuration at position 18 proved to be >98% R. These findings indicated that murine inflammatory exudates exposed in vivo to C20:5, ω-3 and ASA produced 5-lipoxygenase pathway 5-series 5S-HEPE, a product also identified with human PMNs 24, as well as a novel 18R-HEPE, whose route of formation was determined (vide infra). Air pouch inflammatory exudate cells from these ASA- and EPA-treated mice contained predominantly PMN (as in Fig. 1), which were 25–60% lower in number than in exudates formed with TNF-α alone (n = 3). Also, these exudates, when activated with ionophore A23187 (4 μM), generated essentially equivalent amounts of 18R-HEPE (10.2 ± 4.3 ng/106 cells) and 5S-HEPE (10.9 ± 2.9 ng/106 cells).
Figure 1.
Inflammatory exudates from murine dorsal pouches treated with aspirin generate novel compounds: LC/MS/MS. TNF-α–induced leukocyte exudates collected at 6 h from FVB mice given ASA (3.5 h at 500 μg/air pouch) and EPA (4 h at 300 μg/pouch) contained (2.3 ± 0.5 × 106 leukocytes per pouch); see Materials and Methods. (A) Selected ion chromatogram of mono-HEPEs. (B) MS/MS of 18R-HEPE. (C) MS/MS of 5S-HEPE. (D) MS/MS of 12,15,18R-triHEPE (see text for identification of diagnostic ions). Results are representative of n = 4.
Evidence for novel trihydroxy-containing products was also obtained in these inflammatory exudates (Fig. 1 D). Ions present within MS/MS were consistent with a trihydroxy-containing product from C20:5 with a parent ion at m/z 349 = [M-H]− and product ions of structural significance present at m/z 291 and 195 that are consistent with fragmentations denoted in the inset (Fig. 1 D). Also, an observed 270 nm UV absorbance maximum, indicative of a conjugated triene, together with the presence of the m/z = 291 (cleavage C17–C18 positions) as well as the 20-carbon structure, implicated that 18R-HEPE and the triHEPE were biosynthetically related.
It was of interest to determine whether these new compounds were also generated by human cells and if they possess bioactivities. To this end, human ECs known to induce COX-2 with IL-1β 9 or hypoxia (not shown) were pulsed with EPA and treated with ASA, and extracted materials were subject to LC/MS/MS analysis (Fig. 2 A). Selected ion monitoring at m/z 259 revealed that HUVECs treated with ASA converted EPA to 18R-HEPE (Fig. 2 A). Also, HMVECs treated with ASA and EPA generated 18-HEPE (10.6 ng/106 cells) and 15-HEPE (6.0 ng/106 cells) (n = 2, four determinations; data not shown). These observations implicated the involvement of COX-2 in the generation of these compounds, which proved to be the case with recombinant human COX-2 exposure to ASA and ω-3 PUFA (Table ), findings that may be of clinical significance.
Figure 2.
Human ECs and PMNs: novel trihydroxy-containing compounds LC/MS/MS. (A) HUVECs treated with IL-1β (24 h) and ASA. (B) Human PMNs (∼ 30 × 106/ml) incubated (30 min, 37°C) with serum-treated zymosan (100 ng/ml) and acetylated ASA-recombinant COX-2–derived products from EPA. (C) MS/MS of 5,12,18R-triHEPE (see text for ions and fragmentation). (D) MS/MS of 15-epi-LXA5. Results are representative of n = 3–5 for PMNs and n = 3 for ECs.
Table 1.
Hydroxy Compounds Generated from PUFA and Recombinant Human COX-2 with ASA or a Selective COX-2 Inhibitor
| Hydroxy-containing compounds (ng/incubation) | ||||
|---|---|---|---|---|
| PUFA | NSAID | ω-2 | ω-5 | ω-9 |
| 13-HODE | 9-HODE | |||
| C18:2 | ASA | – | 2.9 ± 0.6 | 25.2 ± 17.8 |
| C18:2 | Vehicle alone | – | 55.0 ± 18.7 | 243.0 ± 68.6 |
| 15R-HETE | 11R-HETE | |||
| C20:4 | ASA | – | 234.0 ± 112.5 | 1.4 ± 1.6 |
| C20:4 | Vehicle alone | – | 11.5 ± 8.2 | 1.8 ± 1.0 |
| C20:4 | Selective inhibitor | – | 5.5 ± 0.8 | 0.9 ± 0.1 |
| 18R-HEPE | 15R-HEPE | 11R-HEPE | ||
| C20:5 | ASA | 16.2 ± 3.3 | 16.8 ± 5.8 | 5.4 ± 3.3 |
| C20:5 | Vehicle alone | 7.0 ± 3.3 | 17.9 ± 5.2 | 40.7 ± 10.3 |
| C20:5 | Selective inhibitor | 5.8 ± 2.6 | 0.0 ± 0.6 | 0.0 ± 0.3 |
Results are the mean ± SEM, n = 3. The selective COX-2 inhibitor NS398 was used at 100 μM. All products were extracted, identified, and quantitated using internal standards and LC/MS/MS. Compounds of interest are shown in bold type.
