Abstract
Neisseria gonorrhoeae (GC) is a human pathogen that adheres to and invades genital surfaces. Although pili are required for the initial adherence, the interaction of GC with epithelial cells is also promoted by a family of outer membrane proteins, the opacity (Opa) proteins such as OpaA protein from strain MS11. Studies have demonstrated that the interaction of the OpaA GC with epithelial cells involves binding to heparan sulfate attached to syndecan receptors. However, other Opa proteins interact with CEA gene family member 1 (CGM1) or biliary glycoprotein (BGP), members of the CD66 antigen family. In this study, we demonstrate that, in addition, the 180-kD carcinoembryonic antigen (CEA) is a receptor for Opa proteins. This conclusion was based on the following observations. First, transfected HeLa cells expressing CEA (HeLaCEA) and the CEA-expressing colon cancer cell line (LS 174T) bound and subsequently engulfed the Opa+ bacteria. These interactions were inhibited by anti-CEA antibody, but could not be inhibited by addition of heparin. Furthermore, OpaI E. coli directly bound purified CEA. We also compared the adherence and invasion by Opa+ bacteria of CD66 transfected HeLa cells: HeLa-BGPa, HeLa-CGM6, HeLa-NCA, HeLa-CGM1a, HeLa-CEA, and HeLa-Neo serving as negative control. Using OpaI as the prototype, the relative ability of the transfected HeLa cell lines to support adherence was (CEA = BGPa >CGM1a >NCA >>CGM6 = Neo). The ability to mediate invasion of the transfectant cells was (CGM1a >CEA >BGPa >NCA >CGM6 = Neo). Among the Opa proteins tested, OpaC proved to be bifunctional, able to mediate adherence to both syndecan receptors and to CD66 antigens.
N eisseria gonorrhoeae (GC)1 cause uncomplicated gonorrhea, pelvic inflammatory disease, disseminated gonococcal infection, and ophthalmia neonatorum. These diseases result from the ability of GC to adhere to and penetrate mucosal epithelial cells (1, 2). Pili are the components promoting the initial adherence and infection by GC (3–5).
Pathobiological studies of gonococcal infection are hampered by the highly restricted species specificity of gonorrheal disease so that there is no good animal model available currently. The most informative model has been in vitro infection of human fallopian tube organ culture (FTOC; 6, 7). Initial attachment of GC to FTOC mucosal columnar epithelial cells is mediated by pili. About 18 h after infection, the attached organisms are endocytosed by the nonciliated cell. Phagocytosed GC appear to be rapidly transcytosed through the epithelial cells and enter the submucosal space at the base of the cells (8). Expression of a family of outer membrane proteins, the phase-variable opacity (Opa) proteins, by GC enhances their ability to adhere and to invade the epithelial cells in FTOC (9). Moreover, Opa expressing Escherichia coli can attach and invade human fallopian tube epithelium at low levels (10). These results indicate that Opa proteins alone may be sufficient to promote adherence to and invasion of the human cells. Studies with infected human volunteers have demonstrated a strong selection for Opa+ gonococci among the reisolates suggesting that Opa proteins play a role in gonococcal pathogenesis (11, 12).
Identification of the receptors involved in the interaction with host cells is a crucial step in understanding disease causation by bacteria. Some components on the mammalian cell surface have been shown to act as receptors for pathogenic microorganisms. One of the best defined receptor– ligand relationships is the interaction of Yersinia pseudotuberculosis invasin with β1 chain integrins on the mammalian cell surface (13). E-cadherin has recently been demonstrated as the receptor for internalin, a surface protein for entry of Listeria monocytogenes into epithelial cells (14). In GC, a variety of components participate in the interaction with the eukaryotic cells. These include pili (3), LPS (15), a 36-kD glycolipid-binding adhesin (16), interaction with carbohydrate structures on epithelial cells (17), and the Opa proteins (18). In gonococcal strain MS11, the Opa protein family consists of 11 unlinked opa genes whose sequences are known (19). One distinct Opa protein, the OpaA, has been correlated with adherence and subsequent internalization of GC by Chang conjunctival cells (20–22). The other members of Opa family are able to stimulate PMN adherence and phagocytosis (22, 23). It has been demonstrated that the interaction of the OpaA GC with epithelial cells involves binding to heparan sulfate syndecan receptors that are situated on the cell surface (24, 25). Moreover, recent studies indicated that gene family member (CGM)1 (26) and biliary glycoprotein (BGP) (27), members of the carcinoembryonic antigen (CEA) or CD66 family, serve as receptors for Opa+ bacteria for promoting adherence and internalization. In the instance of BGP or CD66a, it has been shown that the portion of the molecule that interacted with the Opa protein is the NH2-terminal domain that is homologous to the IgG variable domain (IgV-like; 28). This is supported by the ability of CGM1 to interact with Opa proteins since its surface-exposed portion consists solely of one IgV-like domain (26).
However, in addition to CD66a (BGP) and CD66d (CGM1), the CEA family also includes CD66b (CGM6), CD66c (NCA), and CD66e, the classical tumor associated CEA. We have investigated the activity of the different CD66 antigens with Opa proteins by expressing these five members of the CD66 family in HeLa cells and demonstrated that the transfectants differ in their ability to support adherence and internalization. Finally, OpaC protein from MS11 demonstrated a dual function; it interacted with both heparan sulfate syndecan receptors and members of the CEA family.
Materials and Methods
Bacterial Strains, Monoclonal Antibodies, and Cell Lines.
GC strain MS11 was cultured and maintained as previously described (29). Only pilus− GC and LOSb (lacto-N-neotetraose) phenotype were used (30). Recombinant opa genes from GC MS11 were constructed and expressed in E. coli HB101 as described previously (23). The designations of Opa proteins of both GC and E. coli are based on papers of Swanson et al. (11) and Belland et al. (23). E. coli HB101 containing the vector pGEM-3Z is designated as pGEM. E. coli HB101 expressing OpaA, OpaB, OpaC, OpaH, and OpaI genes are designated as pEXA, pEXB, pEXC, pEXH, and pEXI, respectively. Bacterial suspensions were from Luria-Bertani plates containing 50 μg/ml carbenicillin after growth for 16–20 h at 37°C. COL-1 mAb, specific for CGM1 and CEA only, was donated by Z. Shi (Zymed Laboratories Inc., San Francisco, CA) and mAb IB4 reactive with CD18 was provided by S. Wright (Merck Inc., Rahway, NJ).
The human colon adenocarcinoma cell line LS 174T was purchased from American Type Culture Collection (Rockville, MD). HeLa-CEA, HeLa-CGM1a, HeLa-BGPa, HeLa-NCA, and HeLa-CGM6 cells were constructed by transfecting HeLa cells with CEA, CGM1a, BGPa, NCA, and CGM6 cDNAs, and selected for surface antigen expression (31). HeLa-Neo cells are HeLa cells that were transfected with neomycin-resistance gene only (31).
Wild-type Chinese hamster ovary (CHO)-K1 and isogenic mutants 745 and 677 that have specific defects in proteoglycan biosynthesis were provided by J.D. Esko (University of Alabama, Birmingham, AL) (32–34). Mutant CHO cell 745 lacks xylosyltransferase and expresses no heparan sulfate and chondroitin sulfate, while mutant 677 which has defective N-acetylglucosaminyl and glucoronosyltransferases, produces no heparan sulfate, but 2–3 times increased chondroitin sulfate.
