Abstract
Therapeutic efficacy of a tumor cell–based vaccine against experimental B16 melanoma requires the disruption of either of two immunoregulatory mechanisms that control autoreactive T cell responses: the cytotoxic T lymphocyte–associated antigen (CTLA)-4 pathway or the CD25+ regulatory T (Treg) cells. Combination of CTLA-4 blockade and depletion of CD25+ Treg cells results in maximal tumor rejection. Efficacy of the antitumor therapy correlates with the extent of autoimmune skin depigmentation as well as with the frequency of tyrosinase-related protein 2180–188–specific CTLs detected in the periphery. Furthermore, tumor rejection is dependent on the CD8+ T cell subset. Our data demonstrate that the CTL response against melanoma antigens is an important component of the therapeutic antitumor response and that the reactivity of these CTLs can be augmented through interference with immunoregulatory mechanisms. The synergism in the effects of CTLA-4 blockade and depletion of CD25+ Treg cells indicates that CD25+ Treg cells and CTLA-4 signaling represent two alternative pathways for suppression of autoreactive T cell immunity. Simultaneous intervention with both regulatory mechanisms is therefore a promising concept for the induction of therapeutic antitumor immunity.
Keywords: immunotherapy, melanoma, tolerance, TRP-2, depigmentation
Introduction
T cell–mediated immunotherapy represents a promising treatment for human malignancies. In cancer patients, the absence of efficient tumor-specific immunity can be related to inadequate APC function or to T cell tolerance/ignorance towards tumor antigens 1. Nevertheless, extensive studies of melanoma patients revealed that CTLs against a number of melanocyte/melanoma-specific antigens (e.g., gp100, MART-1, and tyrosinase-related protein [TRP]-2) can be retrieved from the blood of such patients 2 3 4. Initiation of T cell responses requires stimulation of the T cell receptor via its peptide/MHC ligand on the APCs, as well as costimulatory signals 5. The major costimulatory molecule on T cells is generally thought to be CD28. CTL–associated antigen (CTLA)-4, which shares its ligands (CD80 and CD86) with CD28, downregulates T cell responsiveness, thereby raising the threshold for T cell activation 6 7 8. By this mechanism CTLA-4 signaling can, amongst others, prevent the initiation of autoreactive T cell responses.
Besides CTLA-4, CD4+ T cells have been associated with suppression of T cell responses. North et al. had already demonstrated that CD4+ T cells from tumor-bearing mice were capable of inhibiting the effect of adoptive T cell therapy 9. Also, recent reports have shown that several subsets of regulatory T (Treg) cells are involved in controlling autoreactive lymphocytes. In particular, Sakaguchi and colleagues 10 showed that Treg cells with strong suppressive capacities are defined by the expression of the CD25 marker (the IL-2R α chain). Elimination of CD25+ T cells results in the development of autoimmune diseases in rodents, whereas administration of such Treg cells prevents development of the autoimmune disease 10 11 12 13. In vitro, CD4+CD25+ T cells are nonproliferative to antigenic stimulation, but strongly inhibit the activation of other CD4+ or CD8+ T cells 14 15 16 17. The mode of action of these Treg cells is currently under intensive investigation.
We have shown previously that disruption of negative regulatory mechanisms, through blockade of CTLA-4, can unleash therapeutic T cell immunity against the poorly immunogenic melanoma B16-BL6 18. Effective treatment of B16-challenged mice was achieved through administration of a combination of a GM-CSF–producing tumor cell vaccine and CTLA-4–blocking antibodies. Tumor rejection was accompanied by skin depigmentation, suggesting that autoreactive immune responses are involved in this process. Furthermore, therapeutic efficacy in this setting was found to depend on the CD8+, but not the CD4+ T cell subset, making this experimental system a highly relevant model for CTL-mediated immunotherapy of human melanoma.
In this study we show that the therapeutic efficacy of the B16-GM-CSF tumor cell vaccine was equally potentiated if combined with prior in vivo depletion of CD25+ T cells, instead of CTLA-4 blockade. Moreover, combination of CD25 depletion and CTLA-4 blockade resulted in an even more potent effect of the antitumor treatment. Increased efficacy of treatment was associated with increased frequencies of CTLs specific for the melanocyte/melanoma differentiation antigen TRP-2, as well as by more profound skin depigmentation. In the absence of CD25+ Treg cells, CTLA-4 blockade enhanced the induction of effector CTLs in vitro as well as in vivo. Our data argue that CTLA-4 blockade and CD25+ T cell depletion affect alternative regulatory mechanisms. The combination of these vaccination strategies strongly enhances autoreactive cellular immunity leading to effective immunotherapy of cancer.
Materials and Methods
Cell Lines and Mice.
B16-BL6 (obtained from I Fidler, MD Anderson Cancer Center, Houston, TX), GM-CSF–producing B16 cell lines BL6/GM-E, BL6/GM18 18, B16/B7.1 18, 9H10 19, and PC61 (American Type Culture Collection) were cultured in Iscove's IMDM (BioWhittaker) supplemented with 1 U/ml penicillin, 1 μg/ml streptomycin, 2 μM l-glutamine, 20 μM β-mercaptoethanol (complete medium), and 4% FCS. GM-CSF production by BL6/GME and BL6/GM18 was confirmed in vitro during the course of the vaccination experiments. T cells were cultured in complete medium supplemented with 8% FCS and 10 Cetus U IL-2 per milliliter.
C57BL/6 female mice were obtained from IFFA Credo, C57BL/6 Nude (nu/nu) female mice were obtained from Bomholtgard and were maintained and treated in accordance with the national guidelines for animal care. Mice were treated and used for tumor experiments when 8–12 wk old.
Antibodies and Peptides.
Anti–murine CTLA-4 antibody 9H10 (hamster) and anti–murine CD25 antibody PC61 (rat) were purified using a protein G agarose column (Roche). For depletions, GK1.5 (anti-CD4), 2.43 (anti-CD8) were purified in our laboratory from hybridoma culture supernatant using protein free hybridoma medium (Life Technologies) and a capillary dialyzer (Fresenius Medical Care, St. Wendel, Germany). Rat IgG was obtained from Sigma-Aldrich. Anti–CD4-APC, anti–CD8b-FITC, anti–CD8a-APC, and anti–CD25-FITC (7D4) were obtained from BD PharMingen and were used to confirm depletions of the relevant cell populations, anti–CD25-PE (PC61) was obtained from Beckman Coulter. We tested GM-CSF production by lines GME and GM18 by an ELISA using commercially available antibodies to murine GM-CSF (BD PharMingen). H-2Kb tetramers were produced as described previously 20 21 and stored frozen in Tris-buffered saline/16% glycerol/0.5% BSA. Peptides were produced by using standard F-moc chemistry. The purity was checked by HPLC and fractions were confirmed to contain >95% of the expected sequence.