As shown in Table , linoleic acid (C18:2) was converted to both 13-hydroxy-9Z,11E-octadecadienoic acid (13-HODE; ω-5 oxygenation) and 9-HODE (ω-9), which were greatly diminished by ASA but not completely abolished. AA was converted to 15R-HETE (ω-5) as well as 11R-HETE (ω-9), consistent with earlier findings 9. ASA triggered the appearance of a lipoxygenase activity that switched to 15R-HETE production by acetylated COX-2 9 14 15, which did not appear to influence 11R-HETE formation (Table ). 11R-HEPE was the major product with EPA and COX-2, with lesser amounts of 15R-HEPE (ω-5) and 18R-HEPE (ω-2). 1-14C–labeled EPA was used to confirm precursor–product relationships (n = 3). ASA acetylation of COX-2 led to an approximately twofold increase in 18R-HEPE (ω-2), with a >85% reduction in 11R-HEPE (the ratio of positional oxygenations with C20:5 was 1:1:0.3, with 18R ≈ 15R > 11R). Hence, together they suggested that acetylated COX-2 in ECs (Fig. 2) was a dominant source of 18R-HEPE and 15R-HEPE.
Interestingly and unlike the isolated COX-2 product profiles, neither 11R-HEPE (from C20:5) nor 11R-HETE (from C20:4) were major products of the vascular ECs (Table and Fig. 2). With the selective COX-2 inhibitor NS398 12 13, these oxygenations were reduced, and only 18R-HEPE formation from EPA appeared to escape inhibition (Table ). These results suggest that ASA treatment at local sites of inflammation along with ω-3 PUFA (i.e., EPA; C20:5, ω-3) administration, as exemplified by cytokine-driven acute inflammation (Fig. 1), can convert EPA via COX-2 to 18R-HEPE and 15R-HEPE.
Because human PMNs convert ASA-triggered, COX-2–derived 15R-HETE to 15-epi-LXA4 9 and EPA is converted to 5-series LX by human leukocytes 25 as well as trout macrophages 26, we next evaluated whether activated human PMNs engaged in phagocytosis handle acetylated COX-2–derived C20:5, ω-3 products 18R-HEPE and 15R-HEPE. Serum-treated zymosan, a phagocytic stimulus, initiated the utilization and conversion of acetylated COX-2 C20:5-derived products to two classes of trihydroxy-containing EPE, determined again by selected ion monitoring at m/z 349.5 [M-H]−, the base peak molecular ion for these products (Fig. 2 B). One, shown in Fig. 2 C, gave essentially the same MS/MS observed in Fig. 1 D from murine cells and was consistent with the 5,12,18R-triHEPE structure depicted in the inset giving diagnostic ions (Fig. 2 C) at m/z 305, 233, 195, and 291 (Fig. 1 D). This product is an 18R-hydroxy–carrying “LTB5-like” structure (see Fig. 2 D, inset). Indeed, when isolated 18R-HEPE was incubated as above with activated PMNs, it was converted to several compounds, including this product. Also, synthetic LTB5 incubated with B. megaterium homogenates and NADPH at pH 8.0 to facilitate hydroxylations 23 was transformed to a trihydroxy product (n = 3) with an m/z 291 ion characteristic for the presence of the 18R alcohol group as obtained from human PMNs shown in Fig. 2 C. These independent lines of evidence indicated that PMNs take up 18R-HEPE, which is converted by their 5-lipoxygenase, to insert molecular oxygen and in subsequent steps to 5-hydro(peroxy)-18R-DiH(p)EPE and 5(6)epoxide formation to 5,12,18R-triHEPE (an 18R-carrying LTB5-like product) that is likely to possess the stereochemistry of LTB5 24, retaining the 18R chirality of the precursor.
