Abstract
Despite permitting uncontrolled intracellular visceral infection for 8 wk, interferon-γ (IFN-γ) gene knockout (GKO) mice infected with Leishmania donovani proceeded to reduce liver parasite burdens by 50% by week 12. This late-developing IFN-γ–independent antileishmanial mechanism appeared to be dependent largely on endogenous tumor necrosis factor-α (TNF-α): L. donovani infection induced TNF-α mRNA expression in parasitized GKO livers and neutralization of TNF-α reversed control at week 12. 7 d of treatment of infected GKO mice with interleukin-12 (IL-12) readily induced leishmanicidal activity and also partially restored the near-absent tissue granulomatous response, observations that for the first time expand the antimicrobial repertoire of IL-12 to include IFN-γ–independent effects. The action of IL-12 against L. donovani was TNF-α dependent and required the activity of inducible nitric oxide synthase. These results point to the presence of an IFN-γ–independent antimicrobial mechanism, mediated by TNF-α, which remains quiescent until activated late in the course of experimental visceral leishmaniasis. However, as judged by the effect of exogenous IL-12 this quiescent mechanism can readily be induced to rapidly yield enhanced intracellular antimicrobial activity.
In endogenous and exogenous form, IFN-γ plays a critical role in the control of experimental visceral leishmaniasis, an intracellular infection caused by the protozoan, Leishmania donovani (1, 2). A role for IFN-γ–independent antileishmanial mechanisms in the control or resolution of visceral leishmaniasis has been suggested (1) but not yet systematically analyzed in an in vivo environment guaranteed to be IFN-γ free. IFN-γ gene knockout (GKO)1 mice (3) provide such an IFN-γ–free system and have been used in a variety of experimental models (3–8). The results reported here in L. donovani–infected GKO mice point clearly to the presence of a novel IFN-γ–independent antileishmanial mechanism. This mechanism is mediated by endogenous TNF-α and can be induced by exogenous IL-12.
Materials and Methods
Animals.
GKO mice with a disruption of the gene encoding IFN-γ, bred on a BALB/c background, were provided by Dr. T. Stewart of Genentech, Inc. (South San Francisco, CA) (3). Homozygous IFN-γ–disrupted (GKO−/−), wild-type (GKO+/+), or heterozygous (GKO+/−) male and female littermates aged between 2–7 mo were used. Mice were genotyped by genomic PCR analysis of tail DNA using standard conditions. Two sets of primers were added to each reaction: one set spanned a 220-bp sequence of the nondisrupted endogenous gene; the other set was specific for a 375-bp sequence in the neomycin-resistance gene that was part of the foreign DNA disrupting the IFN-γ gene. Supernatants of GKO spleen cell cultures (5 × 106 cells/ml) either unstimulated or stimulated for 24 h with 5 μg/ml Con A (Sigma Chemical Co., St. Louis, MO) contained <50 pg/ml of IFN-γ by ELISA analysis (Endogen, Cambridge, MA). In contrast, culture supernatants from spleen cells of normal 20–30-g female BALB/c mice (Charles River Laboratories, Wilmington, MA) contained >3,000 pg/ml of IFN-γ after 24 h of Con A stimulation (data not shown). Southern blot analysis (9) on BamHI-digested genomic DNA from mice used for breeding indicated that the IFN-γ gene was disrupted as originally described (data not shown) (3).
Visceral Infection.
Mice were infected intravenously via the tail vein with 1.5 × 107 L. donovani amastigotes (one Sudan strain) harvested from infected hamster spleen homogenates (10, 11). The course of infection was assessed by microscopic examination of Giemsa-stained liver imprints. Liver parasite burdens were quantitated as Leishman-Donovan units (LDU) using the number of liver amastigotes per 500 nuclei × liver weight in grams (11). Liver tissue was also fixed in 10% formalin, and paraffin sections were stained with hematoxylin and eosin. These slides were scored for granuloma formation by counting the number of infected foci in 25 consecutive microscopic fields. Each focus was scored as (a) no cellular reaction, (b) a core of fused Kupffer cells with no cellular infiltrate, (c) a developing granuloma with Kupffer cell fusion and some cellular infiltrate, or (d) as a mature granuloma with the fused core surrounded by a well-developed mononuclear cell mantle (11).
RNA Extraction and Qualitative Reverse Transcriptase–PCR.
Approximately 50-mg pieces of liver were homogenized in guanidine isothiocyanate-phenol solution (RNAzol B; Tel-Test, Inc., Friendswood, TX) according to manufacturer's instructions. cDNA was synthesized from ∼2 μg of total RNA by standard methods using oligo(dT)18 and MuMLV reverse transcriptase (RT) (9). cDNA was amplified in a 50-μl reaction volume containing the following: each primer at 2 μM, 200 μM dNTP, 5 μl 10× buffer with magnesium supplied by the enzyme manufacturer (Boehringer Mannheim, Indianapolis, IN), and 1 U of Taq polymerase. For qualitative PCR, the number of cycles (e.g., 34– 40) was varied to optimize yield of product. As a control for equal input cDNA, each sample was amplified for GADPH (12).
Primers.
IFN-γ: sense, G AGG GAA TTC CTA GCT CTG AGA CAA TGA ACG CTA; antisense, TCA AGG ATC CGA ATC AGC AGC GAC TCC T (13). GADPH: sense, GAT GAC ATC AAG GTG GT; antisense, TCT TGC TCA GTG TCC TTG CTG (13). TNF-α: commercially available primers from Stratagene (La Jolla, CA). iNOS: sense, TCA CGC TTG GGT CTT GTT CAC T; antisense, TTG TCT CTG GGT CCT CTG GTC A (14).
Treatment with IL-12.
Groups of 3–6 mice were continuously treated for 1 wk with 1 μg/day of murine rIL-12 (7.8 × 106 U/mg; Genetics Institute, Cambridge, MA) suspended in saline containing 1 mg/ml of BSA (10). Subcutaneous osmotic pumps provided 7 d of continuous treatment with IL-12 or saline/BSA (Alzet model 2001; Alza Corp., Palo Alto, CA). Pumps were implanted 14 d after infection.
Treatment of Mice with anti–TNF-α Antiserum.
0.2 ml of rabbit anti-murine TNF-α antiserum or normal rabbit serum was injected intraperitoneally starting on the indicated day after infection and twice-weekly thereafter (15). The anti–TNF-α antiserum contained 3 mg/ml IgG with neutralizing activity of 5 × 104 U/mg of protein (15).