Treatment of B16-BL6 Tumors.
Mice were challenged subcutaneously on the right flank with 2.5 × 103 (or the amount indicated) B16-BL6 tumor cells in 200 μl PBS-0.1% BSA. At the same day, treatment was initiated by injecting 106 irradiated (16,000 rad) GM-CSF–producing B16 cells (clones GME and GM18 in a 1:1 mixture) subcutaneously into the left flank, and repeated 3 and 6 d later. Simultaneously with the cellular vaccinations, the mice were injected (on days 0, 3, and 6) intraperitoneally with 200 μg anti–CTLA-4 antibody 19 in 200 μl PBS. Tumor growth was scored by measuring perpendicular diameters. Mice were killed when the tumors displayed ulceration or reached a size of 150 mm2. Depletion of CD4+ or CD8+ T cells was accomplished by injection of the relevant antibodies (100 μg intraperitoneally) on days −1, 0, 7, 14, and 21 (day of tumor challenge is referred to as day 0). Depletions of CD25+ T cells were performed by intraperitoneal injection of 400 μg PC61 as described previously 22 at day −4. Depletions were confirmed by flow cytometry using noncrossreactive antibodies. Statistical analysis was performed using SPSS data analysis software (SPSS Inc.).
Flow Cytometry and IFN-γ Release Assays.
Analysis of cell surface markers on blood lymphocytes was performed using a FACScalibur™ (Becton Dickinson) and CELLQuest™ software. Blood (50–100 μl) was collected in heparin-coated tubes and erythrocytes were lysed using standard laboratory protocols. The remaining lymphocytes were washed and incubated with the indicated antibodies/tetramers in PBS/1%BSA for 20 min at room temperature. After two washes with PBS, FACS® analysis was performed with the cells in PBS/1%BSA/1 μg/ml propidium iodide. Propidium iodide-negative cells were gated for the lymphocyte population and analyzed. For intracellular IFN-γ cytokine staining, vaccine draining lymph nodes were isolated and single cell suspensions were stimulated for 5 h with TRP-2180–188 peptide or irrelevant (E1A234–243) peptide at a concentration of 5 μg/ml in medium containing Brefeldin A. Cells were then fixed in paraformaldehyde and incubated with PE-conjugated anti–IFN-γ and APC-conjugated anti-CD8 (BD PharMingen). The samples were analyzed on a FACScalibur™. For IFN-γ release assays, splenocytes were restimulated in vitro with 105 irradiated (16,000 rad) B16/B7.1 per 106 splenocytes in IL-2 (10 Cetus U/ml) supplemented culture medium. After 7 d, splenocytes were collected and dead cells removed after a Ficol-Hypaque gradient. Splenocytes (105 cells per well) were stimulated with target cells (2 ×104 cells per well) in 96-well round-bottomed plate. After 24 h, supernatant was tested for the presence of IFN-γ by sandwich ELISA (BD PharMingen). As target cells we used peptide-pulsed naive irradiated splenocytes. For peptide pulsing, we incubated 106 naive splenocytes for 1 h in culture medium containing 25 μg/ml peptide. Unbound peptide was washed away by three washes in culture medium. Specific IFN-γ release was calculated as: IFN-γ release in response to TRP-2180–188 loaded splenocytes − IFN-γ release in response to E1A234–243 loaded splenocytes.
In Vitro Priming Assay.
CD25-depleted naive splenocytes were loaded with TRP-2180–188 peptide, irradiated, and used as stimulatory cells in an in vitro priming assay. Per well of a 24-well plate, 106 CD25-depleted naive splenocytes were incubated with 5 × 105 stimulatory cells in complete medium containing 10 Cetus U of IL-2 per milliliter (and, if indicated, 50 μg/ml CTLA-4–blocking antibody [9H10]). After 3 d, cells were isolated by Ficoll flotation and 4 d later analyzed for specific IFN-γ release in response to TRP-2 peptide or irrelevant peptide. Specific IFN-γ release was calculated as described previously.
Depigmentation Studies.
Mice were scored for skin depigmentation >100 d after vaccination. Depigmentation was scored according to the presence of depigmented areas on the vaccination site (1 point), tumor site (1 point), or elsewhere (1 point), rendering scores from 0 to 3. Depigmented areas were scored positive when >25 mm2 and containing >50% depigmented hairs. Scoring was performed in a blinded fashion.
Results
Depletion of CD4+ or CD25+ T Cells Enhances Efficacy of Antitumor Treatment.