In an analogous biosynthetic fashion, 15R-HEPE was converted by PMN via 5-lipoxygenation to a 5-series LXA5 analogue (Fig. 2 D) that also retains their C15 configuration. Its MS/MS gave prominent ions, m/z 305, 233, and 251, depicted in the MS/MS spectrum, namely 15-epi-LXA5, consistent with 15S-containing LX5 structures (5-series) observed from endogenous sources of EPA in trout macrophages 26. In this case, the chirality of the precursor 15R is retained by human PMN to give 15-epi-LXA5 (Fig. 2 D), which is the 5-series ω-3 analogue of 15-epi-LXA4. As with LX biosynthesis, conversion of both 18R- and 15R-HEPE by activated PMNs with 5-lipoxygenation was accompanied with a reduction in LTB4 formation (not shown). Together, these results indicate that isolated human ECs and PMNs (Fig. 2) can generate the novel products observed with inflammatory exudates (Fig. 1).
Transendothelial migration is a pivotal event in PMN recruitment and inflammation and a recognized locus of action for traditional antiinflammatory therapies 27. Endogenous lipid mediators that can control these cell–cell interactions are of interest. Therefore, we isolated and assessed 5,12,18R-triHEPE and its precursor 18R-HEPE on human PMN transmigration. Both compounds inhibited LTB4-stimulated PMN transendothelial migration (Fig. 3 A) with an apparent IC50 of 5–50 nM for 5,12,18R-triHEPE and IC50 >1.0 μM for 18R-HEPE. Thus, the new 5-series members, namely, 18R-carrying trihydroxy-HEPE and 18R-HEPE, inhibited PMN migration, as did 15-epi-LXA4 and its omega end analogue 10 16, tested in parallel for direct comparison (Fig. 3 A). Their rank order of potency was 15-epi-LXA4 stable analogue > 5,12,18R-triHEPE > 18R-HEPE.
Figure 3.
The novel compounds inhibit human PMN transmigration and inflammation in the murine air pouch. (A) Inhibition of PMN transendothelial migration. PMNs were incubated with isolated compounds [10−10–10−6 M] 18R-HEPE (○), 5,12,18R-triHEPE (•), or ATL analogue used for reference (♦), and transmigration was initiated using an optimal amount of LTB4 [10 nM] (100%) and HUVEC coincubations (90 min, 37°C). Results are the mean ± SEM; n = 3. (B) Competition binding with human recombinant LTB4 receptor. LTB4 receptor stably expressed in HEK293 cells (∼5 × 105 cells/incubation) were incubated with [3H]LTB4 (∼1 nM) plus LTB4 (▪) and 18R-HEPE (○), 5,12,18R-triHEPE (•), or LTB5 (□). Results are the mean ± SEM from n = 3. (C) Inhibition of leukocyte trafficking in 6 d dorsal air pouch. TNF-α (100 ng) was injected intrapouch and 100 ng i.v. tail vein of test compound. ATLa (analogue 15(S)-16(parafluoro)-phenoxy-LXA4; reference 16) was used for comparison. Results represent n = 4.
The G protein–coupled receptor for LTB4 was identified 28, and to determine whether these 18R-containing products interact with the human LTB4 receptors to block PMNs, this receptor was cloned 11 from reported sequences 28 and stably expressed in HEK293 cells for competition binding experiments (Fig. 3 B). The homoligand LTB4 effectively competed (IC50 ≈ 2.5 nM). 18R-HEPE did not, while both LTB5 and 5,12,18R-triHEPE competed (IC50 ∼0.5 μM), giving a trend with LTB5 > 5,12,18R-triHEPE. Although the 5,12,18R-triHEPE and a related structure (i.e., LTB5) were substantially less effective than 4-series LTB4, consistent with the reduced PMN activity of LTB5 24, their potency for displacing [3H]LTB4 was in the range of currently available synthetic LTB4 receptor antagonists (not shown). These findings suggest that 5,12,18R-triHEPE might serve as a damper for LT-mediated responses in vivo if generated in appropriate quantities within the microenvironment as well as a biotemplate for total synthesis of new classes of receptor antagonists. When administered intravenously into tail at low levels (100 ng), 5,12,18R-triHEPE was a potent inhibitor of PMN infiltration into murine dorsal air pouches (Fig. 3 C), as was a 15-epi-LX stable analogue 16 given at equivalent doses for the purpose of direct comparison. 18R-HEPE also carried some activity in vivo (<5,12,18R-triHEPE), whereas it was far less effective with isolated human PMNs in transendothelial migration and apparently did not interact with recombinant LTB4 receptors at these concentrations.
