Abstract
To determine whether DNA immunization could elicit protective immunity to Leishmania major in susceptible BALB/c mice, cDNA for the cloned Leishmania antigen LACK was inserted into a euykaryotic expression vector downstream to the cytomegalovirus promoter. Susceptible BALB/c mice were then vaccinated subcutaneously with LACK DNA and challenged with L. major promastigotes. We compared the protective efficacy of LACK DNA vaccination with that of recombinant LACK protein in the presence or absence of recombinant interleukin (rIL)-12 protein. Protection induced by LACK DNA was similar to that achieved by LACK protein and rIL-12, but superior to LACK protein without rIL-12. The immunity conferred by LACK DNA was durable insofar as mice challenged 5 wk after vaccination were still protected, and the infection was controlled for at least 20 wk after challenge. In addition, the ability of mice to control infection at sites distant to the site of vaccination suggests that systemic protection was achieved by LACK DNA vaccination. The control of disease progression and parasitic burden in mice vaccinated with LACK DNA was associated with enhancement of antigen-specific interferon-γ (IFN-γ) production. Moreover, both the enhancement of IFN-γ production and the protective immune response induced by LACK DNA vaccination was IL-12 dependent. Unexpectedly, depletion of CD8+ T cells at the time of vaccination or infection also abolished the protective response induced by LACK DNA vaccination, suggesting a role for CD8+ T cells in DNA vaccine induced protection to L. major. Thus, DNA immunization may offer an attractive alternative vaccination strategy against intracellular pathogens, as compared with conventional vaccination with antigens combined with adjuvants.
Immunization with plasmid DNA has been shown to induce protective immunity in a variety of experimental models of infection (1–12) through both MHC class I– (13– 15) and class II–restricted T cell responses (16). The mechanism by which DNA vaccination is able to generate these potent immune responses appears to be through the induction of various proinflammatory cytokines elicited in response to certain immunostimulatory sequences (ISS)1 contained in the bacterial plasmid (17). In addition, recent evidence suggests that the route by which DNA is administered plays an important role in determining the type of CD4+ T cell (i.e., Th1 or Th2 type) response generated (18, 19). Thus, DNA vaccination can provide a useful and an effective way in providing effective immunity to particular pathogens depending on the type of immunity required for protection.
In susceptible BALB/c mice infected with Leishmania major, the preferential expansion of parasite specific Th2-type cells, and the relative absence of IFN-γ production results in progressive infection and a fatal outcome (20–23). In addition, it has been demonstrated that manipulation of the immune response at the time of infection with exogenous IL-12 (24, 25), or inhibition of endogenous IL-4 (26, 27), leads to protective immunity mediated by preferential induction of a Th1-type response. Furthermore, vaccination with either soluble leishmanial antigens (SLA) or the single parasite LACK protein in the presence of recombinant IL-12 protein has been shown to induce a protective Th1 response (28, 29).
In the present study, we investigated whether DNA vaccination could be used to induce a protective response in BALB/c mice infected with L. major. For this purpose, we used DNA encoding the recently identified LACK antigen (29). This 36-kD protein is a highly conserved protein among related leishmania species and is expressed in both promastigote and amastigote forms of the parasite. LACK has been found to be the focus of the early immune response directed to the parasite with most LACK-reactive T cells producing IL-4, but not IFN-γ, in response to antigenic stimulation (30). Moreover, BALB/c mice made tolerant to LACK by transgenic expression of LACK in the thymus were found to be resistant to parasite infection, suggesting that the early activation of LACK-reactive T cells contributes to the initial cytokine milieu favoring a nonhealing Th2-type phenotype (30). These observations were further extended in a recent report showing that early IL-4 messenger RNA (mRNA) expression in response to LACK was diminished in Vβ4-deficient BALB/c mice (31). Taken together, these results suggest that early production of LACK-specific IL-4 from Vα8+ Vβ4+ CD4+ T cells in BALB/c mice is required for Th2 development and susceptibility to infection. Thus, because altering the LACK-specific Th2 response in BALB/c mice induces resistance to infection (29–31), we assessed the ability of LACK DNA as a vaccine to induce protective immunity in BALB/c mice infected with L. major.
MATERIALS AND METHODS
Mice.
Female BALB/c mice were purchased from the Division of Cancer Treatment, National Cancer Institute (Frederick, MD) and kept in the National Institute of Allergy and Infectious Diseases Animal Care Facility under pathogen-free conditions. Mice used were between 6–8 wk of age.
Media and Reagents.
HBSS (Biofluids, Rockville, MD) was used as wash medium. RPMI-1640 (Biofluids, Rockville, MD) supplemented with 10% fetal bovine serum (Biofluids, Rockville, MD), penicillin (100 U/ml), l-glutamine (2 mM), sodium pyruvate (1 mM), and 2-ME (0.005 mM) was used to culture splenocytes and lymph node cells. LACK recombinant protein was purified from bacteria as previously described (29).
Plasmid Construction and Purification.
A cDNA encoding a truncated LACK protein (amino acids 143–312) was cloned in frame downstream to a Kozak consensus sequence and an initiation codon into a pECE vector (29). The insert was excised using HindIII and ligated into an expression vector PcDNA-3 downstream to the CMV promoter (Invitrogen, San Diego, CA). Eukaryotic expression vectors carrying cytokine cDNAs (IL-12, IL-4) were provided by J. Haynes (Auragen, Inc., Middleton, WI). Plasmid DNA was purified by double banding cesium chloride gradient ultracentrifugation. The 260/280 ratios ranged from 1.8 to 2.0.
Immunization.
Female BALB/c mice were injected in their hind footpads with 100 μg of plasmid DNA suspended in 50 μl of sterile PBS. In some experiments, 100 μg cytokine of cDNA was combined with 100 μg LACK DNA and injected as above. For immunization with protein, mice were injected as above with 50 μg of recombinant LACK protein with or without 1 mg of rIL-12 (Genetics Institute, Cambridge, MA). Mice were boosted 2 wk later with their initial regimen.