Inhibition of Inducible Nitric Oxide Synthase.
Aminoguanidine (AG) (Sigma), used as an inducible nitric oxide synthase (iNOS) inhibitor (16), was given to mice as a 1% solution in drinking water (wt/vol) starting 12 h after infection and continued for 4 wk. Water was replaced weekly. At 2 and 4 wk, liver parasite burdens were determined in AG-treated and untreated controls.
Analysis of Data.
Data were subjected to a two-tailed Student's t test. P values of <0.05 were considered significant.
Results
Course of L. donovani Infection in GKO Mice.
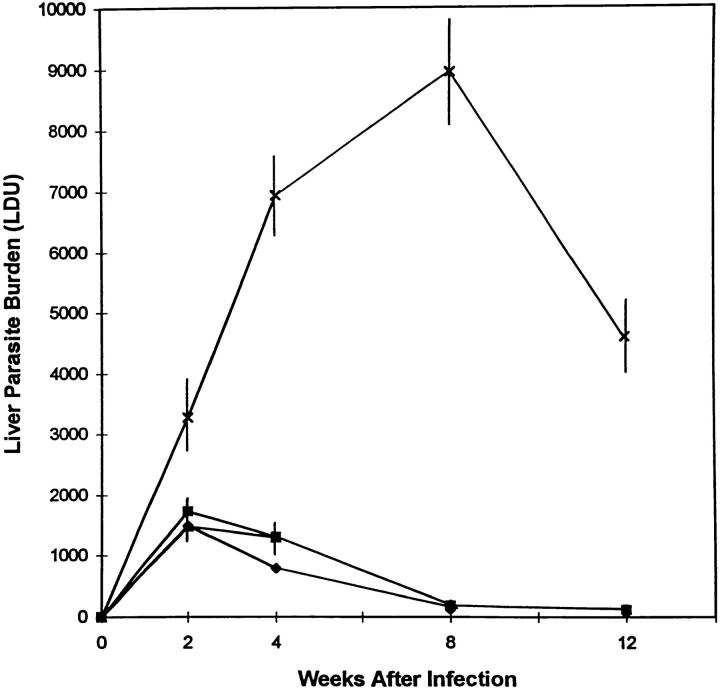
Whereas visceral infection was controlled by week 4 in IFN-γ geneintact littermates (GKO+/+ or GKO+/−) and in normal BALB/c mice, liver parasite burdens increased dramatically in GKO mice during the first 8 wk (Fig. 1). These results confirmed the critical role of endogenous IFN-γ in initial control of L. donovani (1). However, after week 8, parasite burdens in GKO mice decreased significantly, falling to 50% of peak levels by week 12 (P <0.05). The results at week 12 indicated that resolution of visceral leishmaniasis is initiated by a late-acting IFN-γ–independent mechanism. In one experiment, there were sufficient GKO mice to extend limited observations to week 16. In this experiment, mean (± SEM) liver parasite burdens peaked at week 8 (8932 ± 557, n = 3) and declined to 5198 ± 1457 (n = 3) and 4582 ± 60 (mean ± range, n = 2) at weeks 12 and 16, respectively.
Figure 1.
Course of visceral infection in GKO mice. Results represent mean LDU ± SEM from 6–12 animals per timepoint for GKO−/− (X), GKO+/+ (▪), and BALB/c mice (♦); except at week 12 for BALB/c mice (n = 4) and GKO+/− mice (▴, identical to GKO+/+ or BALB/c LDU at all timepoints) (n = 3).
Granulomatous Tissue Response.
Effective control over visceral replication and eventual resistance to L. donovani is expressed in the tissues by the assembly of influxing T cells and monocytes into granulomas at the sites of infection (11). In this model, hepatic granulomas consist of a core of fused parasitized Kupffer cells surrounded by a mononuclear cell mantle comprised of influxing CD4+ and CD8+ cells and monocytes (11, 17). As shown in Table 1, control BALB/c mice displayed the kinetics of granuloma formation already reported in previous studies (11, 17, 18); however, the GKO tissue response in the liver differed considerably.
Table 1.
Liver Histologic Response to L. donovani Infection
| Hepatic infection and cellular response | Weeks after infection | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal BALB/c | GKO | |||||||||||||||
| 2 | 4 | 8 | 12 | 2 | 4 | 8 | 12 | |||||||||
| Liver burden (LDU) | 1,547 | 537 | 143 | 212 | 3,264 | 7,648 | 9,113 | 4,821 | ||||||||
| Infected foci | 128 | 147 | 52 | 70 | 123 | 248 | 265 | 172 | ||||||||
| No cellular reaction (%) | 29 | 1 | 0 | 0 | 80 | 46 | 24 | 22 | ||||||||
| KC fusion alone (%) | 27 | 11 | 2 | 0 | 12 | 35 | 52 | 39 | ||||||||
| KD fusion plus infiltrate (%) | 27 | 33 | 14 | 2 | 5 | 12 | 17 | 21 | ||||||||
| Granulomas (%) | 16 | 56 | 85 | 98 | 3 | 6 | 6 | 18 | ||||||||
Liver histologic sections of normal BALB/c and GKO mice were examined for amastigote burdens (LDU) and tissue cellular reactions. 25 × 40 fields were counted and scored as no cellular reaction including no Kupffer cell fusion (KC); fused KC alone; fusion with a developing cellular infiltrate (early granuloma formation); or fully formed granuloma (11). Results indicate mean values for four mice at each timepoint (eccept BALB/c, week 12; n = 3 mice). SEM was <14%.
2 wk after infection, 70% of all infected foci in BALB/c controls had provoked some tissue response, including mature granuloma formation (Table 1). GKO mice, on the other hand, showed a scant response to L. donovani at week 2 with 80% of parasitized foci consisted of unfused infected Kupffer cells with no surrounding mononuclear cells. By week 4, 56% of BALB/c foci were scored as mature granulomas, structures rarely seen (6%) at this timepoint in GKO mice (Fig. 2). However, by week 8, there was evidence of some response at GKO foci, and by week 12, about one in five GKO foci consisted of fully developed granulomas. Overall, these results clearly indicate that endogenous IFN-γ regulates optimal recruitment of influxing T cells and monocytes and therefore is required for the initiation of the granulomatous response early in infection. Nevertheless, there is also some mature granuloma formation in the absence of IFN-γ, reflecting the effect of a late-acting IFN-γ–independent response.