Adherence and Internalization Assays.
All cell lines were cultured in RPMI 1640 medium (GIBCO BRL, Gaithersburg, MD) with 10% FCS (Hyclone Labs., Logan, UT). For adherence assays, cells were grown to confluence (1–2 × 105 cells/well) in 24-well culture plates (Falcon, Lincoln Park, NJ), and washed twice with serum-free RPMI. E. coli were suspended in RPMI at OD540 of 0.04, and 0.5 ml of the bacterial suspensions was added to each well. The plates were incubated at 37°C with 5% CO2 for 4 h. The incubation period was limited to 2 h when mAb were included to avoid their inactivation. Experiments were terminated by washing three times with 1 ml of serum-free RPMI. Adherent bacteria were counted by suspending the cells in PBS containing 0.5% saponin (Calbiochem Corp., La Jolla, CA) and plating dilutions on Luria-Bertani agar medium containing 50 μg/ml of carbenicillin or on GC plates. The level of adherence of GC and E. coli to cells was calculated by determining the CFU associated with the host cell monolayers. Internalization assays were done in a similar fashion to adherence assays, but after the period of bacterial interaction to cell lines, the monolayers were washed twice and then incubated for 90 min with 1.5 ml of RPMI supplemented with 100 μg/ml gentamicin (GIBCO BRL). For adherence inhibition assays, the bacteria were added as a suspension in RPMI containing heparin at 30 μg/ml or 25 μg/ml mAb. The experiments were performed in duplicate or triplicate.
Binding of CEA to OpaI E. coli.
pGEM or pEXI were suspended in 1 ml of serum-free RPMI at OD540 of 0.8. 4 μg of purified CEA antigen (Calbiochem Corp.) was added to each bacterial suspension and incubated at room temperature for 60 min with gentle shaking. The bacteria were pelleted and washed once with RPMI and subjected to SDS-PAGE. The CEA bound to the bacteria was detected by Western blotting with COL-1 mAb after electrophoretic transfer to Immobilon-P membrane (Millipore Corp., Bedford, MA) (35). The bound COL-1 mAb was detected with HRP-conjugated protein A by chemiluminescence (Amersham Life Science, Arlington Heights, IL).
Results
Adherence and Invasion of Opa+ Bacteria into HeLa-CD66 Transfectants.
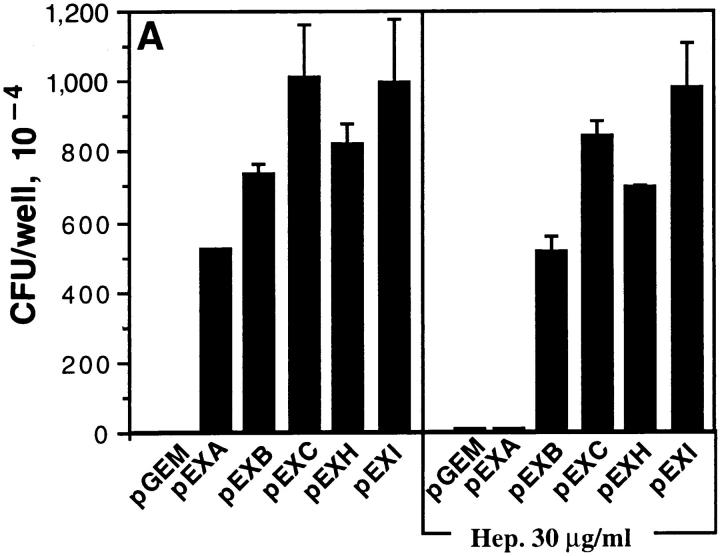
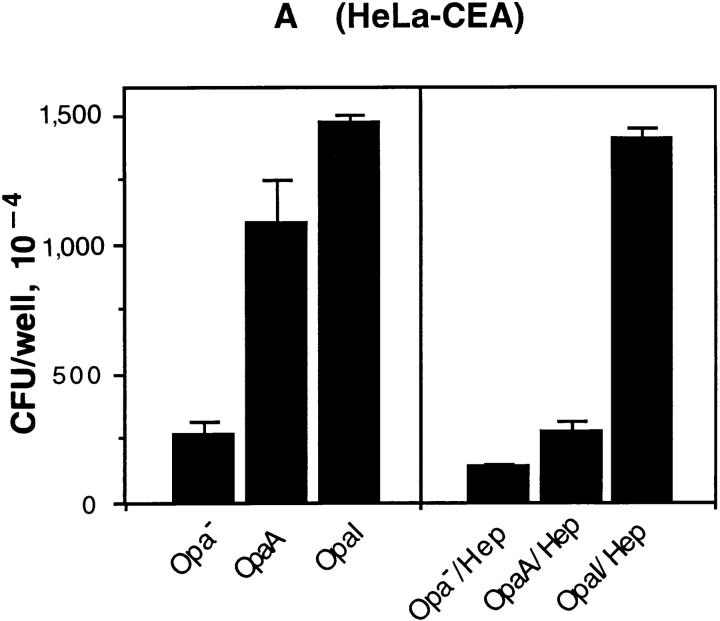
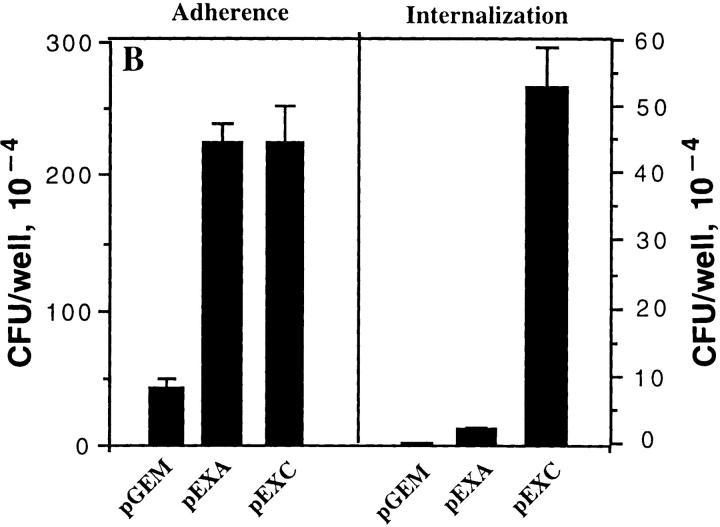
The HeLa-Neo, HeLa-BGPa (CD66a), HeLaCGM6 (CD66b), HeLa-NCA (CD66c), HeLa-CGM1a (CD66d), and HeLa-CEA (CD66e) were examined for their ability to support adherence and invasion of OpaI bacteria. The internalization of Opa+ bacteria into HeLa-CD66 transfectants was measured by gentamicin killing assay and the results were confirmed by electron microscopy. pEXI adhered poorly to HeLa-Neo and CGM6, but attached very well to HeLa-CEA and HeLa-BGP (Fig. 1 A). The adherence of pEXI to HeLa-CGM1a and HeLa-NCA was intermediate.
Figure 1.