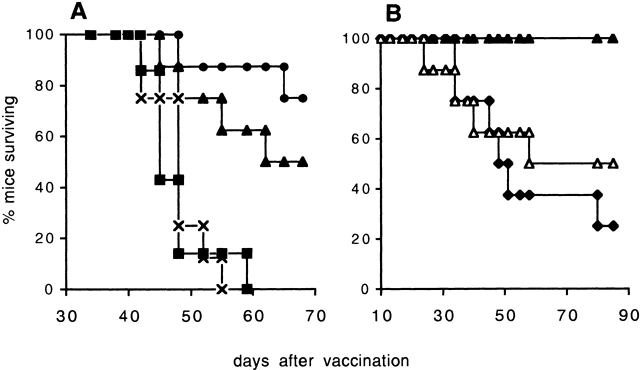
B16-BL6, which was originally selected for its invasiveness 23, is a poorly immunogenic but highly tumorigenic murine melanoma cell line. Although prophylactic vaccination can generally prevent B16 tumor outgrowth 24 25 26 27 28, vaccination after tumor challenge is less effective. We have shown previously 18 that the combination of a GM-CSF–producing tumor cell vaccine and CTLA-4–blocking antibodies can be exploited successfully to treat B16-BL6 tumors. We demonstrated that antitumor treatment is accompanied by autoimmune skin depigmentation and that the mechanism of the tumor rejection is dependent on CD8+ CTLs, whereas CD4+ T cells are not required. Interestingly, we consistently observed a significant increase in the therapeutic efficacy of the vaccination if the mice were depleted of their CD4+ T cells (Fig. 1). In accordance with this, Nagai et al. 29 recently reported enhanced tumor rejection and depigmentation after CD4+ T cell depletion in a study using IL-12–producing B16 cells. These results suggest a possible inhibitory role for CD4+ Treg cells in the control of autoreactive CTLs. Recent reports 16 30 have associated the surface marker CD25 with Treg cells. Since the antitumor response in our model involves CTL-dependent autoimmune depigmentation, we investigated whether CD25+ Treg cells were responsible for limiting the magnitude and/or efficacy of the (autoreactive) immune response in this therapeutic setting. CD4+CD25+ T cells make up ∼5% of the total peripheral CD4+ T cell population in naive C57BL/6 mice (Fig. 2 A). Mice that received a single dose of 0.4 mg of CD25-depleting antibody showed approximately a tenfold reduction in the CD4+CD25+ T cell population (Fig. 2 B), comparable to what was previously reported by others 22. CD25 depletion before tumor challenge resulted in a small but reproducible delay in tumor outgrowth compared with naive mice (Fig. 3 A), indicating that this intervention improved the antitumor immune response of these mice even in the absence of vaccination. Combination of the GM-CSF–producing vaccine with CD25+ T cell depletion was equally effective in preventing tumor outgrowth as combination of the vaccine with CTLA-4 blockade (Fig. 3 A). This indicated that both of these immunomodulation strategies improved the efficacy of the GM-CSF–producing vaccine. Importantly, combination of this vaccine with both CTLA-4 blockade and CD25 depletion resulted in a further increase of treatment efficacy in that all mice were capable of rejecting the B16 tumor (Fig. 3 A).
Figure 1.
CD4+ T cell depletion before vaccination enhances efficacy of B16 treatment. Survival data of mice challenged subcutaneously (day 0) with 5 × 103 B16-BL6 tumor cells. Mice received either no treatment (n = 25, ♦) or B16-GM-CSF tumor cell vaccine in combination with anti–CTLA-4–blocking Ab on days 0, 3, and 6. The vaccinated mice were divided over three groups that received the following Ab on days −1, 0, 7, and 14: depleting anti-CD8 Ab (n = 25, □), depleting anti-CD4 Ab (n = 25, ×), or isotype matched control Ab (n = 25, •). Details on experimental procedure and Ab are described in Materials and Methods. The graph indicates the percentage of surviving mice over time. Significant (P = 0.04, log rank test) difference was found between B16-GM-CSF and anti–CTLA-4–treated mice injected with control antibody (•) and mice injected with CD4-depleting Ab (×).
Figure 2.
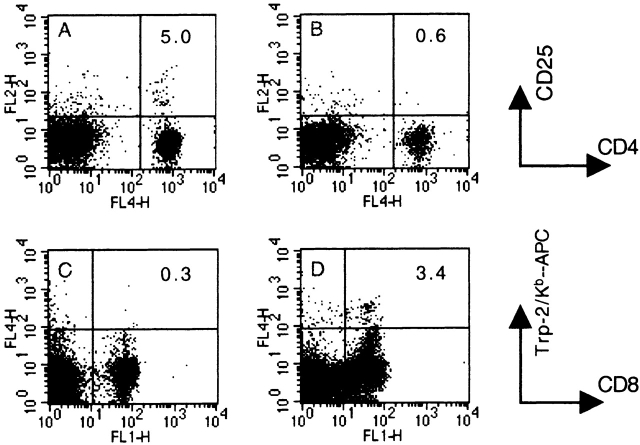
Characterization of blood lymphocytes in CD25-depleted C57BL/6 mice. (A and B) Blood lymphocytes from a naive (A) or a CD25-depleted (B) mouse were stained 21 d after CD25 depletion, with APC-conjugated anti-CD4 and PE-conjugated anti-CD25. Numbers in the upper right quadrant indicate the percentage of CD25+/CD4+ T cells of total CD4+ T cells. (C and D) Blood lymphocytes from a naive (C) or a CD25-depleted/B16-GM-CSF/anti–CTLA-4 vaccinated mouse (D) were stained on day 17 after vaccination for the presence of TRP-2180-188–specific CD8+ T cells using an APC-conjugated TRP-2180–188 Kb-tetramer and anti–CD8-FITC. Numbers in the upper right quadrant indicate the percentage of TRP-2180–188–specific CD8+ T cells of total CD8+ T cells. Representative stainings of three independent experiments are shown.
Figure 3.
CD25+ T cell depletion before vaccination enhances efficacy of treatment. (A) Survival data of mice challenged subcutaneously with 2.5 × 103 B16-BL6 tumor cells. Mice received either no treatment (n = 6, ▪), or depleting anti-CD25 on day −4 (n = 6, ⋄) or vaccination with GM-CSF–producing B16 on days 0, 3, and 6. The vaccinated mice were divided over three groups that received the following Ab: CTLA-4 blocking Ab on days 0, 3, and 6 (n = 8, •); depleting anti-CD25 Ab on day −4 (n = 8, ×); or depleting anti-CD25 Ab on day −4 plus CTLA-4–blocking Ab on days 0, 3, and 6 (n = 8, ▴). (B) Survival data of mice challenged subsutaneously (day 0) with 5 × 103 B16-BL6 tumor cells. Mice received either depleting anti-CD25 Ab on day −4 (n = 6, ⋄) or were vaccinated on days 0, 3, and 6 with GM-CSF–producing B16. The vaccinated mice were divided over two groups that received the following Ab: blocking anti–CTLA-4 Ab on days 0, 3, and 6 (n = 9, •); or depleting anti-CD25 Ab on day −4 combined with blocking anti-CTLA-4 Ab on days 0, 3, and 6 (n = 9, ▴). See Materials and Methods for details. Significant (A, P = 0.025; B, P = 0.0004, log rank test) differences were found between B16-GM-CSF vaccinated mice that received either anti–CTLA-4 Ab (•) or were injected with anti-CD25 Ab plus anti-CTLA-4 Ab (▴). Representative results from two independent experiments are shown.