Infectious Challenge.
L. major (WHOM/IR/−173) promastigotes were grown at 26°C in 199 medium supplemented with 20% HI-FCS (Hyclone Laboratories, Inc., Logan, UT), 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine, 40 mM Hepes, 0.1 mM adenine (in 50 mM Hepes), 5 μg/ml hemin (in 50% triethanolamine), and 1 μg/ml 6-biotin (M199/S). Infective-stage promastigotes (metacyclics) of L. major were isolated from stationary cultures (5–6 d old) by their lack of agglutination with 100 μg/ml of peanut agglutinin (Vector Laboratories, Inc., Burlingame, CA). Mice were challenged with 105 metacyclic promastigotes in their hind footpads 2 wk after the boost. Weekly footpad swelling measurements were recorded using a caliper.
Parasite Quantitation.
Parasite loads in footpads were determined by sequential immersion of footpads in Wescodyne solution (Amsco, Erie, PA), 70% EtOH, and sterile dH2O, before homogenization of weighed tissue in microfuge tubes containing 100 μl of M199/S. Each tissue homogenate was serially diluted in a 96-well flat-bottomed microtiter plate containing biphasic medium, prepared using 50 μl NNN medium with 30% defibrinated rabbit blood, and overlaid with 50 μl M199/S. The number of viable parasites per milligram of tissue was determined from the highest dilution at which promastigotes could be grown out following up to 7 d incubation at 26°C. An identical limiting dilution assay was used to quantitate viable parasites in single cell suspensions of draining lymph nodes obtained at various times after infection.
Treatment of Mice with Neutralizing Antibodies.
Purified neutralizing mAb (1 mg/mouse intraperitoneally) against murine IL-12 (hybridoma c17.8), was obtained from Dr. G. Trinchieri (Wistar Institute, Philadelphia, PA) and injected into BALB/c mice at the time of initial vaccination and then on a weekly basis until 4 wk after infection. Anti-murine CD8 (2.43) was injected into BALB/c mice either at the time of vaccination or infection and then on a weekly basis to ensure continuing depletion. CD8 T cell depletion was confirmed by FACS® analysis of splenocytes from euthanized mice.
Cytokine Production Assay.
At various times points after infection or immediately before infection, mice were killed and the draining lymph nodes were harvested. Single cell preparations from the lymph nodes were plated in triplicate in a 96-well microtiter plate at 3 × 105 cells/200 μl. LACK protein (10 μg/ml) or media were added to cultures and supernatants were collected 48 h later and stored at −20°C.
Measurement of Cytokine Production.
Measurement of IFN-γ and IL-4 were assessed by specific ELISA as previously described (32). The lower limit of detection of IFN-γ and IL-4 was 30 pg/ml and 1.5 pg/ml, respectively.
Measurement of Lack-specific Antibody Responses.
Pooled serum samples (n = 7–12 mice/group) were obtained at various time points after infection and analyzed for the presence of LACK-specific antibodies. In brief, 96-well Immulon-4 plates were coated with LACK protein (10 μg/ml) overnight at 4°C. Plates were blocked with 2% BSA/PBS at 37°C for 1 h to prevent nonspecific binding. Sera was added at serial fivefold dilutions (starting at 1:5) and incubated overnight at room temperature. Horseradish peroxidase–conjugated-goat anti–mouse IgG1 or IgG2a (Southern Biotechnology Associates, Inc., Birmingham, AL) was added for 1 h at 37°C. ABTS peroxidase substrate (KP Laboratories, Gaithersburg, MD) was added and absorbance was read on ELISA plate reader (Dynatech, Chantilly, VA) using a 410-nm filter referenced to 510 nm.
RESULTS
LACK DNA Vaccination Induces Similar Protective Efficacy Compared with LACK Protein Plus rIL-12.
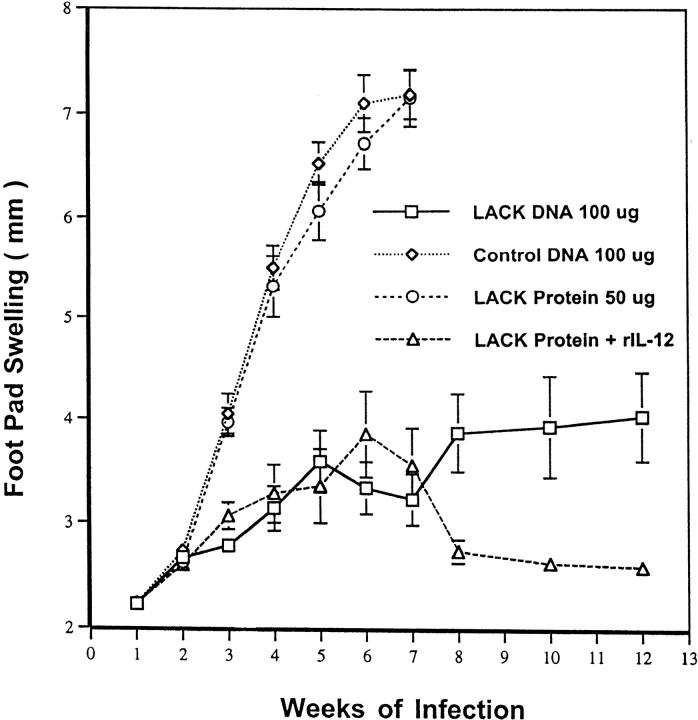
In the initial experiment, the ability of LACK DNA vaccination to induce a protective response was directly compared with that achieved by LACK protein and rIL-12. This latter immunization regimen has been previously shown to be an effective vaccine against L. major infection (29). As shown in Fig. 1, mice vaccinated with either LACK DNA alone, or with LACK protein and rIL-12, effectively controlled disease at 12 wk after infection compared with mice vaccinated with LACK protein alone or control DNA. Although in this particular experiment there is a modest decrease in footpad swelling from mice vaccinated with LACK protein and rIL-12 compared with LACK DNA alone; this did not correlate with a reduction in parasite load (see below). Moreover, in other experiments not shown, mice vaccinated with LACK DNA and LACK protein plus IL-12 had similar degrees of footpad swelling.