Figure 2.
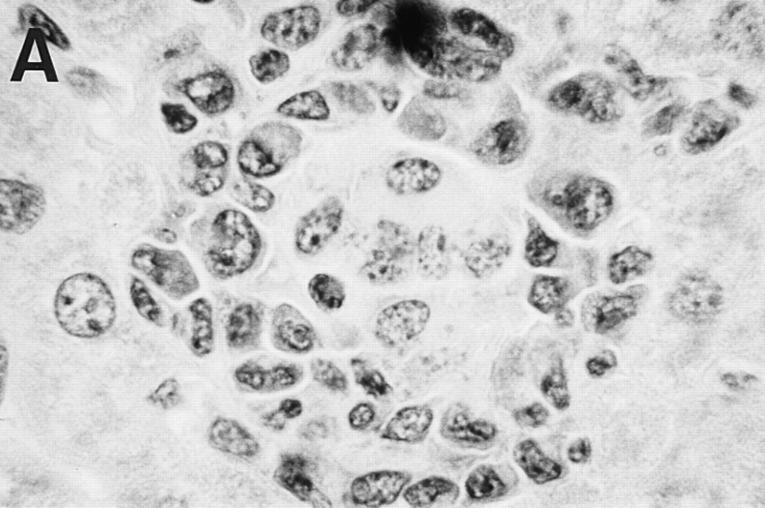
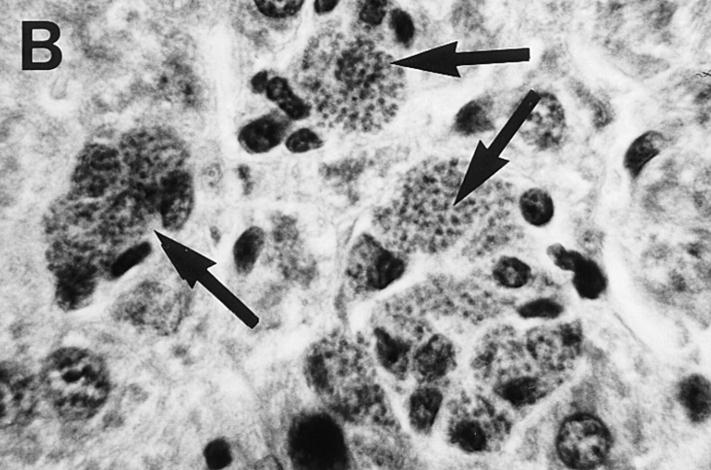
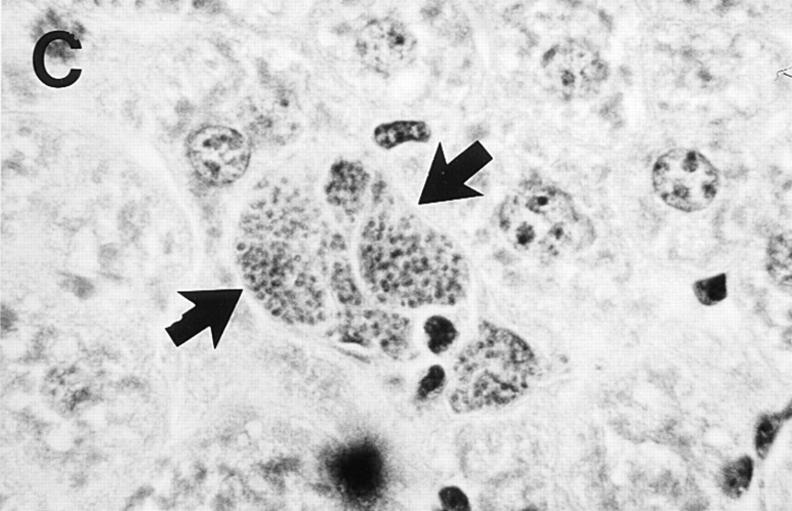
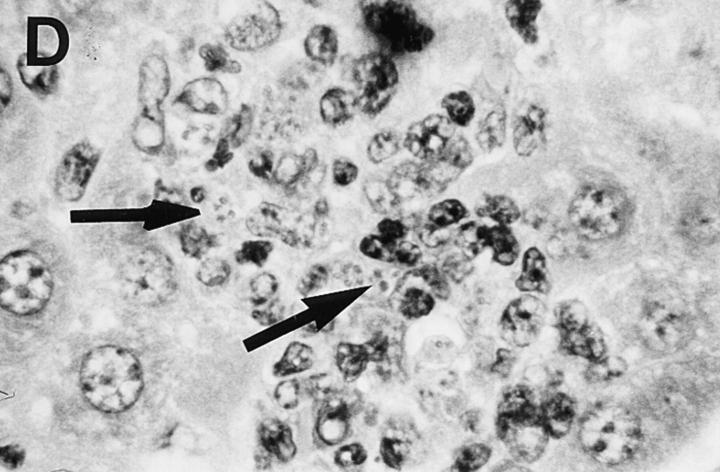
Liver histologic reaction in L. donovani–infected GKO mice and response to IL-12 treatment. (A) Normal BALB/c mouse shows mature granuloma formation surrounding fused parasitized Kupffer cell core 4 wk after infection. (B) 4-wk–infected GKO liver showing three large heavily parasitized foci (arrows) with minimal mononuclear cell infiltrate. (C) 3-wk–infected untreated GKO mouse showing heavily infected Kupffer cell focus with no cellular infiltrate. (D) 3-wk–infected GKO mouse treated with IL-12 during the preceding 7 d shows few parasites (arrow) and clearly enhanced cell influx. Original magnification, ×630.
Role of Endogenous TNF-α in GKO Mice.
Because TNF-α is essential to the resolution of L. donovani infection in normal BALB/c mice (15), we next focused on TNF-α as a mechanism that might permit GKO mice to reduce liver parasite burdens after week 8.