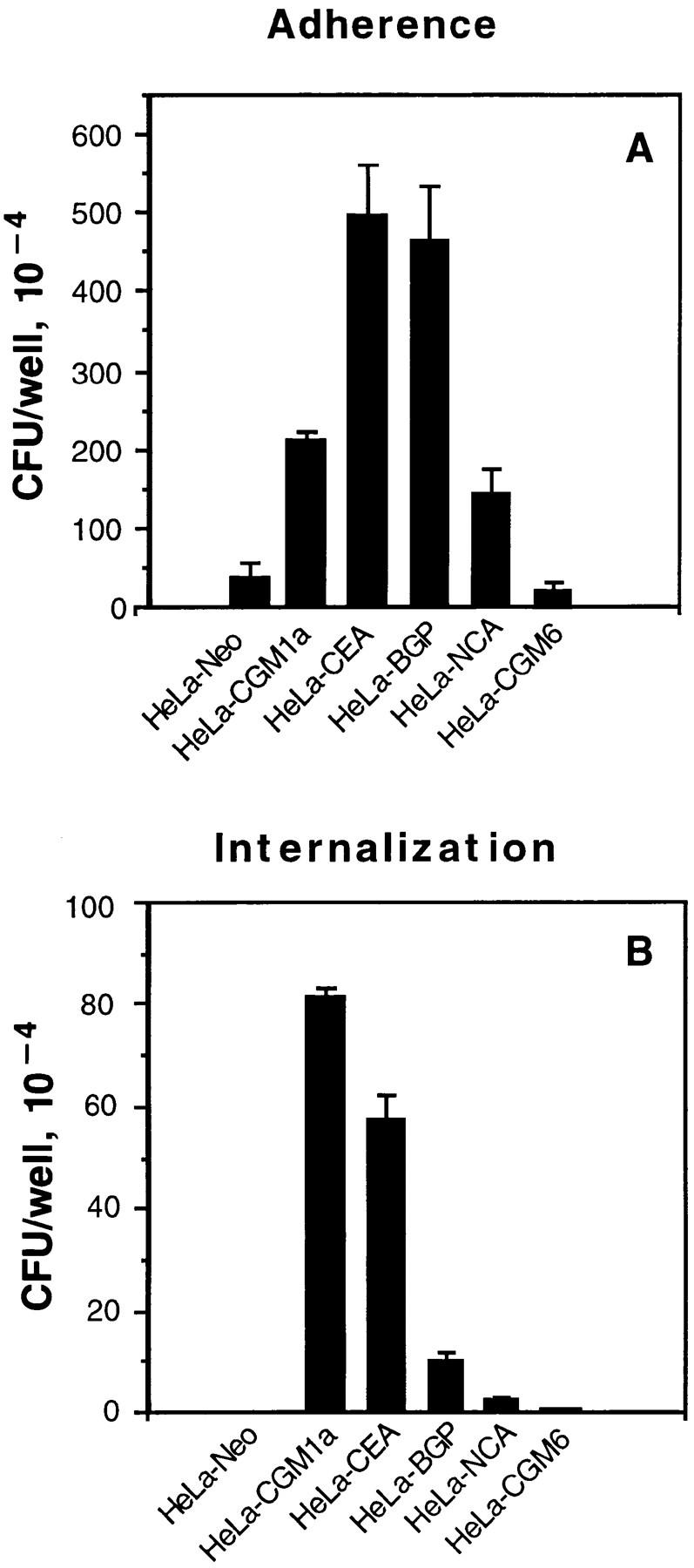
Adherence and internalization of pEXI by HeLa cells transfected with various CD66 antigens. All the cell lines were grown to confluence in 24well culture plates containing RPMI medium 1640 and were incubated with pEXI for 4.5 h. The adherent and intracellular E. coli were distinguished by incubation with gentamicin. (A) The ability of these cell lines to bind pEXI: HeLa-CEA >HeLaBGP >HeLa-CGM1a >NCA >>HeLa-CGM6 = HeLa-Neo = 0. (B) The number of intracellular bacteria. Although HeLaCGM1a showed the highest internalization of pEXI, HeLaCEA could also engulf pEXI. The internalization of pEXI into HeLaBGP was negligible. Bars, SEM.
The ability of these cell lines to promote invasion showed a different pattern. Although the adherence of pEXI to HeLa-BGPa was high, invasion was limited (Fig. 1 B). Electron microscopy demonstrated that HeLa-CEA cells were also able to engulf pEXI (Fig. 1 B) and OpaI GC (Fig. 2). However, compared to HeLa-CGM1a (Fig. 1 B, 2E), HeLaCEA cells demonstrated lower ability to internalize pEXI or OpaI GC (Figs. 1 B and 2 B). Virtually all HeLaCGM1a cells contained large numbers of GC, but a lower degree of cellular invasion by OpaI bacteria was observed with HeLa-CEA cells. HeLa-NCA and HeLa-CGM6 were hardly invaded at all.
Figure 2.
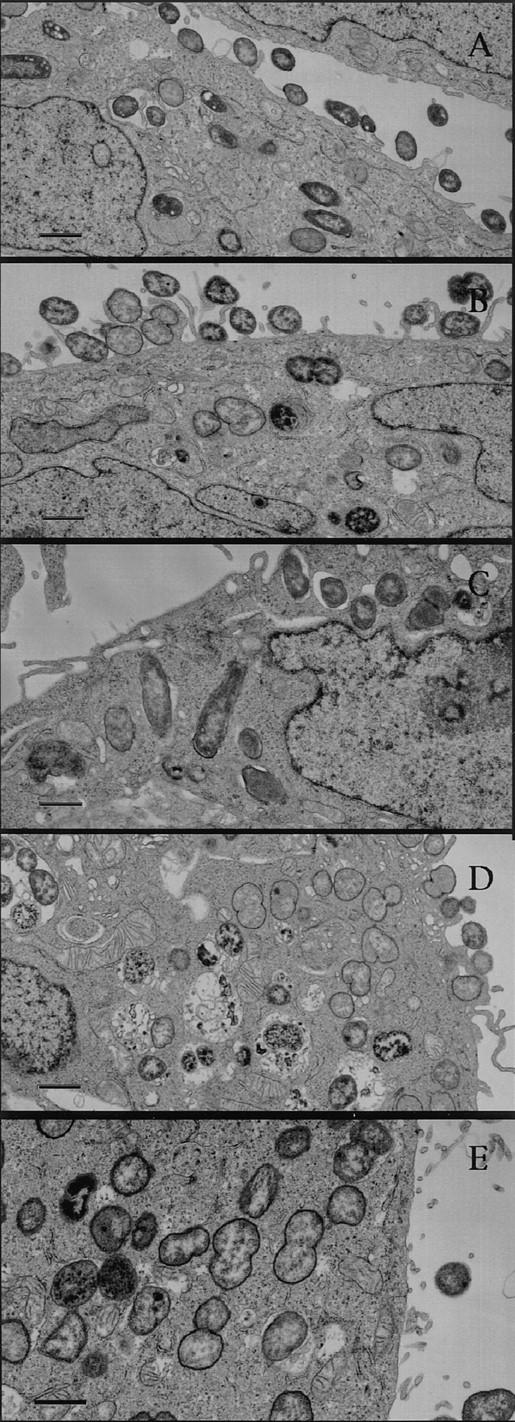
Electron micrographs of internalization of OpaI E. coli and GC by HeLa-CEA and LS 174T. The micrographs demonstrate the ability of HeLa-CEA to internalize OpaI E. coli (A) and OpaI GC (B). CEAexpressing colon cancer cells (LS 174T) were also able to engulf OpaI E. coli (C) and OpaI GC (D). The engulfment of OpaI CG by HeLaCGM1a (E) served as a positive control. Bar, 1 μm.