To more extensively probe the potency of CD25+ T cell depletion in antitumor treatment, mice were challenged with the double number of highly malignant B16-BL6 tumor cells (5 × 103 cells) compared with the experiments described in Fig. 1 and Fig. 3 A. In this particular setting, mice receiving the combination of GM-CSF–producing B16 vaccine plus CTLA-4 blockade experienced a delay in tumor outgrowth but failed to reject the tumor, due to more rapid tumor outgrowth (Fig. 3 B). Interestingly, when the GM-CSF–producing B16 vaccine plus CTLA-4 blockade was applied to mice that had undergone prior CD25 depletion, most mice did reject their tumor (6/9 survival). This illustrates even more dramatically the strong immunoregulatory action of the CD25+ T cell subset in preventing the development of an effective antitumor immunity.
The same conclusion pertains to the role of this T cell subset in immune responses causing skin depigmentation. CD25-depleted, vaccinated animals experienced more pronounced and widespread autoimmune skin depigmentation than mice receiving the GM-CSF–producing B16 vaccine plus CTLA-4 blockade alone (Fig. 4). Taken together, our data show that disruption of immunoregulatory processes through CTLA-4 blockade and CD25 depletion can have synergistic effects on antitumor and autoimmune CTL responses in B16-BL6–challenged mice. This suggests that CD25+ Treg cells still exert their function in the presence of CTLA-4 blockade and, thereby, that immunomodulation through CTLA-4 and the CD25+ T cell subset can constitute alternative regulatory mechanisms.
Figure 4.

Depigmentation of mice vaccinated with GM-CSF–producing B16. Groups of mice were untreated (n = 12, ⋄), treated with B16-GM-CSF and anti–CTLA-4 Ab alone (n = 16, ▵) or in combination with prior CD25 depletion (n = 20, ○) as described in Material and Methods. Each marker represents the average depigmentation score for a single mouse from three independent (blinded) scorings. For each group the mean is shown ± SEM. Significant (P < 0.02, Student's t test) difference was found between B16-GM-CSF vaccinated mice that received either anti–CTLA-4 Ab alone (▵) or in combination with anti-CD25 Ab (○).
Enhanced TRP-2180–188–specific CTL Responses in the Absence of CD25+ Treg Cells.
Our experiments show a clear correlation between efficacy of the antitumor response and the extent of autoimmune depigmentation. Recent data have revealed that both processes can be induced by specific vaccination against the melanocyte/melanoma differentiation antigen TRP-2, either with a DNA-based vaccine encoding the entire antigen 31, or with dendritic cells loaded with the H-2Kb–restricted CTL epitope TRP-2180–188 32. Since the therapeutic effect of the vaccination in our experimental system was shown to depend on CD8+ T cells, we tested the level of CTL immunity against the TRP-2180–188 epitope. Analysis of TRP-2180–188 specific CTL response was performed by flow cytometry using Kb/TRP-2180–188 tetramers on blood of tumor-challenged mice. In vaccinated mice, numbers of tetramer+ CD8+ T cells are clearly increased compared with background levels detected in naive mice (Fig. 2C and Fig. D, and Fig. 5 A, no. 1). Also tumor-bearing mice receiving no treatment at all develop an, albeit small, increase in the percentage of TRP-2180–188–specific CD8+ T cells. When tumor-bearing mice were vaccinated using GM-CSF–producing vaccine and CTLA-4 blockade, frequencies of TRP-2180–188–specific CD8+ T cells are considerably augmented compared with nonvaccinated mice. Importantly, these frequencies are increased most prominently (to ∼2% of the CD8+ population) if the treatment with GM-CSF–producing vaccine and CTLA-4 blockade is performed in CD25-depleted animals (Fig. 5 A, no. 4). This demonstrates directly that CD25+ T cells can inhibit the induction of an autoreactive CTL response, even in the presence of CTLA-4 blockade, and correlates with the in vivo tumor rejection data (Fig. 3A and Fig. B). Notably, these data also indicate that in CD25-depleted mice, vaccine-induced TRP-2180–188–specific CD8+ T cell responses require CD4+ T cell help (Fig. 5 A, no. 6), since this response is absent in CD4-depleted mice (see also below).
Figure 5.
Analysis of TRP-2180–188–specific CD8+ T cells in vaccinated mice. (A) Blood lymphocytes were stained on day 17 after tumor challenge for the presence of TRP-2180–188–specific CD8+ T cells using an APC-conjugated TRP-2180–188 Kb-tetramer and anti–CD8-FITC. The percentage of TRP-2180–188–specific CD8+ T cells of total CD8+ T cells is indicated. Treatments of mice were as follows: group 1, naive mice; groups 2–8, B16-BL6 tumor challenge on day 0; groups 3–7, vaccination on days 0, 3, and 6 with GM-CSF–producing B16. If indicated, 200 μg blocking anti–CTLA-4 Ab was injected on days 0, 3, and 6, depleting anti-CD25 Ab (450 μg) was injected at day −4. Significant difference (P = 0.03, Student's t test) was found between mice from groups 3 and 4. Error bars indicate the SD of three measurements from one experiment. One representative experiment of two is shown. (B) Lymph node cells were stained on day 17 after tumor challenge for the presence of TRP-2–specific IFN-γ–producing CD8+ T cells. Results indicate the percentage of TRP-2–specific CD8+ T cells from the total CD8 population calculated as (percentage of TRP-2 responders) − (percentage of E1A responders). A representative result from two experiments is shown.
To analyze the functional activity of the TRP-2–specific CTLs detected by tetramer staining, we measured, directly ex vivo, intracellular IFN-γ production in CD8+ T cells derived from vaccine draining lymph nodes in response to TRP-2 peptide. Fig. 5 B shows that TRP-2–specific IFN-γ production can be detected in CD8+ T cells from mice that received the B16-GM-CSF vaccine in combination with CD25 depletion and CTLA-4 blockade. In the absence of CD25 depletion or CTLA-4 blockade the numbers of IFN-γ–producing CTLs are decreased, similarly as found for tetramer staining. As expected, the percentage of specific CTLs was higher in the vaccine draining lymph nodes than in the blood. We also analyzed IFN-γ release by splenocyte cultures in the presence of the TRP-2180–188 peptide (Fig. 6). The IFN-γ release data showed that TRP-2180–188–specific CTL immunity can be detected in splenocyte cultures from mice that had received treatment with GM-CSF–producing vaccine and CTLA-4 blockade and that this activity is markedly increased in mice that had also undergone CD25 depletion (Fig. 6, no. 4).