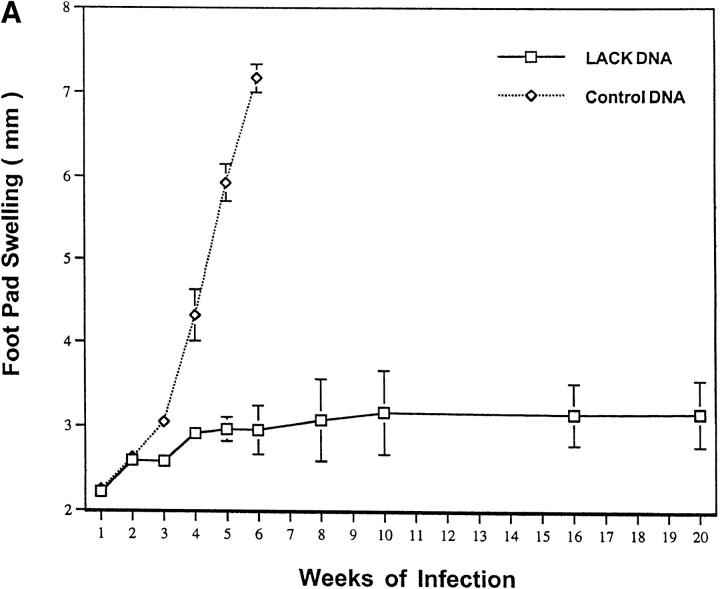
Figure 1.
LACK DNA vaccination provides similar protection as LACK protein plus rIL-12 to BALB/c mice infected with L. major. BALB/c mice (n = 12/group) were initally immunized and boosted 2 wk later with LACK DNA, control DNA (100 μg), or with LACK protein (50 μg) with or without rIL-12 (1 μg). 2 wk after the boost, mice were challenged in the hind footpad with 105 live L. major metacyclic promastigotes. Weekly footpad measurements represent the average foot pad scores ± SEM.
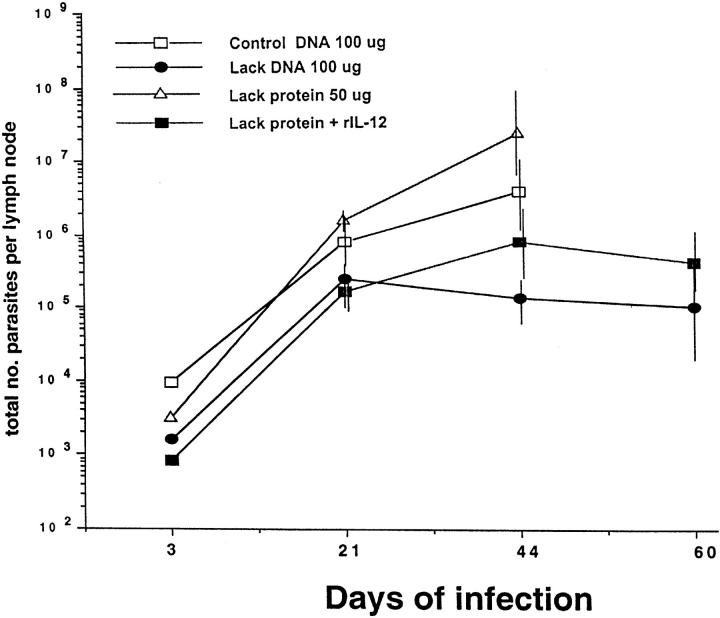
To determine whether the course of disease as assessed by footpad swelling was correlated with the parasitic burden, L. major were quantitated from draining LN at various time points after infection (Fig. 2). Mice vaccinated with LACK DNA had an ∼2-log reduction in parasite burden at 6 wk after infection compared with mice vaccinated with control DNA. Similarly, mice vaccinated with LACK protein plus rIL-12 had a reduction in parasitic load compared with those mice vaccinated with LACK protein alone. Finally, it should be noted that mice vaccinated with LACK DNA or LACK protein plus rIL-12 continued to show control of parasite replication for up to 10 wk after infection. Similar results were seen in a separate experiment when parasitic burden was quantitated from the footpad lesions 8 wk after infection. Mice vaccinated with control DNA had 2.1 × 109 viable parasites/mg of tissue. In contrast, mice vaccinated with LACK DNA were found to have 4-log decrease (3.02 × 105 parasites/mg of tissue) in parasitic burden from their footpad lesions.
Figure 2.
Quantitation of viable parasite from draining lymph nodes. Single cell suspensions were made from draining lymph nodes harvested from infected mice at various time points after infection. The number of viable parasites was determined from the highest dilution at which promastigotes could be grown out 7 d of incubation. Data shown represents an average of three mice ± one SD at each time point.
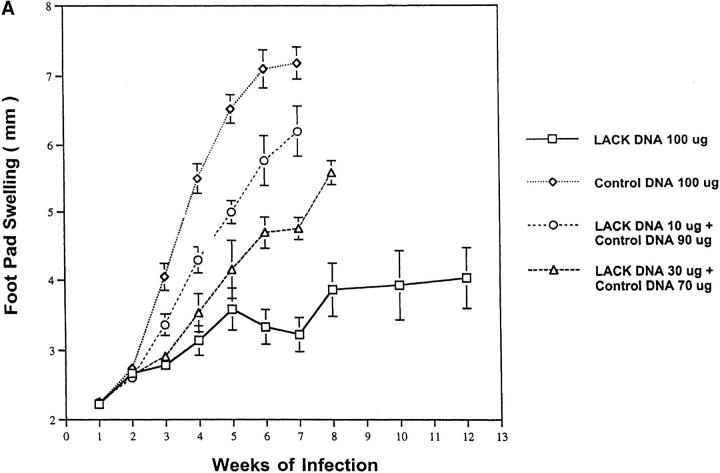
LACK DNA Immunization Protects BALB/c Mice in a Dose-dependent Manner.