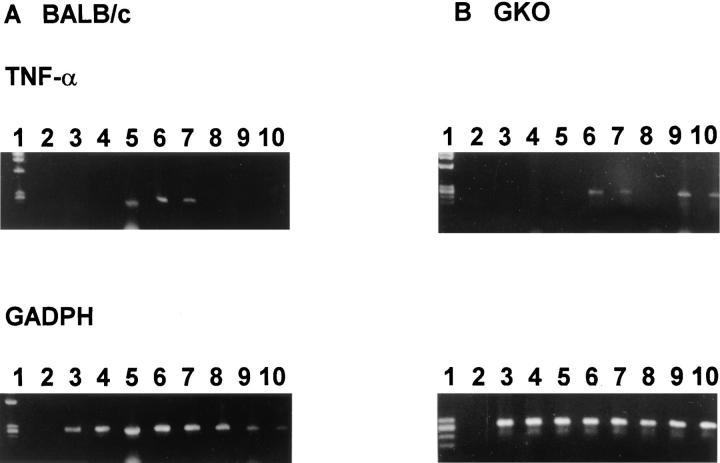
TNF-α mRNA expression in liver was examined using a qualitative RT-PCR assay in which results can be read only as positive (visualized on agarose gel) or negative (not visualized). Although a negative result in this assay does not imply the absence of cytokine mRNA, conversion within groups of mice from negative to largely positive qualitative results, either as infection progressed or after treatment was given, suggests enhanced mRNA expression. As shown in Table 2 and Fig. 3, TNF-α mRNA was not detected in GKO livers using this assay at week 2 but was detected in an increasingly greater proportion of mice as visceral infection peaked (week 8) and began to resolve (week 12). In livers of infected normal BALB/c mice (Table 2), this assay yielded qualitative results that suggested differences in the kinetics of TNF-α mRNA expression in that expression was detected earlier and waned by weeks 8–12. These latter results appeared to mirror the more rapid onset of acquired resistance and control over infection in normal, IFN-γ–intact mice (see Fig. 1).
Table 2.
Expression of TNF-α mRNA in L. donovani–infected Liver
| Weeks after infection | Strain | TNF present no. samples/total (%) | ||
|---|---|---|---|---|
| Uninfected | GKO | 0/4 (0) | ||
| BALB/c | 0/5 (0) | |||
| 2 | GKO | 0/6 (0) | ||
| BALB/c | 3/6 (50) | |||
| 4 | GKO | 3/6 (50) | ||
| BALB/c | 5/6 (83) | |||
| 8 | GKO | 5/6 (83) | ||
| BALB/c | 3/6 (50) | |||
| 12 | GKO | 6/6 (100) | ||
| BALB/c | 1/6 (17) |
Qualitative RT-PCR was performed using cDNA liver samples isolated at the designated timepoints. Results are from 2–3 experiments at each timepoint.
Figure 3.
Kinetics of TNF-α mRNA expression in BALB/c mice (A) and GKO mice (B). Qualitative RT-PCR was performed using cDNA from liver RNA extracted at each timepoint. Each lane represents one mouse. Lane 1, size markers; lane 2, PCR control; lanes 3 and 4, 2 wk after infection; lanes 5 and 6, 4 wk after infection; lanes 7 and 8, 8 wk after infection; lanes 9 and 10, 12 wk after infection. Similar results were obtained from six mice for each timepoint from 2–3 separate experiments.
To analyze the role of TNF-α in the IFN-γ–independent antileishmanial mechanism, infected GKO animals were treated with anti–TNF-α antiserum starting at week 8. In untreated GKO controls, parasite burdens peaked at week 8 and then declined (Table 3). Injections of anti–TNF-α reversed this control, whereas injections of normal rabbit serum had no effect. These results indicated that endogenous TNF-α is required for the control of infection after week 8 in GKO mice, suggesting that TNF-α secretion likely represented a key IFN-γ–independent antileishmanial mechanism.
Table 3.
Effect of Anti–TNF-α on Visceral Infection in GKO Mice
| Treatment during weeks 8–12 | Liver Parasite Burden (LDU) | |||
|---|---|---|---|---|
| Week 8 | Week 12 | |||
| None (control) | 9,788 ± 579 | 4,187 ± 636 | ||
| Normal serum | 3,943 ± 469 | |||
| Anti–TNF-α | 10,202 ± 1,361 | |||
Starting 8 wk after infection, GKO mice were injected twice-weekly with either normal rabbit serum or anti–TNF-α antiserum. Results are mean LDU ± SEM from two experiments with 5–6 mice per group. P <0.002 for anti–TNF-α–treated group versus untreated and normal saline-treated mice.
Response of GKO Mice to Exogenous IL-12.
To determine whether we could activate this initially quiescent IFN-γ–independent mechanism in infected mice, we examined the effect of exogenous IL-12. Whereas IL-12 is a potent inducer of IFN-γ expression (Table 4) (19–23) and stimulates IFN- γ–dependent antileishmanial activity in normal BALB/c mice (10), some reports indicate possible IFN-γ–independent actions of IL-12 as well (6, 22, 23). GKO mice were continuously treated with IL-12 starting 2 wk after infection, and liver burdens were measured 7 d later. During this period, the parasite burden doubled in untreated mice (Table 5). In contrast, liver burdens were reduced in IL-12– treated animals by 34% (week 3 LDU (1687 ± 300) versus week 2 LDU in controls (2556 ± 468)) indicating induction of L. donovani killing. Thus, the IFN-γ–independent mechanism identified here was indeed sensitive to cytokine upregulation, and despite the absence of IFN-γ, IL-12 was nevertheless quite capable of stimulating an antileishmanial response.
Table 4.
IL-12–induced Upregulation of IFN-γ in Normal BALB/c Mice
| Treatment of mice | IFN-γ (pg/ml) | |||
|---|---|---|---|---|
| Supernatant | Serum | |||
| Saline/BSA | 12 ± 9 | 304 ± 271 | ||
| IL-12 | >3,000 | >3,000 | ||
Mice were given daily intraperitoneal injections of 1 μg of IL-12 for 4 d. 24 h after the last injection, serum was collected and spleen cells were harvested and cultured in vitro (5 × 106 cells/ml) for an additional 24 h with no further stimulation. Results are mean ± SEM values from three mice per group from a single experiment.
Table 5.