Figure 6.
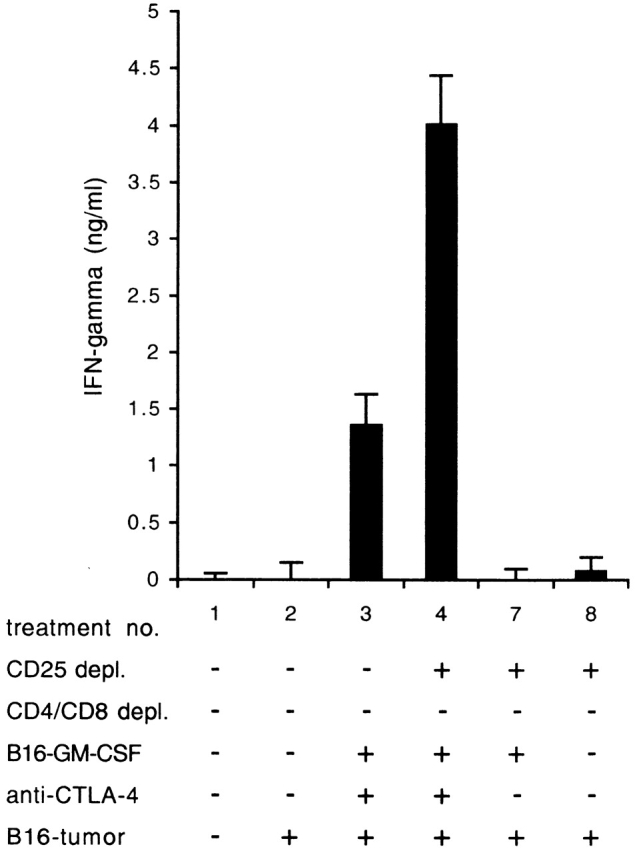
IFN-γ release in response to TRP-2180–188 peptide. Splenocytes from mice were in vitro restimulated with irradiated B16/B7.1 and tested for recognition of TRP-2180–188 peptide-loaded target cells in an IFN-γ release assay 1 wk later. Treatments of mice are described in legend to Fig. 5. Values indicate average from three measurements with SD indicated. One representative experiment of two is shown.
Administration of Anti-CD25 mAb Can Deplete Effector T Cell Populations.
It is well known that CD25, besides being a marker for Treg cells, is an activation marker on lymphocyte subsets 33 34. The depletion of T cells expressing the CD25 marker may therefore not only eliminate Treg cells but also deplete preexisting antigen-experienced T cell populations of both CD4+ and CD8+ lineages. We investigated the latter in an experimental setup in which we established such preexisting populations by preventive vaccination against B16-BL6 tumor challenge. Most mice prevaccinated with B16-GM-CSF in combination with CTLA-4 blockade exhibited tumor-specific memory in that they were capable of rejecting a subsequent tumorigenic dose of B16-BL6 (Fig. 7 A). CD4+ T cell depletion of immunized mice completely abrogated the protective effect of the prophylactic vaccination. This absolute requirement of CD4+ T cells for protective vaccination is in sharp contrast to our findings in the therapeutic setting (Fig. 1), where CD4+ T cell depletion even enhances the efficacy of treatment. Our observations are in line with those of Hung et al. 35, who reported a key role for CD4+ T cells in the vaccination-induced immune response against B16. Importantly, also CD25+ T cell depletion 4 d before tumor challenge, a timing also applied in our therapeutic experiments, negatively affected the capacity of prevaccinated mice to reject B16-BL6. This supports the notion that CD25 depletion can result in the elimination of protective antigen-experienced T cells that were elicited by the vaccine. Since the negative effect of CD25 depletion is less profound than CD4 depletion, it is likely that T cell help provided by CD4+ T cells, that are CD25− at the time of challenge, also plays an important role in the immune defense against B16-BL6.
Figure 7.
Analysis of in vivo anti-CD25 mAb administration. (A) Prophylactic vaccination efficiency is decreased by administration of anti-CD4 mAb or anti-CD25 mAb. Mice received either no treatment (n = 6, ▪) or were vaccinated with B16-GM-CSF and blocking anti-CTLA-4 Ab on days 0, 3, and 6. The vaccinated mice were divided over three groups that received the following Ab in combination with CD4 depletion (n = 8, ×), CD25 depletion (n = 8, ▴), or without depletion (n = 8, •). Administration of depleting mAb was performed on day 17. All mice were challenged with 5 × 103 B16-BL6 tumor cells on day 21. (B) Therapeutic vaccination in CD25-depleted mice is dependent of CD4+ as well as CD8+ T cells. Therapeutic treatment with irradiated GM-CSF–producing B16 cells plus CTLA-4 blockade was performed in mice depleted for CD25+ T cells (n = 8, ▴), in mice depleted for CD25+ T cells and CD8+ T cells (n = 8, ♦), or in mice depleted for CD25+ T cells and CD4+ T cells (n = 8, ▵). Significant (P = 0.025, log rank test) difference was found between CD25 depleted/vaccinated mice (▴) and CD4 plus CD25 depleted/vaccinated mice (▵).
In view of the fact that depletion of the CD25+ T cell subset can indeed result in the elimination of cells that are positively involved in B16 tumor eradication, we reevaluated the importance of the CD4+ and CD8+ T cell subsets in antitumor therapy involving CD25 depletion. We have previously shown that rejection of B16-BL6 after therapeutic vaccination with GM-CSF–producing B16 in combination with CTLA-4 blockade is highly dependent on the CD8+ T cell subset, but not on CD4+ T cells (see Fig. 1 and reference 18). We now demonstrate that in CD25-depleted mice full efficacy of this therapy does not only depend on the action of CD8+ T cells, but also on the availability of help by CD4+CD25− T cells (Fig. 7 B). This result is mirrored by our observation that the number of tetramer-positive CTLs in mice receiving this treatment is decimated if these mice in addition were depleted of their CD4+ T cells (Fig. 5 A, no. 6). It is likely that also a subpopulation of CD4+ T cells positively affects the antitumor response in mice that receive antitumor treatment without CD25 depletion. However, in this setting the inhibitory effect of CD4+CD25+ Treg cells outweighs the beneficial effect of the CD4+CD25− Th cells.
CTLA-4 Blockade Enhances CTL Induction In Vitro and In Vivo.