To determine the optimal amount of LACK DNA needed to confer protection, mice were injected with either 10, 30, or 100 μg of LACK DNA. To control for potential nonspecific immunostimulatory effects of bacterial DNA, control DNA was added to some groups so that all mice received a total of 100 μg of DNA. As shown in Fig. 3 A, LACK DNA vaccination induced effective immunity in a dose-responsive manner. Thus, based on these experiments, we conclude that at least 100 μg of LACK DNA is required for a protective response.
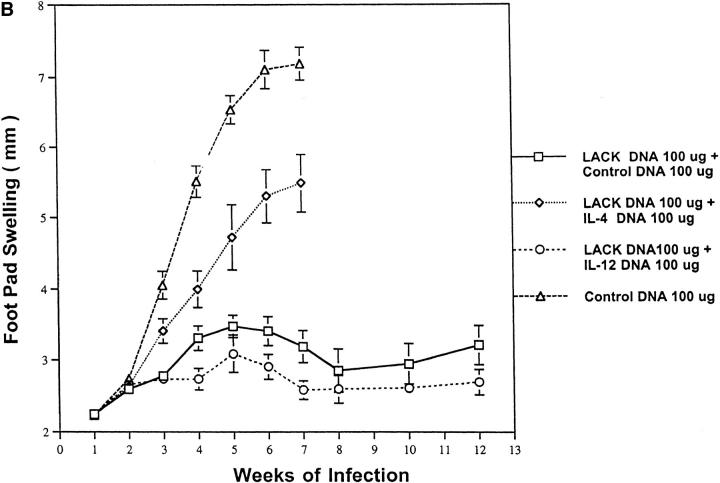
Figure 3.
Cytokine DNA and the amount of DNA used for vaccination influence disease outcome. (A) Mice (n = 12/ group) were immunized and boosted 2 wk later with varying doses of LACK DNA (10, 30, and 100 μg) in combination with varying amounts of control DNA to provide a total of 100 μg of DNA per vaccination. (B) In the same experiment, mice (n = 12/group) were immunized and boosted 2 wk later with LACK DNA (100 μg) plus 100 μg of control DNA, IL-4 DNA, or IL-12 DNA. 2 wk after the boost, mice were challenged in the hind footpad with 105 live L. major metacyclic promastigotes.
Addition of Cytokine DNA Influences the Protective Efficacy of LACK DNA Vaccination.
As alluded to in the Introduction, IL-4 and IL-12 have been shown to be critical cytokines in controlling disease outcome to L. major infection. To evaluate whether combining cytokine DNA to LACK DNA affected the immunity to L. major infection, IL-4 DNA and IL-12 DNA were injected concomitantly with LACK DNA. It should be noted that before these experiments, IL-4 and IL-12 DNA were shown to produce biologically active protein from supernatants of COS cells transfected with these respective DNAs (data not shown). As shown in Fig. 3 B, footpad swelling in mice vaccinated with LACK DNA plus IL-12 DNA was not substantially different than that seen in mice vaccinated with LACK DNA plus control DNA. Because bacterial DNA (by virtue of their immunostimulatory sequences) has been shown to be a potent inducer of IL-12 and IFN-γ, it is likely that the effects of the additional control DNA is comparable to the effect achieved by IL-12 DNA. Remarkably, the addition of IL-4 DNA significantly abrogated the protective efficacy of LACK DNA. This result is consistent with previous observations showing the potent effects of IL-4 in regulating protective immunity to L. major. Moreover, these data suggest that when sufficient amounts of IL-4 are present, they exert a dominant effect over that of IL-12 (32).
LACK DNA Immunization Leads to a Systemic and Durable Immune Response.
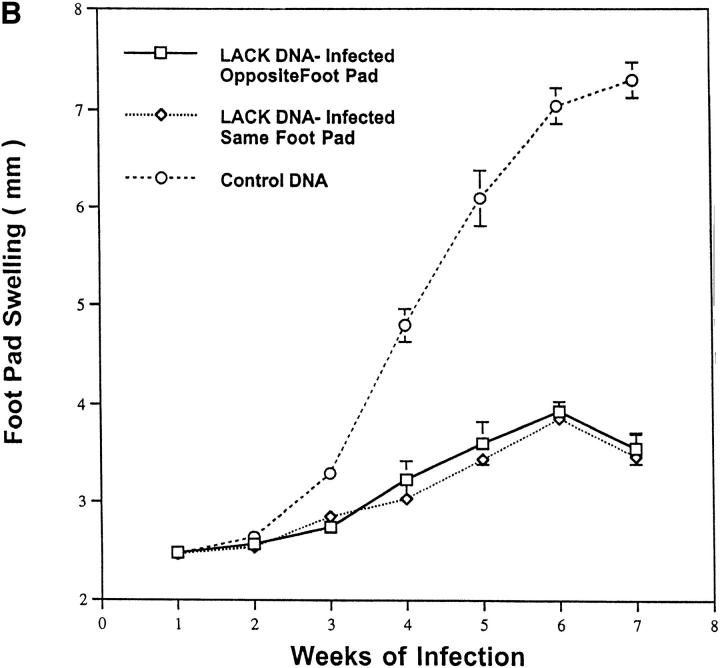
It was of additional interest to determine whether effective immunity induced by LACK DNA vaccination was both systemic and durable over a prolonged period after infection. Moreover, it was important to determine whether the protective response induced by LACK DNA vaccination was maintained when mice were infected beyond 2 wk after the last vaccination. To this end, mice were infected 5 wk after the last vaccination with LACK DNA and measurements of footpad lesions were continued for at least 20 wk after infection. As shown in Fig. 4 A, mice vaccinated with LACK DNA maintained an effective immune response that was sustained for at least 20 wk, even when the infectious challenge was given 5 wk after the last vaccination. The ability of LACK DNA to induce effective immunity at a site distant from the point of vaccination was shown in a separate experiment by infecting mice in the opposite footpad to which they received LACK DNA vaccination. As shown in Fig. 4 B, mice were protected in a similar manner to those mice infected in the same footpad.