Effect of IL-12 on Visceral Infection in GKO Mice
| Treatment | Liver parasite burdens (LDU) | |||
|---|---|---|---|---|
| Week 2 | Week 3 | |||
| None (control) | 2,556 ± 468 (9) | 5,343 ± 777 (8) | ||
| IL-12 | 1,687 ± 300 (8)* | |||
| IL-12 plus anti–TNF-α | 8,514 ± 782 (8)‡ | |||
| IL-12 plus control antiserum | 2,725 ± 288 (9)‡ | |||
| Saline plus control antiserum | 6,428 ± 826 (5) | |||
2 wk after infection, GKO mice were treated by pump for 7 d with IL-12 (1 μg/d) or saline. Where indicated, mice were also injected during the week of IL-12 treatment with either anti–TNF-a antiserum or control serum given every 3 d starting just after pump insertion. Results are from three experiments, and represent mean ± SEM values for (n) mice.
Significantly different (P <0.05) from control mice at week 2 and week 3.
Significantly different (P <0.05) from control mice at week 3.
The IFN-γ–independent action of IL-12 was also evident in the tissue histologic response in GKO mice. In untreated 3-wk infected GKO mice, 2% of infected foci were scored as mature granulomas (two experiments; n = 4 mice; see Fig. 2). In contrast, in the same GKO mice treated with IL-12 for 7 d, 31% of infected foci were scored as mature granulomas (n = 4 mice; see Fig. 2). This 15-fold increase in mature granuloma formation thus correlated with IL-12– induced IFN-γ–independent reduction in liver parasite burdens.
Role of TNF-α in IL-12 Action in GKO Mice.
Effects of IL-12 unrelated to IFN-γ include upregulation of other cytokines including TNF-α and GM-CSF (23–25), both of which induce antileishmanial activity (10, 12). To investigate whether the IFN-γ–independent, IL-12–induced antileishmanial response involved TNF-α, 2-wk infected GKO mice were injected twice-weekly with anti–TNF-α antiserum or normal serum during 7 d of IL-12 administration. As shown in Table 5, there was some nonspecific inhibition of the effect of IL-12 by simultaneous administration of control serum; however, injections of anti–TNF-α totally abolished the antileishmanial activity induced by IL-12.
Interaction of IL-12, TNF-α, and iNOS in GKO Mice.
Activated macrophages use at least two mechanisms for killing intracellular pathogens: the generation of reactive oxygen intermediates and the induction of l-arginine–dependent toxic reactive nitrogen intermediates (26–34). The latter includes nitric oxide (NO), the toxic product of iNOS (30, 35, 36), the induction of which is controlled by cytokines, e.g., TNF-α in combination with IFN-γ (28), and other stimuli including intracellular pathogens as well (27). Therefore, we completed this analysis by investigating the mobilization of iNOS as an antileishmanial effector mechanism in IL-12–treated GKO mice.
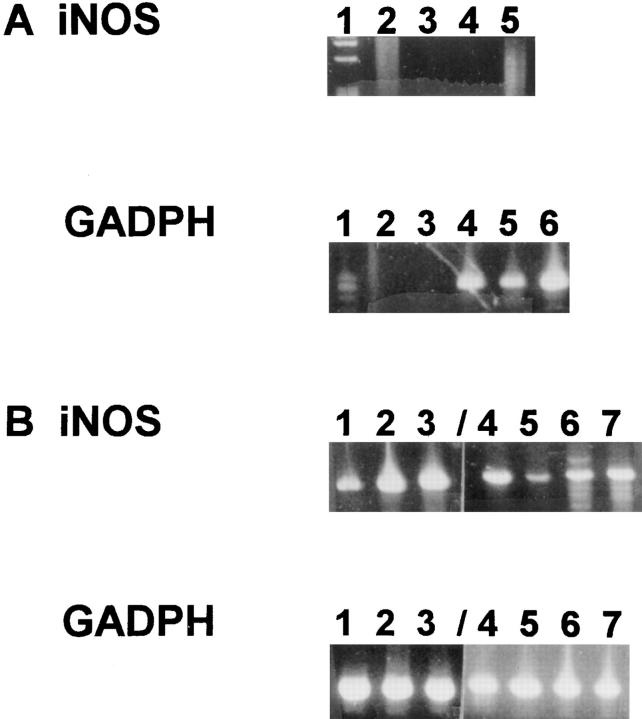
iNOS mRNA was not detected in livers of normal uninfected BALB/c mice by qualitative RT-PCR, but was detected in these animals at week 3 (3 of 6 mice), and week 4 (5 of 5 mice) after infection (Fig. 4). Although not detected in GKO mice at week 3 (0 of 6 mice), iNOS mRNA was detected at week 4 (4 of 8 mice) (Table 6).
Figure 4.
Expression of iNOS mRNA in livers of L. donovani–infected BALB/c mice. Total liver RNA was isolated at 2, 3, and 4 wk after infection and assayed for the presence of iNOS message by qualitative RTPCR. Each lane represents one mouse. GADPH is a control for the amount of cDNA in each sample. (A) iNOS RT-PCR: lane 1, size markers; lane 2, PCR control; lanes 3, 4, and 5, 2-wk samples. GADPH RTPCR: lane 1, size markers; lane 2, PCR control; lane 3, blank, lanes 4, 5, and 6, 2-wk samples. (B) iNOS RT-PCR: lanes 1, 2, and 3: 3-wk samples; lanes 4, 5, 6, and 7, 4-wk samples from a different gel and PCR reaction. GADPH RT-PCR, same as iNOS.
Table 6.
iNOS mRNA Expression in Livers of GKO Mice
| Time after infection and treatment | iNOS Detected | |
|---|---|---|
| No. samples (%) | ||
| Uninfected | 0/3 (0) | |
| 2 Weeks | 1/6 (17) | |
| 3 Weeks | 0/6 (0) | |
| 3 Weeks plus IL-12 | 4/6 (67) | |
| 3 Weeks plus IL-12 plus anti-TNF | 0/6 (0) | |
| 3 Weeks plus IL-12 plus control serum | 4/5 (80) | |
| 4 Weeks | 4/8 (50) |
Liver RNA was assayed for the expression of iNOS message by qualitative RT-PCR. Where indicated, IL-12 treatment (1 μg/d for 7 d) was begun at the end of week 2 with or without simultaneous twiceweekly injections of anti–TNF-α or normal rabbit serum. Results are from 2–3 experiments at each timepoint.
To assess the activity of NO in control of visceral infection in this model, iNOS was inhibited by oral treatment with AG (16). In initial experiments, normal BALB/c mice and wild-type GKO littermates (GKO +/+) were treated continuously for 4 wk starting 1 d after infection. As shown in Table 7, AG treatment inhibited control of visceral infection and allowed a three- to four-fold increase in liver parasite burdens, indicating an antileishmanial role for iNOS in normal animals.