OpaI E. coli Bind the CEA Antigen.
The HeLa-CEA cells supported the highest level of adherence with pEXI (Fig. 1 A), indicating that the CEA protein is a receptor for Opa protein. To demonstrate a direct binding of Opa+ bacteria with CEA, purified CEA was mixed with equal amounts of Opa negative pGEM or positive pEXI bacteria. The bacteria–CEA mixture was incubated for 1 h, pelleted, and solubilized. The amount of CEA antigen bound to the bacteria was detected with COL-1 antibody by Western blotting. As seen in Fig. 3, pEXI bound much more CEA than pGEM.
Figure 3.
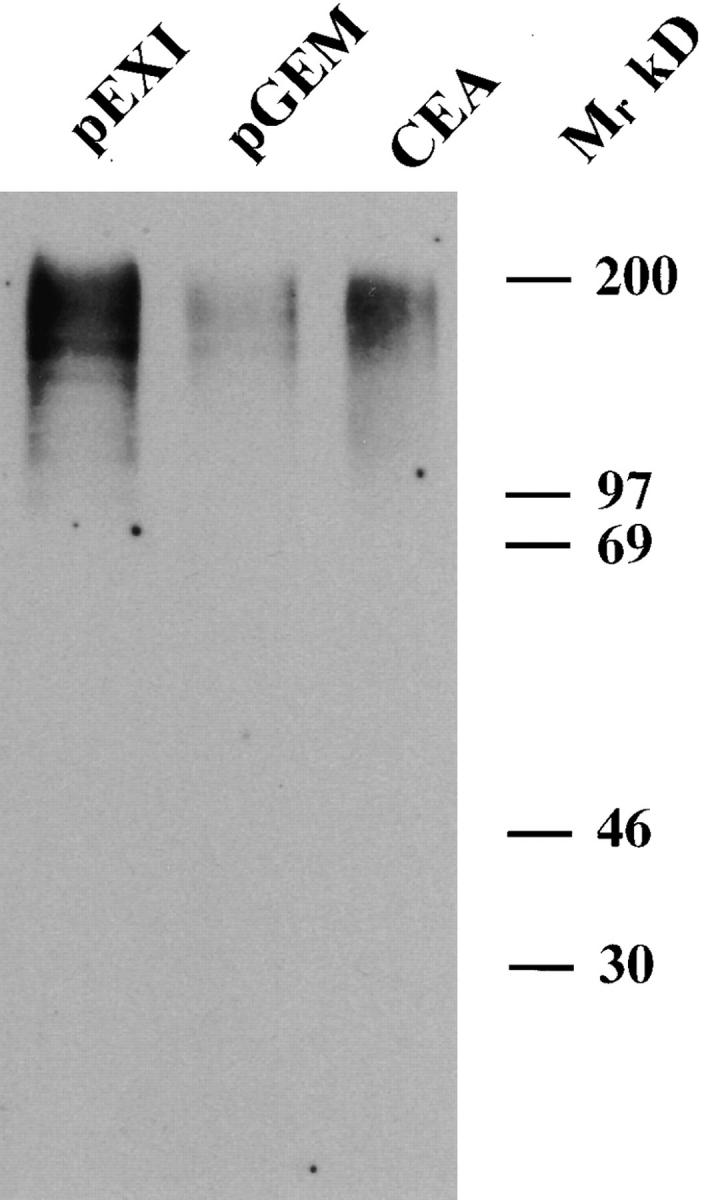
The binding of purified CEA to pEXI. Equal numbers of pGEM (Opa−) and pEXI (OpaI) were suspended in RPMI containing equal amounts of CEA. After incubation, the bacteria were recovered by centrifugation, washed, and lysed, and the lysates were subjected to SDS-PAGE and transfered to Immobilon-P membrane. The CEA bound on the bacteria was detected by anti-CD66 mAb (COL-1). Much more CEA was bound by pEXI than by pGEM.
Multiple Opa Proteins Interact with HeLa-CEA Cells, and Are Inhibited by Anti-CEA Antibody.
Since strain MS11 can express several Opa proteins, we examined whether other Opa proteins possess the same function, as illustrated in Fig. 4 A. HeLa-CEA cells also bound OpaA, OpaB, OpaC, and OpaH E. coli with similar efficiency, but only OpaA-mediated adherence was strongly inhibited by soluble heparin. The adherence mediated by OpaI and OpaC was inhibited by anti-CEA antibody (COL-1), but not by IB4 directed to CD18 antigen (Fig. 4 B). These results demonstrate that CEA antigen serves as receptor for several Opa proteins.
Figure 4.
Multiple Opa proteins promote adherence to HeLa-CEA. pGEM, pEXA, pEXB, pEXC, pEXH, and pEXI were incubated with HeLaCEA (A) for 4 h. All of Opa+ E. coli adhered to the cell lines. (B) Addition of COL-1 antibody (CD66) inhibited adherence of OpaI and OpaC E. coli to HeLaCEA. Control antibody IB4 (CD18) had no effect when added at the same final concentration (25 μg/ml). The interaction of pEXI and pEXC with HeLa-CEA was for 2 h and only accounted for the lower bacterial counts.
OpaI Bacteria Bind HeLa-CEA and CEA-expressing Colon Cancer Cells in a Heparin-independent Manner.