Figure 4.
LACK DNA vaccination induces a systemic and durable immune response to mice infected with L. major. (A) BALB/c mice (n = 6/ group) were initially immunized with LACK or control DNA (100 μg) and boosted 2 wk later. 5 wk after the boost, mice were challenged in the hind footpad with 105 live L. major metacyclic promastigotes. (B) In a separate experiment, BALB/c mice (n = 6/group) were initially immunized and boosted 2 wk later with LACK or control DNA (100 μg) in the right hind footpad. 2 wk after the boost, mice were challenged with 105 live L. major metacyclic promastigotes in the same or the opposite footpad to which they recieved LACK DNA vaccination.
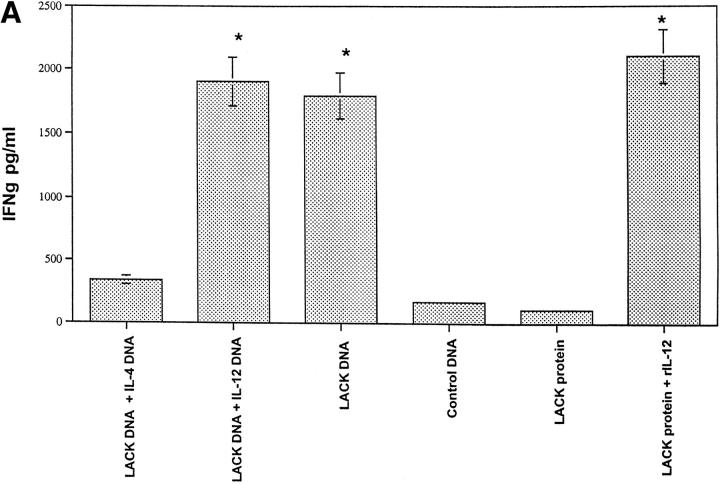
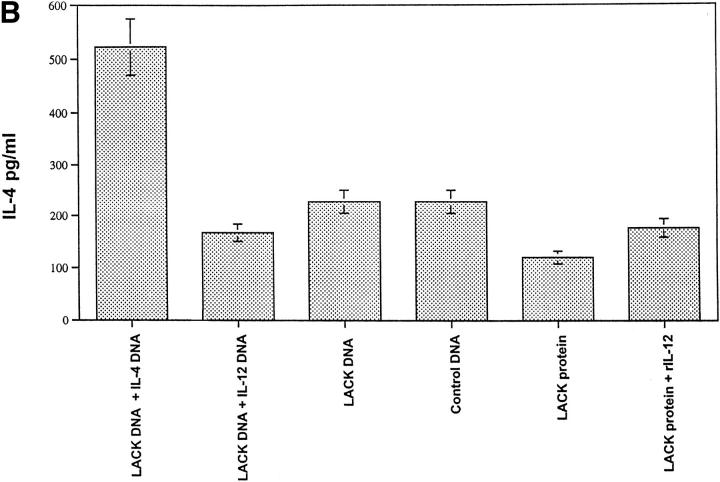
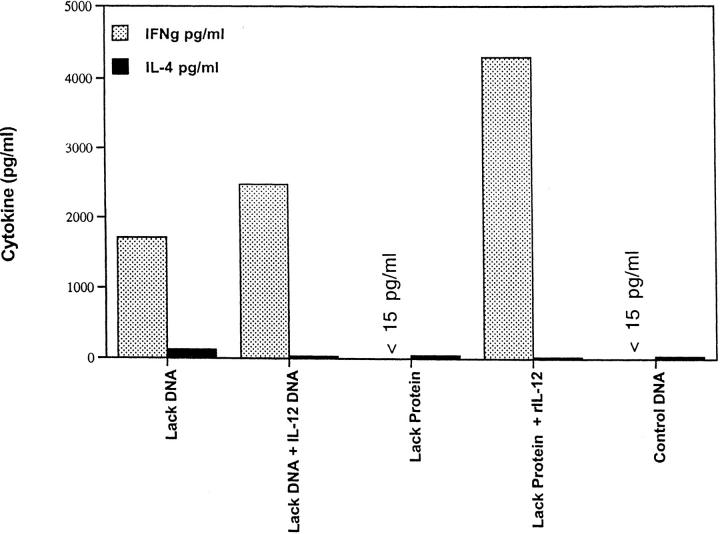
LACK DNA Vaccination Enhances IFN-γ Production In Vitro from Draining Lymph Node Cells of Mice Infected with L. Major.
Production of IFN-γ and IL-4 from draining LN was assessed after in vitro stimulation with LACK protein 6 wk after infection. As shown in Fig. 5 A, LN cells from mice that developed a healing phenotype (i.e., LACK DNA, LACK DNA plus IL-12 DNA, and LACK protein plus rIL-12 protein) made significantly more LACK-specific IFN-γ compared with mice (control DNA or LACK protein) with progressive disease (P < 0.005). Furthermore, mice vaccinated with LACK DNA plus IL-4 DNA had a decrease in production of LACK-specific IFN-γ, consistent with the fact that these mice were unable to control infection (see Fig. 3 B). In the same experiments, IL-4 production was also assessed from LN cells stimulated with LACK antigen. Unexpectedly, production of IL-4 was similar in all groups regardless of the disease outcome (Fig. 5 B); however, it should be noted that there was an increase in the production of antigen-specific IL-4 from mice vaccinated with LACK DNA plus IL-4 DNA. Finally, similar results with regard to production of IFN-γ and IL-4 were observed using a class II MHC peptide for LACK or SLA for stimulation, although the magnitude of the SLA response was less than that seen in response to LACK protein or specific peptide (data not shown).