Other widely used NSAIDs (i.e., acetaminophen and indomethacin) were also tested with recombinant COX-2 and C20:5 as in Table to determine whether they altered conversion to HEPE. Each inhibited 11-HEPE by >95%. Interestingly, 18R-HEPE and 15R-HEPE formation persisted (∼1:1 ratio) in the presence of either acetaminophen or indomethacin at concentrations as high as 2 mM, even though the levels of 15R- and 18R-HEPE were reduced by three to eight times their levels in the absence of inhibitors (n = 3). These findings indicate that the oxygenation of ω-3 fatty acids to R-containing monohydro(peroxy)-containing products is not restricted to ASA treatment and arachidonate. Hence, these commonly used NSAIDs and selective COX-2 inhibitors (Table ) may still permit PUFA oxygenation by activated ECs exposed to NSAIDs (Fig. 2) and at sites of inflammation where the degree of COX-2 interactions with drugs may be “leaky,” permitting novel oxygenation of PUFAs.
Despite the many reports of ω-3 PUFAs (i.e., C20:5) possible beneficial impact in humans 1 2 3 4 5 6, oxygenation by COX-2 to generate novel bioactive compounds has not been addressed in humans or isolated cells. In fish, both C20:5 and C20:4 are mobilized in macrophages and platelets to produce 5- and 4-series eicosanoids, including PG, LT, and LX, with essentially equal abundance 26. Our results indicate that inflammatory exudates from mice treated with ASA and EPA generate novel compounds (Fig. 1) that are also produced by human ECs, recombinant COX-2, and PMNs (Fig. 2). Given the milligram to gram amounts of ω-3 PUFA taken as dietary supplements 1 2 3 4 5 6 and the large area of microvasculature that can carry upregulated COX-2, the conversion of EPA by ECs and neighboring cells as observed in our experiments (Fig. 1 Fig. 2 Fig. 3 Fig. 4) may represent a significant amount at local microenvironments. These COX-2–NSAID-dependent conversions of ω-3 PUFA are likely to be elevated within inflamed or diseased tissues where COX-2 is upregulated and a determinant that impacts fatty acid metabolism 14 when NSAIDs might be of therapeutic benefit, namely with microinflammation.
Figure 4.
Proposed scheme for generating functional arrays of lipid signals from ω-3 PUFA via transcellular processing: endogenous inhibitors of microinflammation. At sites where COX-2 is upregulated and treated with NSAIDs, PG formation from C20:4 is blocked. Systemic ω-3 PUFAs are converted via a COX-2–NSAID lipoxygenase–type mechanism that abstracts hydrogen in a stereospecific fashion at C16 or C13 in C20:5 to give R insertions of molecular O2 to yield 15R-H(p)EPE or 18R-H(p)EPE to form epoxides or are reduced to alcohols. The complete stereochemistry for each of the trihydroxy compounds remains to be determined, and the compounds are depicted in their likely configurations. These compounds interact with cells in the local microenvironment, inhibiting PMN recruitment. COX-2–NSAID-dependent hydrogen abstraction and insertion of molecular oxygen occurs with all ω-3 PUFAs containing 1,4-cis pentadiene units tested (see text for details). Thus, sequential oxygenations of ω-3 PUFA during cell–cell interactions generate novel arrays of signals of interest in microinflammation.
Analogous to 15-epi-LX biosynthesis, EPA COX-2–derived 15R-HEPE was converted by 5-lipoxygenation with 5(6)-epoxide formation in leukocytes to give the 15-epi-LX5 series (Fig. 4). The stable analogues of 15-epi-LXA4, modified at their C15 position through position 20 with bulky groups, resist inactivating enzymes and are more potent in vivo, inhibiting PMN traffic as well as formation and actions of key proinflammatory cytokines 10 16. Hence, 5-series 15-epi-LXs should act in a similar fashion, as they possess a Δ17–18 double bond and thus could function as an ω-3–derived 15-epi-LX analogue.