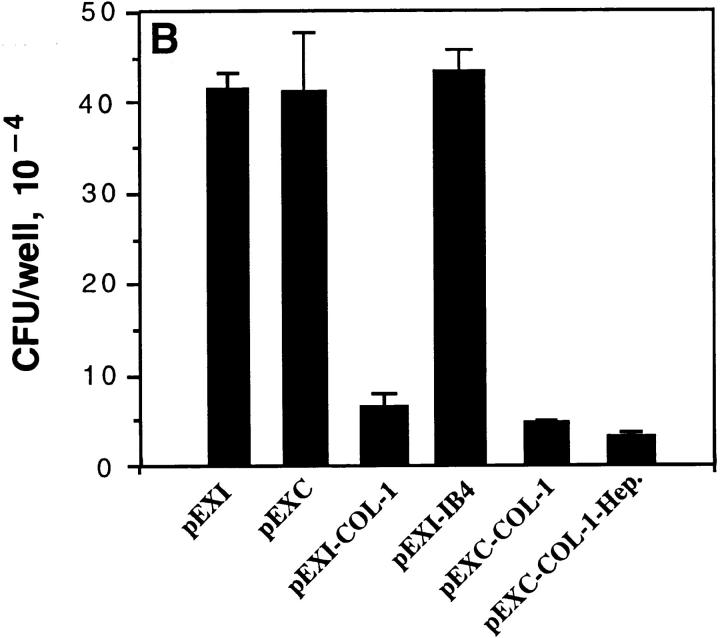
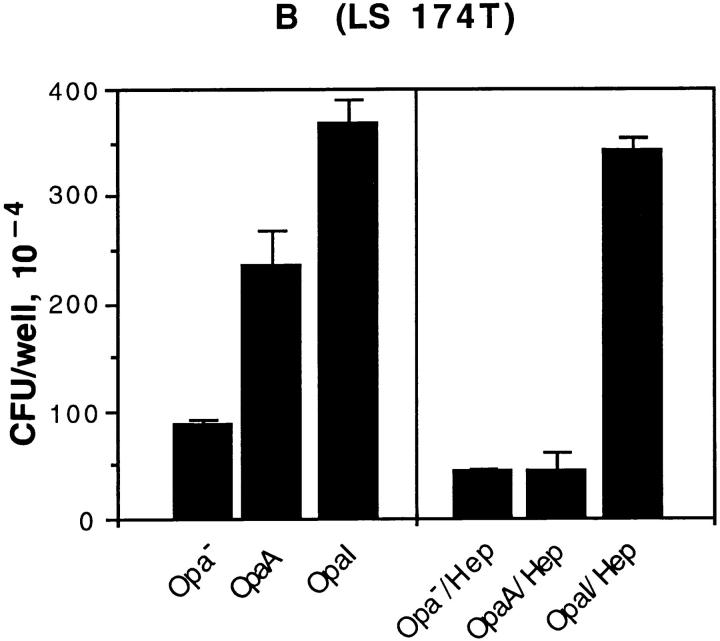
To further substantiate that the binding of Opa+ GC with CEA is due to ligand–receptor interaction, we compared the well-characterized CEA-expressing colon cancer cell line LS 174T with HeLa-CEA in adhesion assays. As seen in Fig. 5 both HeLa-CEA and LS 174T bound the OpaI GC in a heparinindependent fashion, in sharp contrast to the OpaA-mediated interaction. The colon cancer cell line supported adherence about threefold less efficiently than HeLa-CEA, but the overall pattern was identical.
Figure 5.
The interaction of Opa+ GC with HeLa-CEA cells and CEA-expressing colon cancer cells LS 174T. Opa+ GC were incubated with HeLaCEA (A) in RPMI medium 1640 buffer with or without soluble heparin (30 μg/ml). OpaA and OpaI promoted adherence to HeLa-CEA, and OpaA-mediated adherence was inhibited by heparin. Similar results were observed with CEA-expressing colon cancer line LS 174T.
OpaC Bacteria Interact with Both Heparan Sulfate and Members of the CEA Family.
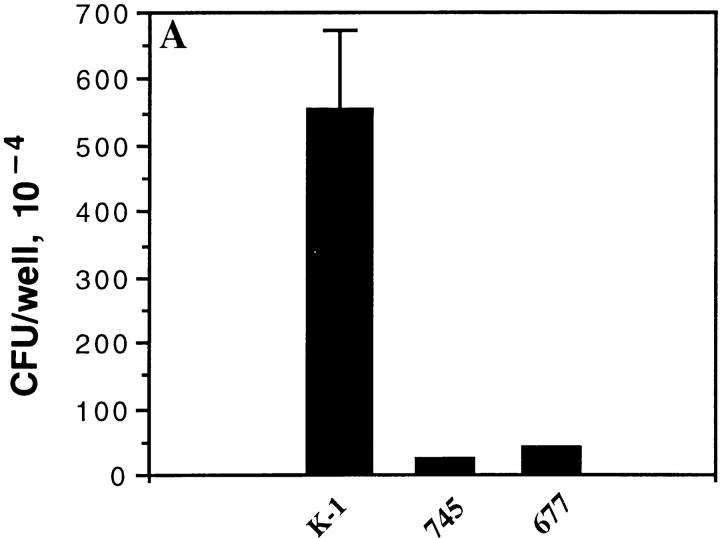
Previously published studies demonstrated that OpaA-mediated adherence to Chang conjunctival and CHO cells is dependent on binding to heparan sulfate syndecan receptors (24, 25). These studies also showed that OpaC had this activity, and that OpaC GC were able to bind tritiated heparin. However, the data shown in Fig. 4, A and B indicated that pEXC was able to bind the HeLaCEA, and that this binding was inhibited by treatment with CD66 antibody, suggesting that OpaC was able to mediate both adherence to syndecans and CD66 antigens. To provide further evidence that OpaC-mediated adherence to CHO epithelial cells is heparan sulfate dependent, we examined the adherence to heparan sulfate-deficient CHO mutants 745 and 677. Compared to wild-type CHO-K1, OpaC GC adhered to mutant 745 and 677 at a very low level (Fig. 6 A). Further proof for reactivity with CD66 antigens is provided in Fig. 6 B showing that pEXC, but not pEXA, is internalized by HeLa-CGM1a.
Figure 6.
Binding specificity of OpaC bacteria for both heparan sulfate and CD66 receptors. OpaC GC were incubated with wild-type CHO-K1 cells and the isogenic mutants 745 and 677 lacking surface heparan sulfate. OpaC GC adhered to CHO-K1 cells, but not the 745 and 677 mutants (A). (B) pGEM, pEXA, and pEXC were incubated with HeLa-CGM1a cells in RPMI 1640 medium for 4.5 h. The adherent and intracellular E. coli were distinguished by incubation with gentamicin. Only pEXC was recovered in large numbers after gentamicin treatment, although both pEXA and pEXC adhered to the HeLa-CGM1a.
Discussion
GC isolated either from the male urethra or from the cervix of infected females are most often Opa+ GC (36–38). Swanson et al. infected male volunteers intraurethrally with an Opa− strain of GC, and found that isolates recovered from the infection were predominantly Opa+ (11). Similar findings were also obtained with different challenge strains in further volunteer studies (12). Furthermore, high levels of Opa-specific antibody after gonococcal infection have been reported (39). Taken together, these data strongly suggest in vivo expression of Opa proteins plays an important role in gonococcal pathogenesis.
The present studies demonstrate that one of the mechanisms for Opa protein-mediated adherence was binding to CEA and CEA-related proteins. To provide this evidence, we chose to use a highly defined system, namely the use of E. coli expressing recombinant Opa proteins and a single cell line, HeLa transfected with various CD66 antigens. This strategy circumvents the unavoidable tendency of GC to alter their Opa phenotype by antigenic variation and allows certainty that the observed effects are strictly attributable to the expression of specific Opa proteins. To confirm that the results were reflective of gonococcal pathogenesis, we included experiments using Opa+ GC and also extended them to a well-characterized CEA-expressing colon cancer cell line, LS 174T. The CEA HeLa cell transfectants (HeLa-CEA) and the CEA-expressing colon cancer cell line bound and engulfed Opa+ bacteria and this interaction was inhibited by anti-CEA mAb. In addition, OpaI E. coli bound purified CEA antigen more effectively than Opa− E. coli.
CEA, a 180-kD tumor-associated cell-surface glycoprotein, was first described in 1965 (40). Later, it was found that antibodies to CEA reacted at low level with normal cells. Also, a number of closely related, cross-reacting antigens were identified that were initially termed nonspecific cross-reacting antigens (NCA; 41–43). The gene encoding CEA is located on human chromosome 19 (44), and is a member of a family of >20 expressible closely related genes (45) that belong to the Ig gene superfamily (46). The human CEA family consists of CEA, NCA, BGP (47), CGM1 including splicing variants CGM1a, CGM1b, and CGM1c (31, 48), CGM2, CGM6, CGM7, and pregnancyspecific glycoproteins (49–51). Although the in vivo function of CEA has not been determined, evidence shows that CEA functions as an intercellular adhesion molecule (52– 54). The binding of CEA to E. coli has been reported (55– 57), and CEA may also play a role as an accessory molecule in binding tumor cells to collagen type I (58).