The synergistic effect of CTLA-4 blockade and CD25 depletion on CTL induction and antitumor response described here could in theory still be caused by differences in biokinetics of the two antibodies used. To prove that CTLA-4 blockade and Treg cell function are indeed alternative pathways for regulation of autoreactive CTL responses, we separated the anti–CTLA-4 and anti-CD25 antibody treatments in space and time and analyzed the effects with regard to CTL induction in vitro as well as in vivo. For this purpose we used CD25-depleted splenocytes. CD25 depletion was accomplished by injection of 450 μg of anti-CD25 antibody on 2 d consecutively and harvesting the splenocytes 3 d later. Flow cytometry analysis with noncrossreactive anti-CD25 (7D4) confirmed >95% depletion of CD25+ T cells. The CD25− splenocytes were then used in an in vitro priming assay to induce in vitro TRP-2180–188–specific CTLs. Fig. 8 A shows that CD25− splenocytes stimulated with TRP-2180–188 peptide and cultured for 1 wk specifically recognize TRP-2180–188–loaded target cells. CTLA-4 blockade during the first 3 d of culturing markedly increased the induction of the TRP-2180–188–specific response. We also used 5 × 107 CD25− splenocytes to reconstitute T cell–deficient C57BL/6 Nude (nu/nu) recipients. The nude recipients were subsequently vaccinated with GM-CSF–producing B16 with or without CTLA-4–blocking antibodies. 3 wk after the last vaccination, the spleens were harvested and the splenocyte cultures were analyzed for the presence of TRP-2180–188–specific CTLs. In T cell cultures from recipients reconstituted with complete splenocytes and vaccinated with or without CTLA-4 blockade no TRP-2180–188–specific reactivity was demonstrable (Fig. 8 B). This is probably due to the decreased T cell numbers in reconstituted nude recipients compared with normal C57BL/6 mice rendering it more difficult to initiate a detectable CTL response. However, when nude mice were reconstituted with CD25− splenocytes, specific IFN-γ release in response to TRP-2180–188 was detected, but only if the GM-CSF–producing B16 vaccine was combined with CTLA-4 blockade. These in vitro and in vivo results show that CTLA-4 blockade acts in the complete absence of CD25+ T cells, probably by directly releasing the brakes on costimulation in responding T cells, including effector CTLs.
Figure 8.
CTLA-4 blockade enhances CTL induction in the absence of CD25+ Treg cells. CD25− splenocytes were used to analyze the effect of CTLA-4 blockade on the induction of effector CTL in vitro (A) and in vivo (B). (A) Naive CD25− splenocytes were, if indicated, stimulated with TRP-2 peptide loaded target cells and anti–CTLA-4 (50 μg/ml) during the first 3 d of culture. At day 7, specific IFN-γ release in response to TRP-2 peptide was measured. (B) C57BL/6 nude recipients were reconstituted with 5 × 107 splenocytes from wild-type C57BL/6 mice on day 0 and vaccinated with GM-CSF–producing B16 cells on days 4, 7, and 10. If indicated, CD25− splenocytes were used to reconstitute the recipients and 200 μg of CTLA-4–blocking Ab was administered on days 4, 7, and 10. 3 wk after the last vaccination splenocytes from mice were in vitro restimulated with irradiated B16/B7.1 and tested for recognition of TRP-2180–188 peptide-loaded target cells in an IFN-γ release assay 1 wk later. Values indicate average from three measurements with SD indicated. Representative results from two independent experiments are shown. Significant difference (A, P < 0.03; B, P < 0.01, Student's t test) was found between treatments no. 3 and 4.
Discussion
The combined results show that the efficacy of therapeutic vaccination against experimental melanoma is markedly increased by interfering with mechanisms that normally keep autoimmune responses in check. Antitumor treatment is strongly improved if vaccination is either accompanied by CTLA-4 blockade or preceded by a depletion of CD25+ Treg cells. These intervention strategies act synergistically, in that combination of CTLA-4 blockade and depletion of CD25+ Treg cells results in maximal efficacy of therapeutic vaccination. The potency of the different treatment modalities for preventing B16 melanoma outgrowth strongly correlates with the extent of autoimmune skin depigmentation in the treated mice as well as with the frequency of TRP-2180–188–specific CTLs detected in the periphery. Furthermore, antitumor protection was abrogated upon depletion of the CD8+ T cell subset. Therefore, our data indicate that the CTL response against melanoma-associated autoantigens is an important component of the therapeutic antitumor immune response, and that this response can be enhanced through interference with immunoregulatory mechanisms. The synergism in the effects of CTLA-4 blockade and depletion of CD25+ Treg cells suggest that these intervention strategies act on different regulatory pathways. Indeed, in the complete absence of CD25+ Treg cells, CTLA-4 blockade markedly augments the CTL response in vitro and in vivo, clearly supporting independent and synergistic activities.
Although the role of CD25+ Treg cells in the suppression of autoimmune responses is by now generally acknowledged, the mechanism by which these cells exert their regulatory effects is still a matter of debate. In particular, it has recently been proposed that CTLA-4 would play an essential role in Treg cell function. This was, amongst others, suggested by the observation by two laboratories that CTLA-4 is constitutively expressed by CD4+CD25+ Treg cells, but not or to a much lower extent by other CD4+ T cell subsets 36 37. Analysis was performed on permeabilized cells, allowing detection of both membrane and cytoplasmic localized CTLA-4. Therefore, although these data do not directly assess whether CD25+ Treg cells constitutively display functional CTLA-4 at their surface, it is clear that in these T cells intracellular supplies of this immunoregulatory molecule are readily available for mobilization to the membrane. The functional importance of the CTLA-4 regulatory signal was demonstrated in a murine model for CD4+ T cell–induced colitis by the fact that the capacity of CD25+ Treg cells to inhibit the pathogenic CD4+ cells was abolished by administration of CTLA-4–blocking Ab 36. However, as acknowledged by the authors, these experiments do not rule out the possibility that CTLA-4 blockade has a direct effect on the pathogenic T cells. In the second study, it is demonstrated that CTLA-4 is critically involved in the suppressive action of CD25+ Treg cells in vitro 37. Further experiments showed that repeated administration of high doses of anti–CTLA-4 Ab induced autoimmune disease in normal Balb/c mice, whereas lower doses of this Ab failed to do so unless combined with high doses of depleting anti-CD25 mAb. Administration of anti-CD25 Ab alone did not induce overt signs of autoimmune disease. Since this treatment results in a strong reduction, but not in a complete elimination of CD4+CD25+ cells, the data were interpreted as showing that CTLA-4 blockade on the remaining CD25+ Treg cells with low dose anti–CTLA-4 Ab suffices to eliminate the remaining suppressive action by these cells. Importantly, these in vivo experiments, similar to those published by Reed and coworkers 36, do not exclude a direct effect of CTLA-4 blockade on the pathogenic effector T cells. Taken together, the available data argue that CTLA-4 is likely to contribute to the action of CD25+ Treg cells, but there is ample evidence that this immunoregulatory molecule also has a direct suppressive effect on the responder T cell population 6 8 38. Moreover, the current study shows that CTLA-4 blockade in the absence of CD25+ Treg cells has a direct effect on the in vitro and in vivo induction of autoreactive effector CTLs (Fig. 8). Accordingly, intervention with immune regulatory mechanisms through both CTLA-4 blockade and elimination of CD25+ Treg cells can result in more profound induction of autoimmune responses than intervention with only one of these mechanisms (Fig. 3).