Because COX-2–NSAID-dependent oxygenation (e.g., 18R and15R) led to bioactive compounds in vivo that block PMN transendothelial migration and infiltration, our findings provide a basis for a novel mechanism of action of NSAIDs and dietary ω-3 supplementation, namely the generation of endogenous functional arrays of lipid signals (Table and Fig. 1 and Fig. 2) that could, by dampening key events in microinflammation, mediate some of the beneficial actions noted for ω-3 therapies in human trials 1 2 3 4 5 6. In this context, 13-HODE, a recognized lipoxygenase product that downregulates platelet–EC interactions 29, is also generated by COX-2 (Table ) and joins this pathway array and class of mediators. As inappropriate inflammatory responses are now recognized as contributors to cardiovascular disease 30 as well as in clinical syndromes where PMN-mediated tissue injury is important 11, our identification of these novel ω-3 PUFA processing pathways evoked by cell–cell interactions and the unexpected impact of NSAIDs opens new avenues for considering the potential clinical protection provided by ω-3 PUFA–based supplementation (as from reference 6) and mechanisms to generate potent local endogenous mediators that control microinflammation (Fig. 3 and Fig. 4). Moreover, our findings offer a basis to circumvent unwanted effects of current antiinflammatory therapies as well as potential biochemical indices and/or markers of effective dietary ω-3 supplementation.
Acknowledgments
We thank Mary Halm Small for assistance in manuscript preparation and Rachel Cochran for technical assistance.
This work was supported in part by National Institutes of Health grants GM38765 (to C.N. Serhan), HL60569 (to S.P. Colgan), and P01-DE13499 (to C.N. Serhan). C. Clish is the McDuffie Postdoctoral Fellow of the Arthritis Foundation, and K. Gronert is a recipient of a National Research Service Award (F32-AI10389).
Footnotes
Presented in part at the 11th International Conference on Advances in Prostaglandin and Leukotriene Research, plenary session June 5, 2000, Florence, Italy.
References
- De Caterina R., Endres S., Kristensen S.D., Schmidt E.B. n-3 Fatty Acids and Vascular Disease 1993. Springer-Verlag; London: pp. 166 [Google Scholar]
- Lands W.E.M. Proc. AOCS Short Course on Polyunsaturated Fatty Acids and Eicosanoids 1987. American Oil Chemists' Society; Champaign, IL: pp. 574 [Google Scholar]
- Iigo M., Nakagawa T., Ishikawa C., Iwahori Y., Asamoto M., Yazawa K., Araki E., Tsuda H. Inhibitory effects of docosahexaenoic acid on colon carcinoma 26 metastasis to the lung. Br. J. Cancer. 1997;75:650–655. doi: 10.1038/bjc.1997.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billman G.E., Kang J.X., Leaf A. Prevention of sudden cardiac death by dietary pure ω-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–2457. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P., Leaf A., Salem N., Jr. Workshop on the Essentiality of and Recommended Dietary Intakes for Omega-6 and Omega-3 Fatty Acids. J. Am. Coll. Nutr. 1999;18:487–489. doi: 10.1080/07315724.1999.10718888. [DOI] [PubMed] [Google Scholar]
- Marchioli R. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarctionresults of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- Weissmann G. Aspirin. Sci. Am. 1991;264:84–90. doi: 10.1038/scientificamerican0191-84. [DOI] [PubMed] [Google Scholar]
- Marcus A.J. Plateletstheir role in hemostasis, thrombosis, and inflammation. In: Gallin J.I., Snyderman R., editors. InflammationBasic Principles and Clinical Correlates. Lippincott Williams & Wilkins; Philadelphia: 1999. pp. 77–95. [Google Scholar]
- Clària J., Serhan C.N. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc. Natl. Acad. Sci. USA. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C.N., Maddox J.F., Petasis N.A., Akritopoulou-Zanze I., Papayianni A., Brady H.R., Colgan S.P., Madara J.L. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- Chiang N., Gronert K., Clish C.B., O'Brien J.A., Freeman M.W., Serhan C.N. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J. Clin. Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H.R. Recent progress in the cellular and molecular biology of prostaglandin synthesis. Trends Cardiovasc. Med. 1998;8:145–150. doi: 10.1016/S1050-1738(98)00004-8. [DOI] [PubMed] [Google Scholar]