We investigated the relative ability of the individual CD66 antigens to mediate adherence and internalization of Opa+ bacteria using HeLa cell transfectant cell lines. HeLa- CEA and HeLa-BGPa showed the highest level of adherence to OpaI, but BGP internalized the bacteria poorly. On the other hand, HeLa-CGM1a and HeLa-NCA showed intermediate levels of adherence, and only CGM1a promoted aggressive phagocytosis of OpaI E. coli (26) and GC (Fig. 2 E). HeLa-CGM6 (CD66b) could neither bind or engulf Opa+ bacteria (Fig. 1 A), although CGM6 is highly expressed on this HeLa cell line (59). Since CEA is not expressed in neutrophils (60), the most likely candidate for promoting phagocytosis of Opa+ bacteria is CGM1a. These results indicated that CD66 family showed variable ability in promoting adherence and internalization of Opa+ bacteria although they share homology in their NH2 termini (60).
The mechanism of CGM1a-promoted internalization may be that in CGM1a there is a cytoplasmic domain containing the YLYL motif, termed the immunoreceptor tyrosine activation motif, which is found in several receptor subunits that associate with cytoplasmic tyrosine kinases (61, 62). Activation of this motif leads to actin polymerization and phagocytosis (for review see reference 63). BGPa, which does not promote internalization of bacteria, does not contain immunoreceptor tyrosine activation motif in its cytoplasmic domain, but rather, two copies of immunoreceptor tyrosine inhibitory motif that are separated by 26 amino acids (47). This motif has been found in a number of immunoreceptors that mediate inhibitory effects (64, 65). However, CEA found on LS 174T cells is anchored to the membrane through a glycosyl-phosphatidylinositol anchor (66, 67). The GPI-anchored proteins mediate many functions in cell growth and cell–cell communication, and can initiate signal transduction (68–70). Thus, it is possible that the phagocytosis of Opa+ bacteria by means of CGM1a in neutrophils and CEA in epithelial cells uses the two distinct signaling mechanisms.
Among the Opa proteins tested, only OpaA showed no reactivity with CEA or CGM1a. OpaA has previously been shown to be reactive with heparan sulfate borne by syndecans and to bind radioactive heparin (24, 25). These studies showed that OpaC also had this specificity. Thus, the question of whether OpaC protein can interact with both heparan sulfate syndecan receptors and members of the CEA family arose. The data shown in Fig. 6 A demonstrates that OpaC GC bound to normal CHO cells, but failed to bind to CHO mutant cells defective in heparan sulfate synthesis, which was further proof for this specificity. On the other hand, OpaC promoted heparin-independent adherence to HeLa-CEA cells (Fig. 4 A), and induced the phagocytosis of OpaC E. coli into HeLa-CGM1a (Fig. 6 B). Thus, MS11 OpaC protein appears to target the two distinct receptors.
Gonorrhea is a genital disease that affects exclusively human beings, and adherence and invasion of epithelial cells is one of the important steps in this disease, as well as bacterial infections in general (71). However, the factors determining the efficient adherence and internalization of GC with or into specific cells are still poorly understood and are very dependent on the specific tissue culture cell line used (72). Identification of CEA and BGP as a receptor for Opa proteins is an important step in our understanding of GC pathogenesis. Normal adult human colon epithelium is known to produce small amounts of CEA-like antigens (73). GC, when isolated from rectal infections, express Opa proteins (38). It has also been shown that BGP is expressed on normal epithelia located in many organs including the uterus, cervix, prostate, and colon (74). Thus, it is likely that most of the tissues susceptible to GC infection express one or another member of the CD66 antigen family.
Acknowledgments
We thank Dr. Asesh Banerjee, Dr. Vijay Pancholi, and Mr. James Parker for useful suggestions and editorial comments on the manuscript.
This work was supported by Public Health Service grants AI 10615 and AI 26558.
Footnotes
1 Abbreviations used in this paper: BGP, biliary glycoprotein; CEA, carcinoembryonic antigen; CGM, CEA gene family member; CHO, Chinese hamster ovary; FTOC, fallopian tube organ culture; GC, Neisseria gonorrhoeae; NCA, nonspecific cross-reacting antigens; Opa, opacity.
References
- 1.Ward ME, Watt PJ. Adherence of Neisseria gonorrhoeaeto urethral mucosal cells: an electron-microscopic study of human gonorrhea. J Infect Dis. 1972;126:601–605. doi: 10.1093/infdis/126.6.601. [DOI] [PubMed] [Google Scholar]
- 2.Wigfield AS. 27 years of uninterrupted contact tracing. Br J Vener Dis. 1972;48:37–50. doi: 10.1136/sti.48.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973;137:571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watt, P.J., and M.E. Ward. 1980. Adherence of Neisseria gonorrhoeae and other Neisseria species to mammalian cells. In Bacterial Adherence. Series B, 6th ed. E.H. Beachey, editor. Chapman & Hall, Ltd., New York. 253–288.
- 5.Swanson J, Robbins K, Barrera O, Corwin D, Boslego J, Ciak J, Blake M, Koomey JM. Gonococcal pilin variants in experimental gonorrhea. J Exp Med. 1987;165:1344–1357. doi: 10.1084/jem.165.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward ME, Watt PJ, Robertson JN. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974;129:650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- 7.McGee ZA, Johnson AP, Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic microorganisms. Infect Immun. 1976;13:608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGee ZA, Gorby GL, Wyrick PB, Hodinka R, Hoffman LH. Parasite-directed endocytosis. Rev Infect Dis. 1988;10:S311–S316. doi: 10.1093/cid/10.supplement_2.s311. [DOI] [PubMed] [Google Scholar]
- 9.Gorby GL, Shaefer GB. Effect of attachment factors (pili plus Opa) on Neisseria gonorrhoeaeinvasion of human fallopian tube tissue in vitro: quantitation by computerized image analysis. Microb Pathog. 1992;13:93–108. doi: 10.1016/0882-4010(92)90070-5. [DOI] [PubMed] [Google Scholar]
- 10.Gorby G, Simon D, Rest R. Escherichia coli that express Neisseria gonorrhoeaeopacity-associated proteins attach to and invade human fallopian tube epithelium. Ann NY Acad Sci. 1994;730:286–289. doi: 10.1111/j.1749-6632.1994.tb44267.x. [DOI] [PubMed] [Google Scholar]
- 11.Swanson J, Barrera O, Sola J, Boslego J. Expression of outer membrane protein II by gonococci in experimental gonorrhea. J Exp Med. 1988;168:2121–2129. doi: 10.1084/jem.168.6.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jerse AE, Cohen MS, Drown PM, Whicker LG, Isbey SF, Seifert HS, Cannon JG. Multiple gonococcal opacity proteins are expressed during experimental urethral infection in the male. J Exp Med. 1994;179:911–920. doi: 10.1084/jem.179.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isberg R, Leong J. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 14.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenesinto epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 15.Wang, J., H. Schneider, and J. Griffiss. 1996. Neisseria gonorrhoeae must express the paraglobosyl LOS in order to invade human genitourinary epithelial cells. In Tenth International Pathogenic Neisseria Conference. Baltimore. 112–113.