Figure 5.
In vitro production of IL-4 and IFN-γ from lymph node cells of vaccinated mice infected with L. major at 6 wk after infection. Individual mice (n = 3) were euthanized and the draining lymph nodes were harvested 6 wk after infection. Single cell preparations were plated in triplicate in 96-well microtiter plates at 3 × 105 cells/200 μl in media alone or with LACK protein (10 μg/ml). 48 h later, supernatants were harvested and IFN-γ and IL-4 content were assayed by ELISA. Production of IFN-γ in media alone was <30 pg/ml. Production of IL-4 in media alone was usually 125 pg/ml or less. Data as shown represents the amount of IL-4 and IFN-γ averaged from three individual mice ± SEM. *P <0.005 in comparing IFN-γ produced from mice vaccinated with LACK DNA or LACK protein plus rIL-12 versus that produced from mice vaccinated with control DNA or LACK protein alone.
LACK-specific Production of IgG1 and IgG2A Are Increased in Vaccinated Mice Infected with L. major.
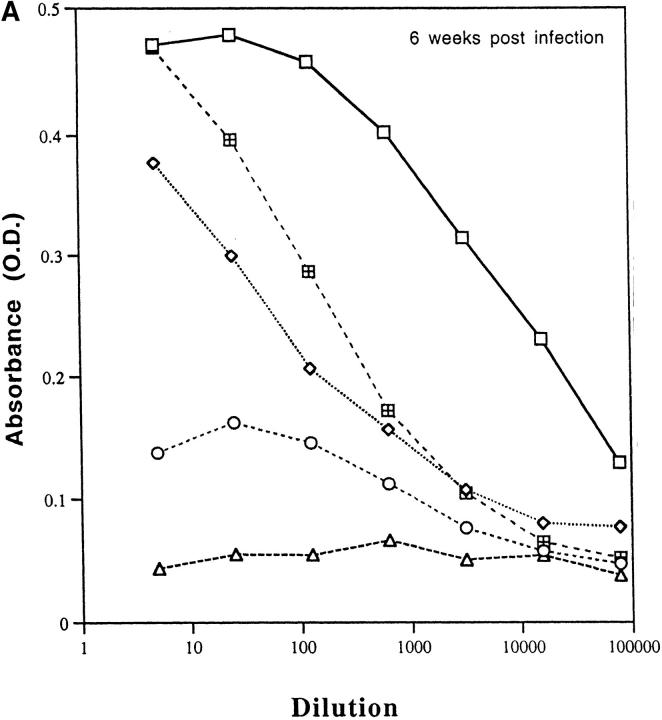
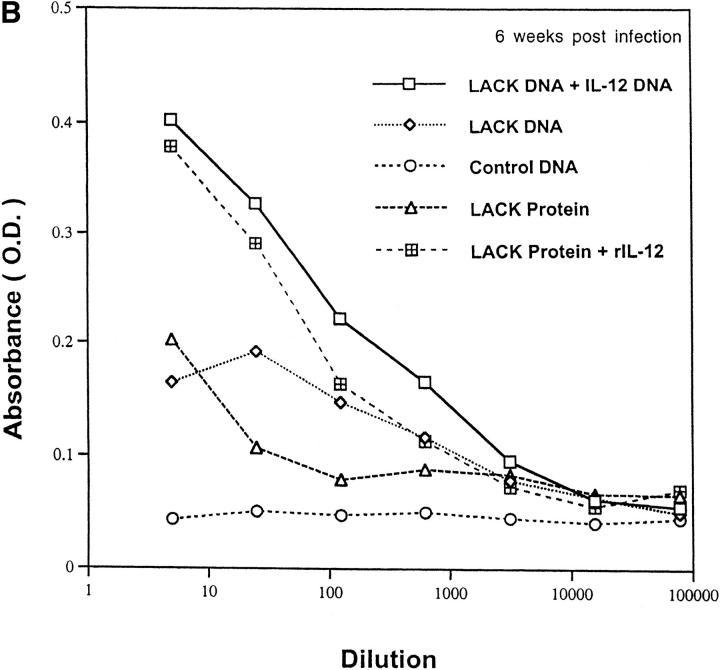
Because cytokines such as IL-4 and IFN-γ direct immunoglobulin class switching for IgG1 and IgG2a, respectively, we measured LACK-specific production of these antibody isotypes to provide an indirect, but physiologic in vivo assessment of the pattern of cytokine production. As shown in Fig. 6 A, mice that developed an effective immune response (LACK DNA plus or minus IL-12 DNA or LACK protein plus rIL-12) had substantially higher levels of LACK-specific IgG2a antibody titers compared with nonhealing mice (control DNA or LACK protein). Furthermore, as shown in Fig. 6 B, it was remarkable that LACK-specific IgG1 remained elevated in mice that had developed a protective immune response consistent with the data shown above (see Fig. 5 B). Finally, total serum IgE were found to be similar in all groups of mice regardless of disease outcome, providing further evidence that IL-4 levels in vivo were not substantially different in any of the mice (data not shown). These results are consistent with the in vitro assessment of cytokine production shown above in that IFN-γ is increased from groups that are protected compared with control mice, but IL-4 is not significantly different among the various groups.
Figure 6.
LACK-specific production of IgG1 and IgG2A in vaccinated mice infected with L. major. Pooled sera (n = 7–12 mice per group) was collected 6 wk after infection from mice vaccinated with LACK DNA with or without IL-12 DNA, or LACK protein in the presence or absence of rIL-12. Sera was tested for LACK-specific antibody (A) IgG2a or (B) IgG1. Data shown is an average of duplicate absorbance (OD) values using serial fivefold dilutions.
Enhanced Production of IFN-γ Before Infection Is Predictive of Disease Outcome.