CD25 is not exclusively expressed by CD4+ Treg cells, but also by antigen-experienced T cells. In particular, activated effector T cells of both CD4+ and CD8+ lineages can be positive for this surface marker 33. This explains why treatment of prevaccinated mice with anti-CD25 Ab diminished the protective antitumor effect of the vaccination (Fig. 7 A) and underscores the importance of proper timing when applying in vivo depletion of CD25+ T cells in combination with vaccination for the immunotherapy of cancer. Even though administration of depleting anti-CD25 Ab greatly enhances the efficacy of antitumor therapy involving vaccination and CTLA-4 blockade, also in this case it results in loss of a valuable T cell subset. This loss is indicated by the fact that the efficacy of antitumor treatment by vaccination and CTLA-4 blockade does not depend on CD4+ T cell help, whereas in CD25-depleted mice additional loss of CD4+ T cell help markedly reduces the efficacy of the therapy (Fig. 7 B) as well as the magnitude of the TRP-2180–188–specific CTL response (Fig. 5 A). Possibly, the CD8+ T cell population in normal mice harbors a CD25+ subset that is activated upon therapeutic vaccination in combination with CTLA-4 blockade, but that is eliminated when mice are treated with anti-CD25 Ab before vaccination. Our observations in this murine melanoma model are reminiscent of recent reports that describe the presence of circulating melanoma/melanocyte antigen-specific CTLs in the blood of healthy human beings, although it should be noted that these latter CTLs appeared to have a naive rather than an antigen-experienced phenotype 39 40. We hypothesize that such preexisting CD8+ CTLs, which may be naive or antigen experienced, are kept in check by CTLA-4– and CD25+ Treg-dependent mechanisms and that it is the activity of these CTL that is unleashed upon blockade of these immunoregulatory mechanisms.
In conclusion, the data presented in this paper reveal that combination of CTLA-4 blockade and elimination of CD25+ Treg cells can result in more effective autoreactive and therapeutic antitumor immunity than when these intervention strategies are applied separately. Our findings support the notion that CD25+ Treg cells and CTLA-4 represent two alternative pathways for suppression of autoreactive T cell immunity, but do not exclude that functional overlap between these pathways exists. Notwithstanding this possible overlap, simultaneous intervention with both regulatory mechanisms appears to be a highly promising strategy for the induction of T cell immunity against tumor-associated autoantigens in the immunotherapy of cancer.
Acknowledgments
The authors wish to thank Drs. S. Zwaveling and A. van Halteren for critically reading the manuscript and Dr. A. Zwinderman for assistance with statistical analysis.
A. van Elsas was a recipient of a postdoctoral fellowship of the Dutch Cancer Society (Nederlandse Kankerbestrijding). This study was supported by the Dutch Cancer Foundation (grant 96-1354).
Footnotes
Abbreviations used in this paper: CTLA, CTL–associated antigen; Treg, regulatory T cell; TRP, tyrosinase-related protein.
References
- Melief C.J., Toes R.E., Medema J.P., van der Burg S.H., Ossendorp F., Offringa R. Strategies for immunotherapy of cancer. Adv. Immunol. 2000;75:235–282. doi: 10.1016/s0065-2776(00)75006-1. [DOI] [PubMed] [Google Scholar]
- Kawakami Y., Rosenberg S.A. T-cell recognition of self peptides as tumor rejection antigens. Immunol. Res. 1996;15:179–190. doi: 10.1007/BF02918248. [DOI] [PubMed] [Google Scholar]
- Bakker A.B., Schreurs M.W., de Boer A.J., Kawakami Y., Rosenberg S.A., Adema G.J., Figdor C.G. Melanocyte lineage-specific antigen gp100 is recognized by melanoma-derived tumor-infiltrating lymphocytes. J. Exp. Med. 1994;179:1005–1009. doi: 10.1084/jem.179.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.F., Appella E., Kawakami Y., Kang X., Rosenberg S.A. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J. Exp. Med. 1996;184:2207–2216. doi: 10.1084/jem.184.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison J.P. CD28-B7 interactions in T-cell activation. Curr. Opin. Immunol. 1994;6:414–419. doi: 10.1016/0952-7915(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Walunas T.L., Lenschow D.J., Bakker C.Y., Linsley P.S., Freeman G.J., Green J.M., Thompson C.B., Bluestone J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- Chambers C.A., Kuhns M.S., Allison J.P. Cytotoxic T lymphocyte antigen-4 (CTLA-4) regulates primary and secondary peptide-specific CD4+ T cell responses. Proc. Natl. Acad. Sci. USA. 1999;96:8603–8608. doi: 10.1073/pnas.96.15.8603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K.M., Chuang E., Griffin M., Khattri R., Hong D.K., Zhang W., Straus D., Samelson L.E., Thompson C.B., Bluestone J.A. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- North R.J., Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1+2− suppressor T cells down-regulate the generation of Ly-1-2+ effector T cells. J. Exp. Med. 1984;159:1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Salomon B., Lenschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., Bluestone J.A. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Stephens L.A., Mason D. CD25 is a marker for CD4+ thymocytes that prevent autoimmune diabetes in rats, but peripheral T cells with this function are found in both CD25+ and CD25− subpopulations. J. Immunol. 2000;165:3105–3110. doi: 10.4049/jimmunol.165.6.3105. [DOI] [PubMed] [Google Scholar]
- Suri-Payer E., Amar A.Z., Thornton A.M., Shevach E.M. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J. Immunol. 1998;160:1212–1218. [PubMed] [Google Scholar]