- Needleman P., Isakson P.C. The discovery and function of COX-2 J. Rheumatol. 24Suppl. 491997. 6 8 [PubMed] [Google Scholar]
- Chiang N., Takano T., Clish C.B., Petasis N.A., Tai H.-H., Serhan C.N. Aspirin-triggered 15-epi-lipoxin A4 (ATL) generation by human leukocytes and murine peritonitis exudatesdevelopment of a specific 15-epi-LXA4 ELISA. J. Pharmacol. Exp. Ther. 1998;287:779–790. [PubMed] [Google Scholar]
- Xiao G., Tsai A.-L., Palmer G., Boyar W.C., Marshall P.J., Kulmacz R.J. Analysis of hydroperoxide-induced tyrosyl radicals and lipoxygenase activity in aspirin-treated human prostaglandin H synthase-2. Biochemistry. 1997;36:1836–1845. doi: 10.1021/bi962476u. [DOI] [PubMed] [Google Scholar]
- Clish C.B., O'Brien J.A., Gronert K., Stahl G.L., Petasis N.A., Serhan C.N. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc. Natl. Acad. Sci. USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D.C., Liao J.K. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethi S., Eastman A.Y., Eaton J.W. Inhibition of phagocyte-endothelium interactions by oxidized fatty acidsa natural anti-inflammatory mechanism? J. Lab. Clin. Med. 1996;128:27–38. doi: 10.1016/s0022-2143(96)90111-0. [DOI] [PubMed] [Google Scholar]
- Levy G.N. Prostaglandin H synthases, nonsteroidal anti-inflammatory drugs, and colon cancer. FASEB J. 1997;11:234–247. [PubMed] [Google Scholar]
- Gronert K., Clish C.B., Romano M., Serhan C.N. Transcellular regulation of eicosanoid biosynthesis. In: Lianos E.A., editor. Eicosanoid Protocols. Humana Press; Totowa, NJ: 1999. pp. 119–144. [DOI] [PubMed] [Google Scholar]
- George H.J., Van Dyk D.E., Straney R.A., Trzaskos J.M., Copeland R.A. Expression purification and characterization of recombinant human inducible prostaglandin G/H synthase from baculovirus-infected insect cells. Protein Expr. Purif. 1996;7:19–26. doi: 10.1006/prep.1996.0003. [DOI] [PubMed] [Google Scholar]
- Capdevila J.H., Wei S., Helvig C., Falck J.R., Belosludtsev Y., Truan G., Graham-Lorence S.E., Peterson J.A. The highly stereoselective oxidation of polyunsaturated fatty acids by cytochrome P450BM-3. J. Biol. Chem. 1996;271:22663–22671. doi: 10.1074/jbc.271.37.22663. [DOI] [PubMed] [Google Scholar]
- Ruettinger R.T., Fulco A.J. Epoxidation of unsaturated fatty acids by a soluble cytochrome P-450-dependent system from Bacillus megaterium . J. Biol. Chem. 1981;256:5728–5734. [PubMed] [Google Scholar]
- Lee T.H., Menica-Huerta J.M., Shih C., Corey E.J., Lewis R.A., Austen K.F. Characterization and biologic properties of 5,12-dihydroxy derivatives of eicosapentaenoic acid, including leukotriene B5 and the double lipoxygenase product. J. Biol. Chem. 1984;259:2383–2389. [PubMed] [Google Scholar]
- Serhan C.N., Wong P.Y., Samuelsson B. Nomenclature of lipoxins and related compounds derived from arachidonic acid and eicosapentaenoic acid. Prostaglandins. 1987;34:201–204. doi: 10.1016/0090-6980(87)90243-7. [DOI] [PubMed] [Google Scholar]
- Hill D.J., Griffiths D.H., Rowley A.F. Trout thrombocytes contain 12- but not 5-lipoxygenase activity. Biochim. Biophys. Acta. 1999;1437:63–70. doi: 10.1016/s1388-1981(98)00007-9. [DOI] [PubMed] [Google Scholar]
- Cronstein B.N., Kimmel S.C., Levin R.I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroidsthe glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. USA. 1992;89:9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomizo T., Izumi T., Chang K., Takuwa T., Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- Buchanan M.R., Horsewood P., Brister S.J. Regulation of endothelial cell and platelet receptor-ligand binding by the 12- and 15-lipoxygenase monohydroxides, 12-, 15-HETE and 13-HODE. Prostaglandins Leukot. Essent. Fatty Acids. 1998;58:339–346. doi: 10.1016/s0952-3278(98)90069-2. [DOI] [PubMed] [Google Scholar]
- Ridker P.M., Cushman M., Stampfer M.J., Tracy R.P., Hennekens C.H. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N. Engl. J. Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]