It is clear from previous studies that the cytokine milieu at the initiation of infection is critical in determining disease outcome (33–35). In this regard, antigen-specific in vitro production of IL-4 and IFN-γ were assayed from draining LN of immunized mice before infection with L. major. As shown in Fig. 7, mice that controlled infection (LACK DNA plus or minus IL-12 DNA or LACK protein plus rIL-12) made substantial amounts of IFN-γ in response to LACK protein, whereas nonhealing mice had no detectable IFN-γ. As a control, there was no production of IFN-γ in any of the groups in response to SLA (data not shown). These results suggest that enhanced production of LACK-specific IFN-γ before infection is predictive of disease outcome and further underscores the ability of LACK DNA vaccination to induce IFN-γ preferentially.
Figure 7.
Enhanced production of IFN-γ before infection is predictive of disease outcome. Pooled draining lymph nodes from mice (n = 5) were harvested 2 wk after the boost and before mice were infected with L. major. IL-4 and IFN-γ protein production were determined in a similar manner to that outlined in Fig. 5.
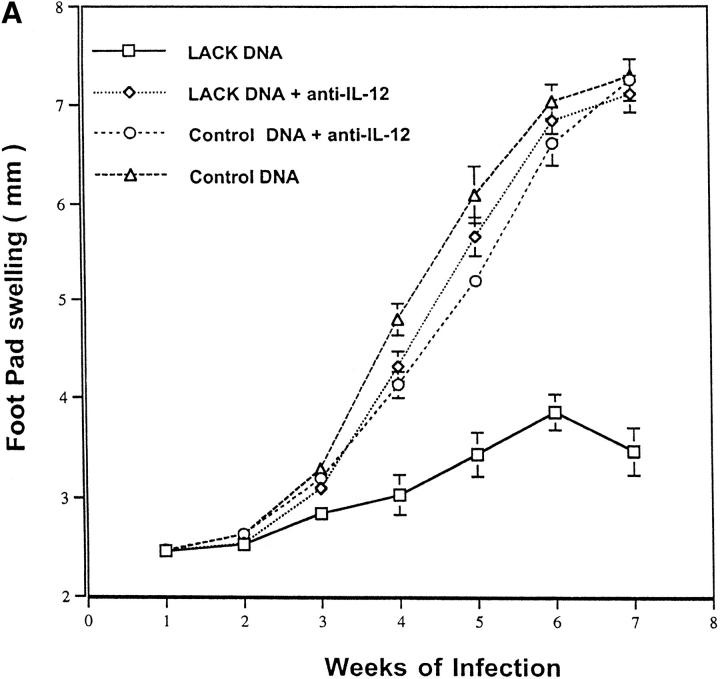
LACK DNA Vaccination Confers Protection Through an IL-12 and CD8-dependent Mechanism.
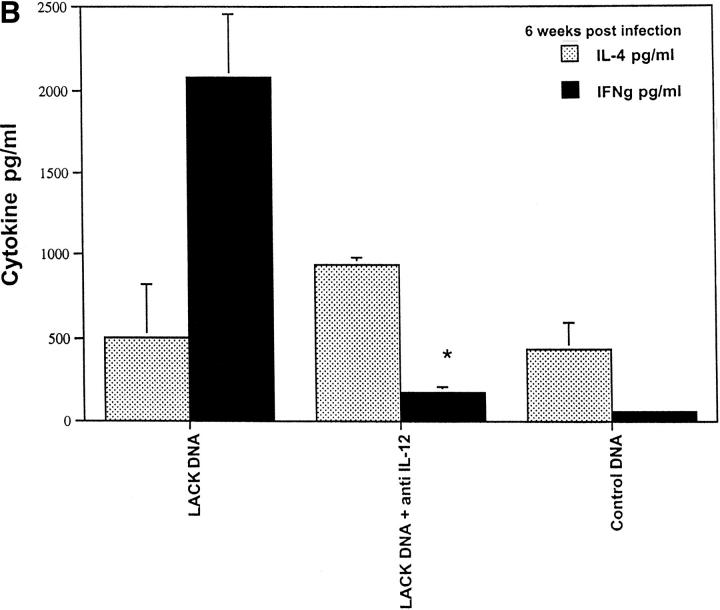
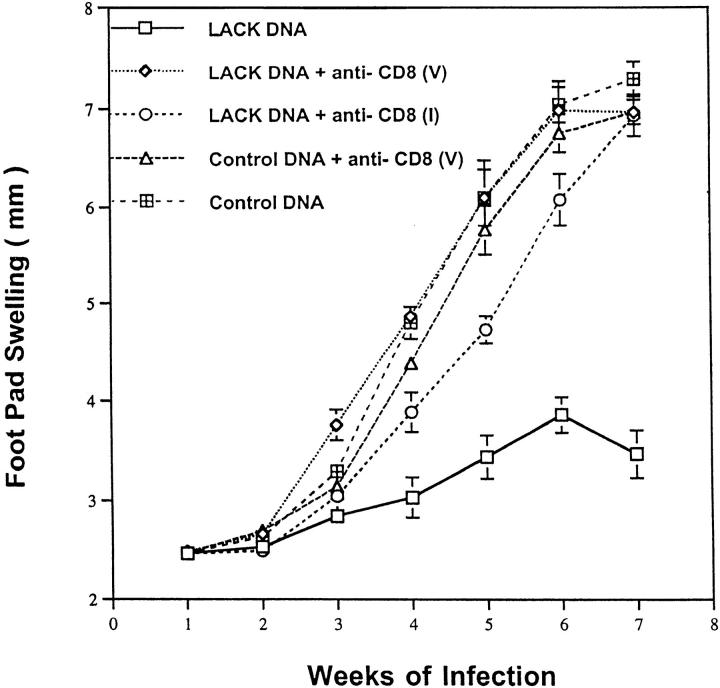
To assess the mechanism by which LACK DNA vaccination enhanced production of IFN-γ, mice were vaccinated with LACK DNA and treated weekly from the time of initial vaccination until 4 wk after infection with a neutralizing antibody against IL-12. As shown in Fig. 8 A, anti–IL-12 treatment completely abrogated protection. Furthermore, vaccinated mice treated with anti–IL-12 had a striking inhibition of in vitro production of IFN-γ (Fig. 8 B), suggesting that LACK DNA induced protective immunity through IL-12–dependent production of IFN-γ. In addition to the ability of DNA vaccination to enhance Th1 responses, it has also been shown to be a potent inducer of MHC class I–restricted CD8 responses (13–15). Furthermore, because CD8+ T cells typically secrete IFN-γ and have been previously shown to have a role in secondary infection to L. major (36–40), we evaluated the effect of CD8+ T cell depletion at both the time of vaccination and the time of infection. Depletion of CD8+ T cells at the time of vaccination would potentially eliminate an initial source of IFN-γ that may be important in generating a strong Th1 response. Alternatively, by delaying the depletion of CD8+ T cells until the time of infection, we could evaluate the requirement for these cells in the effector phase of the response while allowing for the potential production of IFN-γ from CD8+ T cells up to the time of infection. Surprisingly, anti-CD8 treatment at the time of infection completely abrogated the protective response elicited by immunization with LACK DNA (Fig. 9). These results suggest that CD8+ T cells have a role in mediating an effective immune response during a primary infection from mice vaccinated with LACK DNA.