Table 7.
Effect of Aminoguanidine on L. donovani–infected Normal Mice
| Strain | AG Treatment | Liver parasite burden (LDU) | ||||
|---|---|---|---|---|---|---|
| Week after infection | ||||||
| 2 | 4 | |||||
| BALB/c | − | 1,748 ± 125 (6) | 569 ± 127 (9) | |||
| + | 1,746 ± 74 (6) | 2,450 ± 255 (9)* | ||||
| GKO +/+ | − | 2,854 ± 214 (3) | 978 ± 213 (6) | |||
| + | 2,166 ± 81 (3) | 2,919 ± 194 (6)* | ||||
12 h after L. donovani challenge, treatment with 1% AG was begun and given continuously for 4 wk. Results are mean ± SEM values for (n) mice from two experiments in BALB/c mice and one experiment in GKO +/+ mice.
Significantly different from 4-wk untreated control value (P <0.05)
Whereas untreated GKO mice did not express detectable iNOS mRNA 3 wk after infection (0 of 6 mice), iNOS mRNA was detected in the same mice treated for the previous 7 d with IL-12 (4 of 6 mice; Table 6). The in vivo relevance of this effect of IL-12 was tested by simultaneously treating GKO mice with IL-12 and AG. As shown in Table 8, in the presence of AG, the capacity of IL-12 to induce antileishmanial activity was abrogated. Finally, to determine whether IL-12–stimulated upregulation of iNOS involved TNF-α, a recognized participant in iNOS induction (37), we analyzed liver RNA from GKO mice treated with both IL-12– and anti–TNF-α. Anti–TNF-α injections abolished qualitative detection of iNOS mRNA in IL-12–treated animals (see Table 6).
Table 8.
Effect of iNOS Inhibitor in IL-12–treated GKO Mice
| Treatment started after week 2 | Liver parasite burden (LDU) | |||
|---|---|---|---|---|
| Weeks after infection | ||||
| 2 | 3 | |||
| None (control) | 2,251 ± 578 | 4,665 ± 611 | ||
| IL-12 | − | 1,539 ± 338* | ||
| IL-12 plus AG | − | 4,545 ± 717 | ||
Starting 2 wk after infection, GKO mice were treated with IL-12 (1 μg/day) for 7 d with or without the addition of 1% AG to the drinking water. Results are mean LDU ± SEM values from two experiments with 6–10 mice per timepoint.
Significantly different (P <0.05) from control mice at week 3.
Discussion
We used GKO mice and a well-established model of experimental visceral leishmaniasis to characterize host defense responses to intracellular infection in the absence of IFN-γ. Not surprisingly (1), without endogenous IFN-γ, high parasite burdens developed and persisted weeks beyond the time infection was controlled in IFN-γ–intact animals. However, despite the key role of macrophageactivating endogenous IFN-γ in the early phases of this infection (1, 2), untreated GKO mice nonetheless prevented unrestrained infection and reduced liver parasite burdens after week 8. Thus, because GKO mice produce no IFN-γ, the presence of a late onset IFN-γ–independent antileishmanial mechanism appears unequivocal. GKO mice have also been used in other experimental models to demonstrate IFN-γ–independent control over infection (4, 8, 45).
While the nature of the antileishmanial mechanism uncovered here may be multifactorial, our results point to endogenous TNF-α as a primary effector component. In addition, although persistently quiescent (for unclear reasons) in the initial stages of visceral infection, this mechanism can be readily activated early on, as judged by the effects of treatment with exogenous IL-12. This latter result also clearly expands the host defense repertoire of IL-12 beyond the induction of IFN-γ (10, 22, 24), the most widely studied antimicrobial action of IL-12. Although our data characterize the IFN-γ–independent effect of exogenous IL-12, we have not yet determined the possible role of endogenous IL-12 in the control of L. donovani in GKO mice.
In addition to inducing IFN-γ, IL-12 is also known to enhance expression of TNF-α (23), a cytokine with separate effects not necessarily dependent on the presence of IFN-γ as a cofactor (28, 38). In addition, whereas IFN-γ normally induces TNF-α expression by the activated macrophage, some reports indicate IFN-γ–independent induction of TNF-α expression and have suggested that in the absence of TNF-α, IFN-γ is ineffective in control of infection (30, 31, 37, 39). This latter conclusion is supported by results from a prior study in IFN-γ–intact normal BALB/c mice (10) in which the antileishmanial effect of exogenous IL-12 was reversed equally well by either anti–IFN-γ or anti–TNF-α treatment. Whereas these latter results raise the possibility of a direct effect for TNF-α independent of IFN-γ in normal mice, they appear more consistent with the notion that both endogenous TNF-α and IFN-γ are required for optimal expression of IL-12–induced antileishmanial activity (10). However, our results here in GKO mice clearly suggest that TNF-α induced by exogenous IL-12 (or by L. donovani infection alone) can act by itself in the absence of IFN-γ. In addition, other cytokines with documented antileishmanial activity such as GM-CSF (12) may also play a role in the IL-12–stimulated control observed here; for instance, IL-12 also induces GM-CSF mRNA expression in livers of infected GKO mice (data not shown). However, the clear-cut effect of anti–TNF-α in IL-12– treated GKO mice indicates that TNF-α is both necessary and sufficient to mediate the effect of IL-12 in the absence of IFN-γ.
It should also be pointed out that treatment of GKO mice with IL-12 has been reported to induce no protective effect in models of infection caused by M. tuberculosis and T. gondii (40, 41). The use of different test pathogens, and variations in doses of IL-12, or in administration routes and schedules may explain these contrary results. However, another possible explanation, at least in mycobacterial models, may relate to the apparently more strict requirement for endogenous IFN-γ in defense against these particular pathogens (42, 43).
TNF-α and IFN-γ act synergistically to induce synthesis of NO in normal mice (36), and depletion of either cytokine increases mortality after sublethal challenge with various microorganisms (31, 44). In GKO mice, NO synthesis in response to Bacillus Calmette-Guérin infection is depressed but not absent (3, 35). Similarly, in our model, while qualitative detection of iNOS transcription in livers of infected GKO mice was delayed and apparently incomplete, iNOS mRNA was detected in 50% of these animals at week 4. In addition, IL-12 treatment enhanced iNOS mRNA in GKO mice, also supporting the notion that this antimicrobial mechanism can be mobilized in the absence of endogenous IFN-γ, likely via endogenous TNF-α (Table 8). The capacity of the iNOS inhibitor, AG (16), to abolish the antileishmanial effect of IL-12 in GKO mice suggests in vivo relevance for these observations.