- 16.Purachuri DK, Seifert HS, Ajioka RS, Karlsson K-A, So M. Identification and characterization of a Neisseria gonorrhoeaegene encoding a glycolipid-binding adhesin. Proc Natl Acad Sci USA. 1990;87:333–337. doi: 10.1073/pnas.87.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stromberg N, Deal C, Nyberg G, Normark S, So M, Karlsson K-A. Identification of carbohydrate structures that are possible receptors for Neisseria gonorrhoeae. . Proc Natl Acad Sci USA. 1988;85:4902–4906. doi: 10.1073/pnas.85.13.4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bessen D, Gotschlich EC. Interactions of gonococci with HeLa cells: attachment, detachment, replication, penetration, and the role of protein II. Infect Immun. 1986;54:154–160. doi: 10.1128/iai.54.1.154-160.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhat KS, Gibbs CP, Barrera O, Morrison SG, Jahnig F, Stern A, Kupsch E-M, Meyer TF, Swanson J. The opacity proteins of Neisseria gonorrhoeaestrain MS11 are encoded by a family of 11 complete genes. Mol Microbiol. 1991;5:1889–1901. doi: 10.1111/j.1365-2958.1991.tb00813.x. [DOI] [PubMed] [Google Scholar]
- 20.Weel JFL, Hopman CTP, van Putten JPM. In situ expression and localization of Neisseria gonorrhoeaeopacity proteins in infected epithelial cells: apparent role of Opa proteins in cellular invasion. J Exp Med. 1991;173:1395–1405. doi: 10.1084/jem.173.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makino S-I, van Putten JPM, Meyer TF. Phase variation of the opacity outer membrane protein controls invasion of Neisseria gonorrhoeaeinto human epithelial cells. EMBO (Eur Mol Biol Organ) J. 1991;10:1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kupsch EM, Knepper B, Kuroki T, Heuer I, Meyer TF. Variable opacity (Opa) outer membrane proteins account for cell tropisms displayed by Neisseria gonorrhoeaefor human leukocytes and epithelial cells. EMBO (Eur Mol Biol Organ) J. 1993;12:641–650. doi: 10.1002/j.1460-2075.1993.tb05697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belland RJ, Chen T, Swanson J, Fischer SH. Human neutrophil response to recombinant neisserial Opa proteins. Mol Microbiol. 1992;6:1729–1737. doi: 10.1111/j.1365-2958.1992.tb01345.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen T, Belland R, Wilson J, Swanson J. Adherence of pilus− Opa+gonococci to epithelial cells in vitro involves heparan sulfate. J Exp Med. 1995;182:511–517. doi: 10.1084/jem.182.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Putten J, Paul S. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrhoeaeentry into human mucosal cells. EMBO (Eur Mol Biol Organ) J. 1995;14:2144–2154. doi: 10.1002/j.1460-2075.1995.tb07208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen T, Gotschlich E. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virji M, Makepeace K, Ferguson DJP, Watt M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 28.Virji M, Watt M, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. . Mol Microbiol. 1996;22:926–939. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 29.Swanson J, Barrera O. Immunological characteristics of gonococcal outer membrane protein II assessed by immunoprecipitation, immunoblotting, and coagglutination. J Exp Med. 1983;157:1405–1420. doi: 10.1084/jem.157.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swanson, J. 1991. Some effects of LOS and Opa on surface properties of gonococci. In Proceedings of the Seventh International Pathogenic Neisseria Conference. Walter de Gruyter & Co., Berlin. 391–396.
- 31.Nagel G, Grunert F, Kuijpers T, Watt S, Thompson J, Zimmermann W. Genomic organization, splice variants and expression of CGM1, a CD66-related member of the carcinoembryonic antigen gene family. Eur J Biochem. 1993;214:27–35. doi: 10.1111/j.1432-1033.1993.tb17892.x. [DOI] [PubMed] [Google Scholar]
- 32.Esko JD, Stewart TE, Taylor WH. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc Natl Acad Sci USA. 1985;82:3197–3201. doi: 10.1073/pnas.82.10.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esko J, Weinke J, Taylor W, Ekborg G, Roden L, Anantharamaiah G, Gawish A. Inhibition of chondroitin and heparan sulfate biosynthesis in Chinese hamster ovary cell mutants defective in galactosyltransferase I. J Biol Chem. 1987;262:12189–12195. [PubMed] [Google Scholar]
- 34.Shieh M, WuDunn D, Montgomery R, Esko J, Spear P. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J Cell Biol. 1992;116:1273–1281. doi: 10.1083/jcb.116.5.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.James, J.F., and J. Swanson. 1978. Color/opacity colony variants of Neisseria gonorrhoeae and their relationship to the menstrual cycle. In Immunobiology of Neisseria gonorrhoeae. G.F. Brooks, editor. American Society for Microbiology, Washington DC. 338–343.
- 37.James JF, Swanson J. Studies on gonococcus infection. XIII. Occurrence of color/opacity colonial variants in clinical cultures. Infect Immun. 1978;19:332–340. doi: 10.1128/iai.19.1.332-340.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ison, C., and G. Brooks. 1991. Expression of protein II by clinical isolates of Neisseria gonorrhoeae. In Neisseriae 1990. M. Achtman, editor. Walter de Gruyter & Co., Berlin. 597–602.