- Itoh M., Takahashi T., Sakaguchi N., Kuniyasu Y., Shimizu J., Otsuka F., Sakaguchi S. Thymus and autoimmunityproduction of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J. Immunol. 1999;162:5317–5326. [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J. Exp. Med. 1998;188:287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Kuniyasu Y., Toda M., Sakaguchi N., Itoh M., Iwata M., Shimizu J., Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ naturally anergic and suppressive T cellsinduction of autoimmune disease by breaking their anergic/suppressive state. Int. Immunol. 1998;10:1969–1980. doi: 10.1093/intimm/10.12.1969. [DOI] [PubMed] [Google Scholar]
- Thornton A.M., Shevach E.M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- van Elsas A., Hurwitz A.A., Allison J.P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel M.F., Allison J.P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J.D., Moss P.A.H., Goulder P.J.R., Barouch D.H., McHeyzer-Williams M.G., Bell J.I., McMichael A.J., Davis M.M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- Haanen J.B., Toebes M., Cordaro T.A., Wolkers M.C., Kruisbeek A.M., Schumacher T.N. Systemic T cell expansion during localized viral infection. Eur. J. Immunol. 1999;29:1168–1174. doi: 10.1002/(SICI)1521-4141(199904)29:04<1168::AID-IMMU1168>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Onizuka S., Tawara I., Shimizu J., Sakaguchi S., Fujita T., Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- Hart I.R. The selection and characterization of an invasive variant of the B16 melanoma. Am. J. Pathol. 1979;97:587–600. [PMC free article] [PubMed] [Google Scholar]
- Schreurs M.W., de Boer A.J., Figdor C.G., Adema G.J. Genetic vaccination against the melanocyte lineage-specific antigen gp100 induces cytotoxic T lymphocyte-mediated tumor protection. Cancer Res. 1998;58:2509–2514. [PubMed] [Google Scholar]
- DeMatos P., Abdel-Wahab Z., Vervaert C., Hester D., Seigler H. Pulsing of dendritic cells with cell lysates from either B16 melanoma or MCA-106 fibrosarcoma yields equally effective vaccines against B16 tumors in mice. J. Surg. Oncol. 1998;68:79–91. doi: 10.1002/(sici)1096-9098(199806)68:2<79::aid-jso3>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Ashley D.M., Faiola B., Nair S., Hale L.P., Bigner D.D., Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J. Exp. Med. 1997;186:1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arca M.J., Krauss J.C., Strome S.E., Cameron M.J., Chang A.E. Diverse manifestations of tumorigenicity and immunogenicity displayed by the poorly immunogenic B16-BL6 melanoma transduced with cytokine genes. Cancer Immunol. Immunother. 1996;42:237–245. doi: 10.1007/s002620050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuting T., Steitz J., Bruck J., Gambotto A., Steinbrink K., DeLeo A.B., Robbins P., Knop J., Enk A.H. Dendritic cell-based genetic immunization in mice with a recombinant adenovirus encoding murine TRP2 induces effective anti-melanoma immunity. J. Gene Med. 1999;1:400–406. doi: 10.1002/(SICI)1521-2254(199911/12)1:6<400::AID-JGM68>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Nagai H., Hara I., Horikawa T., Oka M., Kamidono S., Ichihashi M. Elimination of CD4+ T cells enhances anti-tumor effect of locally secreted interleukin-12 on B16 mouse melanoma and induces vitiligo-like coat color alteration. J. Invest. Dermatol. 2000;115:1059–1064. doi: 10.1046/j.1523-1747.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Toda M., Asano M., Itoh M., Morse S.S., Sakaguchi N. T cell-mediated maintenance of natural self-toleranceits breakdown as a possible cause of various autoimmune diseases. J. Autoimmun. 1996;9:211–220. doi: 10.1006/jaut.1996.0026. [DOI] [PubMed] [Google Scholar]
- Bowne W.B., Srinivasan R., Wolchok J.D., Hawkins W.G., Blachere N.E., Dyall R., Lewis J.J., Houghton A.N. Coupling and uncoupling of tumor immunity and autoimmunity. J. Exp. Med. 1999;190:1717–1722. doi: 10.1084/jem.190.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreurs M.W., Eggert A.A., de Boer A.J., Vissers J.L., van Hall T., Offringa R., Figdor C.G., Adema G.J. Dendritic cells break tolerance and induce protective immunity against a melanocyte differentiation antigen in an autologous melanoma model. Cancer Res. 2000;60:6995–7001. [PubMed] [Google Scholar]
- Allouche M., Sahraoui Y., Augery-Bourget Y., Perrakis M., Jasmin C., Georgoulias V. Interleukin 2 receptors. Leuk. Res. 1990;14:699–703. doi: 10.1016/0145-2126(90)90097-s. [DOI] [PubMed] [Google Scholar]
- Waldmann T.A., Pastan I.H., Gansow O.A., Junghans R.P. The multichain interleukin-2 receptora target for immunotherapy. Ann. Intern. Med. 1992;116:148–160. doi: 10.7326/0003-4819-116-2-148. [DOI] [PubMed] [Google Scholar]
- Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S., Malmstrom V., Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25 +CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Tagami T., Yamazaki S., Uede T., Shimizu J., Sakaguchi N., Mak T.W., Sakaguchi S. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C.A., Allison J.P. Costimulatory regulation of T cell function. Curr. Opin. Cell Biol. 1999;11:203–210. doi: 10.1016/s0955-0674(99)80027-1. [DOI] [PubMed] [Google Scholar]
- Ogg G.S., Rod Dunbar P., Romero P., Chen J.L., Cerundolo V. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J. Exp. Med. 1998;188:1203–1208. doi: 10.1084/jem.188.6.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet M.J., Valmori D., Dunbar P.R., Speiser D.E., Lienard D., Lejeune F., Fleischhauer K., Cerundolo V., Cerottini J.C., Romero P. High frequencies of naive Melan-A/MART-1-specific CD8+ T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J. Exp. Med. 1999;190:705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]