Two particular aspects of granuloma formation in GKO mice, which was delayed and persistently immature but not altogether absent, warrant comment. First, single parasitized Kupffer cells with no evidence of fusion or any surrounding cellular reaction were present at all timepoints even up to 12 wk after infection. These results (Table 1) provide firm evidence of the critical role of endogenous IFN-γ in macrophage fusion, cell recruitment, and granuloma assembly. Second, however, the capacity of exogenous IL-12 to accelerate and enhance the development of mature granulomas, an effect not apparent in a previous study in IFN-γ–intact mice (10), also demonstrated a separate IFN-γ–independent pathway active in the tissue granulomatous response. Because endogenous TNF-α is not essential for granuloma formation in this model (15), we suspect that factors other than TNF-α are involved in the tissue reaction stimulated by IL-12. The activity of GM-CSF, the expression of which is induced by IL-12 in L. donovani–infected GKO mice, might be a possible compensatory mechanism of granuloma assembly in the absence of endogenous IFN-γ. GM-CSF readily stimulates myelomonocytic cell influx into areas of developing granulomas in L. donovani–parasitized tissues of normal mice (12).
In summary, our results demonstrate the presence of spontaneously developing and IL-12–inducible intracellular antileishmanial effects in the absence of endogenous IFN-γ, indicate that this IFN-γ–independent mechanism is largely mediated by TNF-α, and suggest that the iNOS system appears to be a major antimicrobial effector mechanism in this IFN-γ–independent pathway.
Acknowledgments
We are grateful to Genetics Institute and Amgen Biologicals for providing IL-12 and IFN-γ, respectively, and to J. Hariprashad and D. Kwok for technical assistance.
This work was supported by National Institutes of Health reasearch grant AI-16963.
Footnotes
1 Abbreviations used in this paper: AG, aminoguanidine; GKO, gene knockout mice; iNOS, inducible nitric oxide synthase; LDU, Leishman-Donovan units; NO, nitric oxide; RT, reverse transcriptase.
References
- 1.Squires KE, Schreiber RD, McElrath MJ, Rubin BY, Anderson SL, Murray HW. Experimental visceral leishmaniasis: role of endogenous IFN-γ in host defense and tissue granulomatous response. J Immunol. 1989;143:4244–4249. [PubMed] [Google Scholar]
- 2.Murray HW. Effect of continuous administration of interferon-γ in experimental visceral leishmaniasis. J Infect Dis. 1990;161:992–994. doi: 10.1093/infdis/161.5.992. [DOI] [PubMed] [Google Scholar]
- 3.Dalton DK, Pitts-Meek S, Keshav S, Figari IS, Bradley A, Stewart TA. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science (Wash DC) 1993;159:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 4.Graham MB, Dalton DK, Giltinan D, Braciale VL, Stewart TA, Braciale TJ. Response to influenza infection in mice with a targeted disruption in the interferon-γ gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper. A.M., D.K. Dalton, T.A. Stewart, J.P. Griffin, D.G. Russell, and I.M. Orme. Disseminated tuberculosis in interferon-γ gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Zheng S, Corry D, Dalton DK, Seder RA, Reiner SL, Locksley RM. Interferon-γ-independent effects of interleukin 12 administered during acute or established infection due to Leishmania major. . Proc Natl Acad Sci USA. 1994;91:12932–12936. doi: 10.1073/pnas.91.26.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Reiner SL, Sheng S, Dalton DK, Locksley RM. CD4+ effector cells default to the Th2 pathway in interferon-γ–deficient mice infected with Leishmania major. . J Exp Med. 1994;179:1367–1371. doi: 10.1084/jem.179.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amiri P, Haak-Frendscho M, Robbins K, McKerrow JH, Stewart T, Jardieu P. Anti-immunoglobulin E treatment decreases worm burden and egg production in Schistosoma mansoni–infected normal and interferon-γ knockout mice. J Exp Med. 1994;180:43–51. doi: 10.1084/jem.180.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 10.Murray HW, Hariprashad J. Interleukin-12 is effective treatment for an established systemic intracellular infection: experimental visceral leishmaniasis. J Exp Med. 1995;181:387–391. doi: 10.1084/jem.181.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray HW, Stern JJ, Welte K, Rubin BY, Carriero SM, Nathan CF. Experimental visceral leishmaniasis: production of interleukin 2 and interferon-γ, tissue immune reaction, and response to treatment with interleukin 2 and interferon-γ. J Immunol. 1987;138:2290–2297. [PubMed] [Google Scholar]
- 12.Murray HW, Cervia JS, Hariprashad J, Taylor AP, Stoeckle MY, Hockman H. Effect of granulocyte–macrophage colony-stimulating factor in experimental visceral leishmaniasis. J Clin Invest. 1995;95:1183–1192. doi: 10.1172/JCI117767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miralles GD, Stoeckle MY, McDermott DF, Finkelman FD, Murray HW. Th1 and Th2 cell-associated cytokines in experimental visceral leishmaniasis. Infect Immun. 1994;62:1058–1063. doi: 10.1128/iai.62.3.1058-1063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stenger S, Thuring H, Rolinghoff M, Bogdan C. Tissue expression of inducible nitric oxide synthase is closely associated with resistance to Leishmania major. . J Exp Med. 1994;180:783–793. doi: 10.1084/jem.180.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumang MCT, Keogh C, Moldawer LL, Helfgott DC, Teitelbaum R, Hariprashad J, Murray HW. Role and effect of TNF-α in experimental visceral leishmaniasis. J Immunol. 1994;153:768–775. [PubMed] [Google Scholar]
- 16.Chan J, Tanaka K, Carroll D, Flynn J, Bloom BR. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. . Infect Immun. 1995;63:730–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McElrath MJ, Murray HW, Cohn ZA. Dynamics of granuloma formation in experimental visceral leishmaniasis. J Exp Med. 1988;167:1927–1937. doi: 10.1084/jem.167.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray HW, Miralles GD, Stoeckle MY, McDermott DF. Role and effect of IL-2 in experimental visceral leishmaniasis. J Immunol. 1993;151:929–938. [PubMed] [Google Scholar]