- 39.Plummer F, Chubb H, Simonsen J, Bosire M, Slaney L, Nagelkerke N, Maclean I, Ndinya-Achola J, Waiyaki P, Brunham R. Antibodies to opacity proteins (Opa) correlate with a reduced risk of gonococcal salpingitis. J Clin Invest. 1994;93:1748–1755. doi: 10.1172/JCI117159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gold P, Freedman SO. Demonstration of tumor-specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Von Kleist S, Chavanel G, Burtin P. Identification of an antigen from normal human tissue that cross-reacts with the carcinoembryonic antigen. Proc Natl Acad Sci USA. 1972;69:2492–2494. doi: 10.1073/pnas.69.9.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mach J-P, Pusztaszeri G. Carcinoembryonic antigen (CEA): demonstration of a partial identity between CEA and a normal glycoprotein. Immunochemistry. 1972;9:1031–1034. doi: 10.1016/0019-2791(72)90113-9. [DOI] [PubMed] [Google Scholar]
- 43.Bordes M, Knobel S, Martin F. Carcinoembryonic antigen (CEA) and related antigens in blood cells and haematopoietic tissues. Eur J Cancer. 1975;11:783–786. doi: 10.1016/0014-2964(75)90171-1. [DOI] [PubMed] [Google Scholar]
- 44.Tynan K, Olsen A, Trask B, de Jong P, Thompson J, Zimmermann W, Carrano A, Mohrenweiser H. Assembly and analysis of cosmid contigs in the CEA-gene family region of human chromosome 19. Nucleic Acids Res. 1992;20:1629–1636. doi: 10.1093/nar/20.7.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson J, Zimmermann W, Osthus-Bugat P, Schleussner C, Eades-Perner A, Barnert S, Von Kleist S, Willcocks T, Craig I, Tynan K, et al. Long-range chromosomal mapping of the carcinoembryonic antigen (CEA) gene family cluster. Genomics. 1992;12:761–772. doi: 10.1016/0888-7543(92)90307-e. [DOI] [PubMed] [Google Scholar]
- 46.Paxton R, Mooser G, Pande H, Lee TD, Shively JE. Sequence analysis of carcinoembryonic antigen: identification glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci USA. 1987;84:920–924. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watt S, Fawcett J, Murdoch S, Teixeira A, Gschmeissner S, Hajibagheri N, Simmons D. CD66 identifies the biliary glycoprotein (BGP) adhesion molecule: cloning, expression, and adhesion functions of the BGPc splice variant. Blood. 1994;84:200–210. [PubMed] [Google Scholar]
- 48.Oikawa S, Inuzuka C, Kuroki M, Arakawa F, Matsuoka Y, Kosaki G, Nakazato H. A specific heterotypic cell adhesion activity between members of carcinoembryonic antigen family, W272 and NCA, is mediated by N-domains. J Biol Chem. 1991;266:7995–8001. [PubMed] [Google Scholar]
- 49.Tatarinov YS, Masyukevich VN. Immunological identification of a new β1-globulin in the blood serum of pregnant woman. Bull Exp Biol Med. 1970;69:66–68. [Google Scholar]
- 50.Oikawa S, Inuzuka C, Kosaki G, Nakazato H. Exon–intron organization of a gene for pregnancy-specific beta 1–glycoprotein, a subfamily member of CEA family: implications for its characteristic repetitive domains and C-terminal sequences. Biochem Biophys Res Commun. 1988;156:68–77. doi: 10.1016/s0006-291x(88)80806-4. [DOI] [PubMed] [Google Scholar]
- 51.Oikawa S, Inuzuka C, Kuroki M, Matsuoka Y, Kosaki G, Nakazato H. A pregnancy-specific beta 1–glycoprotein, a CEA gene family member, expressed in a human promyelocytic leukemia cell line, HL-60: structures of protein, mRNA and gene. Biochem Biophys Res Commun. 1989;163:1021–1031. doi: 10.1016/0006-291x(89)92324-3. [DOI] [PubMed] [Google Scholar]
- 52.Oikawa S, Kuroki M, Matsuoka Y, Kosaki G, Nakazato H. Homotypic and heterotypic Ca(++)-independent cell adhesion activities of biliary glycoprotein, a member of carcinoembryonic antigen family, expressed on CHO cell surface. Biochem Biophys Res Commun. 1992;186:881–887. doi: 10.1016/0006-291x(92)90828-9. [DOI] [PubMed] [Google Scholar]
- 53.Rojas M, Fuks A, Stanners C. Biliary glycoprotein, a member of the immunoglobulin supergene family, functions in vitro as a Ca2(+)-dependent intercellular adhesion molecule. Cell Growth Differ. 1990;1:527–533. [PubMed] [Google Scholar]
- 54.Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners C. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57:327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 55.Leusch H, Hefta S, Drzeniek Z, Hummel K, MarkosPusztai Z, Wagener C. Escherichia coliof human origin binds to carcinoembryonic antigen (CEA) and non-specific crossreacting antigen (NCA) FEBS Lett. 1990;261:405–409. doi: 10.1016/0014-5793(90)80603-g. [DOI] [PubMed] [Google Scholar]
- 56.Leusch H, Drzeniek Z, Hefta S, Markos-Pusztai Z, Wagener C. The putative role of members of the CEAgene family (CEA, NCA and BGP) as ligands for the bacterial colonization of different human epithelial tissues. Int J Med Microbiol. 1991;275:118–122. doi: 10.1016/s0934-8840(11)80775-9. [DOI] [PubMed] [Google Scholar]
- 57.Johnson J, Skubitz K, Nowicki B, Jacques-Palaz K, Rakita R. Nonlethal adherence to human neutrophils mediated by Dr antigen-specific adhesins of Escherichia coli. . Infect Immun. 1995;63:309–316. doi: 10.1128/iai.63.1.309-316.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pignatelli M, Durbin H, Bodmer W. Carcinoembryonic antigen functions as an accessory adhesion molecule mediating colon epithelial cell–collagen interactions. Proc Natl Acad Sci USA. 1990;87:1541–1545. doi: 10.1073/pnas.87.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berling B, Kolbinger F, Grunert F, Thompson J, Brombacher F, Buchegger F, von Kleist S, Zimmermann W. Cloning of a carcinoembryonic antigen gene family member expressed in leukocytes of chronic myeloid leukemia patients and bone marrow. Cancer Res. 1990;50:6534–6539. [PubMed] [Google Scholar]
- 60.Skubitz, K.M., K. Micklem, and E. Van Der Schoot. 1995. CD66 and CD67 cluster workshop report. In Leucocyte Typing V. S.F. Schlossman, editor. Oxford University Press, Oxford. 889–899.
- 61.Keegan A, Paul W. Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol Today. 1992;13:63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- 62.Reth M. The tyrosine activation motif as a target of protein tyrosine kinases and SH2 domains. Semin Immunol. 1995;7:21–27. doi: 10.1016/1044-5323(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 63.Greenberg S. Signal transduction of phagocytosis. Trends Cell Biol. 1995;5:93–99. doi: 10.1016/s0962-8924(00)88957-6. [DOI] [PubMed] [Google Scholar]
- 64.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig M, Ravetch J. A 13–amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature (Lond) 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 65.Daeron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, Fridman W. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 66.Hefta S, Hefta L, Lee T, Paxton R, Shively J. Carcinoembryonic antigen is anchored to membranes by covalent attachment to a glycosylphosphatidylinositol moiety: identification of the ethanolamine linkage site. Proc Natl Acad Sci USA. 1988;85:4648–4652. doi: 10.1073/pnas.85.13.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mayne K, Pulford K, Jones M, Micklem K, Nagel G, van der Schoot C, Mason D. Antibody By114 is selective for the 90 kD PI-linked component of the CD66 antigen: a new reagent for the study of paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1993;83:30–38. doi: 10.1111/j.1365-2141.1993.tb04627.x. [DOI] [PubMed] [Google Scholar]
- 68.Deckert M, Ticchioni M, Bernard A. Endocytosis of GPI-anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McConville M, Ferguson M. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem J. 1993;294:305–324. doi: 10.1042/bj2940305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lisanti M, Tang Z, Scherer P, Kubler E, Koleske A, Sargiacomo M. Caveolae, transmembrane signaling and cellular transformation. Mol Membr Biol. 1995;12:121–124. doi: 10.3109/09687689509038506. [DOI] [PubMed] [Google Scholar]
- 71.Falkow S. Bacterial entry into eukaryotic cells. Cell. 1991;65:1099–1102. doi: 10.1016/0092-8674(91)90003-h. [DOI] [PubMed] [Google Scholar]
- 72.Shaw JH, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. . Infect Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thompson J, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 74.Prall F, Nollau P, Neumaier M, Haubeck H, Drzeniek Z, Helmchen U, Loning T, Wagener C. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J Histochem Cytochem. 1996;44:35–41. doi: 10.1177/44.1.8543780. [DOI] [PubMed] [Google Scholar]