- 19.Gazzinelli R, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin-12 is required for the T-lymphocyte–independent induction of interferon-γ by an intracellular parasite and induces resistance in T-cell–deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wynn TA, Eltoum I, Oswald I, Cheever AW, Sher A. Endogenous interleukin-12 (IL-12) regulates granuloma formation induced by eggs of Schistosoma mansoniand exogenous IL-12 both inhibits and prophylactically immunizes against egg pathology. J Exp Med. 1994;179:1551–1561. doi: 10.1084/jem.179.5.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+T cells to enhance priming for interferon-γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridges innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–162. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 23.Morris SC, Madden KB, Adamovicz JJ, Gause WC, Hubbard BR, Gately MK, Finkelman FD. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994;152:1047–1056. [PubMed] [Google Scholar]
- 24.Brunda MJ. Interleukin-12. J Leuk Biol. 1994;55:280–288. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 25.Naume B, Johnsen AC, Espevik T, Sondam A. Gene expression and secretion of cytokines and cytokine receptors from highly purified CD56+natural killer cells stimulated with interleukin-2, interleukin-7, and interleukin-12. Eur J Immunol. 1993;23:1831–1838. doi: 10.1002/eji.1830230815. [DOI] [PubMed] [Google Scholar]
- 26.Liew FY, Millott S, Parkinson C, Palmer RM, Moncada S. Macrophage killing of Leishmaniaparasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990;144:4794–4797. [PubMed] [Google Scholar]
- 27.Green SJ, Meltzer MS, Hibbs JB, Nacy CA. Activated macrophages destroy intracellular Leishmania majoramastigotes by an L-arginine–dependent killing mechanism. J Immunol. 1990;144:278–283. [PubMed] [Google Scholar]
- 28.Appelberg R, Castro AG, Pedrosa J, Silva RA, Orme IM, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium aviuminfection. Infect Immun. 1994;62:3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dumarey CH, Labrousse V, Rastogi N, Vargaftig BB, Bachelet M. Selective Mycobacterium avium–induced production of nitric oxide by human monocyte-derived macrophages. J Leuk Biol. 1994;56:36–40. doi: 10.1002/jlb.56.1.36. [DOI] [PubMed] [Google Scholar]
- 30.Roach TI, Kiderlen AF, Blackwell JM. Role of inorganic nitrogen oxides and tumor necrosis factor-alpha in killing Leishmania donovaniamastigotes in gamma-interferon lipopolysaccharide-activated macrophages from Lshs and Lshr congenic mouse strains. Infect Immun. 1991;59:3935–3944. doi: 10.1128/iai.59.11.3935-3944.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng H, Popov VL, Walker DH. Depletion of gamma interferon and tumor necrosis factor-alpha in mice with Rickettsia conorii–infected endothelium: impairment of rickettsicidal nitric oxide production resulting in fatal, overwhelming rickettsial disease. Infect Immun. 1994;62:1952–1960. doi: 10.1128/iai.62.5.1952-1960.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray HW, Juangbhanich CW, Nathan CF, Cohn ZA. Macrophage oxygen-dependent antimicrobial activity. II. The role of oxygen intermediates. J Exp Med. 1979;150:950–964. doi: 10.1084/jem.150.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nathan CF, Murray HW, Cohn ZA. The macrophage as an effector cell. N Engl J Med. 1980;303:622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- 34.Murray HW. Cell-mediated immune response in experimental visceral leishmaniasis. II. Oxygen-independent killing of intracellular Leishmania donovani. . J Immunol. 1982;129:351–357. [PubMed] [Google Scholar]
- 35.Chan J, Xing Y, Magliosso RS, Bloom BR. Killing of virulent Mycobacterium tuberculosisby reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–3064. [PubMed] [Google Scholar]
- 37.Nacy CA AI, Meierovics, Belosevic M, Green SJ. Tumor necrosis factor-alpha: central regulatory cytokine in the induction of macrophage antimicrobial activities. Pathobiology. 1991;59:182–184. doi: 10.1159/000163640. [DOI] [PubMed] [Google Scholar]
- 38.Pawelec G, Schaudt K, Rehbein A, Busch FW. Differential secretion of tumor necrosis factor-α and granulocyte/macrophage colony stimulating factors but not interferon-γ from CD4+ compared to CD8+human T cell clones. Eur J Immunol. 1989;19:197–200. doi: 10.1002/eji.1830190132. [DOI] [PubMed] [Google Scholar]
- 39.Liew FY, Parkinson C, Millott S, Severn A, Carrier M. TNF-α mediates host protection against cutaneous leishmaniasis. Immunology. 1990;69:570–573. [PMC free article] [PubMed] [Google Scholar]
- 40.Flynn JL, Goldstein MM, Triebold KJ, Sypek J, Wolf S, Bloom B. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosisinfection. J Immunol. 1995;155:2515–2524. [PubMed] [Google Scholar]
- 41.Scharton-Kersten TM, Wynn TA, Denkers EY, Bala S, Grunvald E, Heiny S, Gazzinelli RT, Sher A. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondiiwhile failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 42.Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile J, Newport M, Levin M, Blanche S, Fischer A, Casanova J. Interferon-γ-receptor deficiency in an infant with fatal Bacille Calmette–Guerin infection. N Engl J Med. 1996;335:1956–1961. doi: 10.1056/NEJM199612263352604. [DOI] [PubMed] [Google Scholar]
- 43.Newport MJ, Huxley CM, Huston S, Hawrylowicz CM, Oostra BA, Williamson R, Levin M. A mutation in the interferon-γ-receptor gene and susceptibility to mycobacterial infection. N Engl J Med. 1996;335:1941–1949. doi: 10.1056/NEJM199612263352602. [DOI] [PubMed] [Google Scholar]
- 44.Green SJ, Nacy CA, Schrieiber RD, Granger DL, Crawford RM, Meltzer MS, Fortier AH. Neutralization of gamma interferon and tumor necrosis factor-alpha blocks in vivo synthesis of nitrogen oxides from l-arginine and protection against Francisella tularensis infection in Mycobacterium bovisBCG-treated mice. Infect Immun. 1993;61:689–698. doi: 10.1128/iai.61.2.689-698.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenesin the absence of IFNγ. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]