Figure 8.
LACK DNA vaccination confers protection in an IL-12–dependent manner. (A) BALB/c mice immunized and boosted 2 wk later with LACK or control DNA (100 μg) were challenged with live 105 L. major metacyclic promastigotes 2 wk after the boost. Some of the vaccinated mice (n = 10/group) were treated with 1 mg of anti–IL-12 intraperitoneally weekly from the time of vaccination to 4 wk after infection. (B) In the same experiment, draining lymph nodes from individual mice were harvested 6 wk after infection and production of IL-4 and IFN-γ were determined in a similar manner to that outlined in Fig. 5. *P <0.005 in comparing IFN-γ produced from mice vaccinated with LACK DNA versus that produced from mice vaccinated with LACK DNA plus anti– IL-12.
Figure 9.
CD8+ T cells have an important role in mediating protection induced by LACK DNA vaccination. Mice (n = 10/group) receiving LACK or control DNA vaccination were treated with anti-CD8 antibody intraperitoneally starting at the time of vaccination (V) or at the time of infection (I) and then weekly until 4 wk after infection.
DISCUSSION
In this report, we show that immunization of genetically susceptible BALB/c mice using LACK DNA protected virtually all of the mice against progressive, nonhealing infections with L. major. Furthermore, we report here an effective vaccine (given subcutaneously) in BALB/c mice against cutaneous leishmaniasis where immunomodulating adjuvants such as IL-12 were not required. In the only reported use of DNA vaccination in this model, it was not demonstrated that control of infection was effectively achieved in a sustained manner (41). However, the immunity conferred by LACK DNA was maintained for at least 20 wk after infection and provided effective systemic immunity at a site distant from the point of vaccination. Furthermore, previous studies using LACK protein or SLA combined with rIL-12 (28, 29) showed protective efficacy when animals were infected 2 wk after the last vaccination. In our experiments, both LACK protein plus rIL-12 and LACK DNA induced a protective response when mice were infected 2 wk after the last vaccination. Further studies are in progress to compare the ability of protein vaccination plus rIL-12 versus DNA vaccination to induce long lasting immunity. Finally, these data show that LACK DNA immunization without adjuvants elicits a potent immune response that is similar to that induced by LACK protein plus rIL-12, but is qualitatively very different from that induced by LACK protein alone because the latter, as previously reported and confirmed here, induced no protection whatsoever (29). Thus, these results clearly indicate that DNA vaccination can substitute for potential cytokine adjuvants like IL-12.
DNA vaccination has been shown to elicit a long-lasting immune response in a variety of animal models (42, 43). Although the immunological mechanisms of DNA vaccination have still not been completely elucidated, there is evidence to suggest that the route (18, 19) or the dose of DNA play critical roles in determining the type of response induced. In our initial studies, we were unable to show any protective immunity when LACK DNA (5 μg) was administered using a gene gun (data not shown). These results are consistent with previous work showing that this route (and/or dose) of immunization induces a preferential Th2-type response, which would fail to provide protective immunity in this model. By contrast, immunizing mice subcutaneously with LACK DNA allowed us to administer much larger amounts of DNA, showing that protective immunity was dose dependent. At least 100 μg of LACK DNA was required for optimal protection. Based on the recent demonstration that bacterial DNA containing CpG motifs are potent activators of the immune system (44), it is likely that these immunostimulatory sequences (17) played a critical role in the efficacy of the vaccine through their preferential induction of IL-12 and IFN-γ (45–48). Thus, the critical protective dose of subcutaneously administered LACK DNA was due to either a requirement for a threshold dose of immunostimulatory sequence, antigen encoding sequence, or both.
Previous studies have shown that the cytokine milieu present at the initiation of infection plays an important role in the subsequent development of either a protective Th1 or a noncurative Th2 response (34–36). In particular, treatment of susceptible BALB/c mice with repeated doses of IL-12 at the time of infection mediates a protective response and is associated with the production of Th1 type cytokines (24, 25), whereas neutralization of IL-12 leads to disease susceptibility in resistant mice (25). Alternatively, treatment of susceptible mice with anti-IL-4 at the time of infection has been previously shown to enhance IFN-γ leading to control of infection (26, 27). In addition, anti– IL-4 treatment at the time of infection was recently shown to be important in maintaining early responsiveness to IL-12 (35). Thus, successful vaccination against this infection must provide sufficient IL-12 to overcome the potent immunoregulatory effects of the early IL-4 burst that accompanies inoculation of these parasites into BALB/c mice.
Our analysis of the immune response in protected mice is consistent with the ability of LACK DNA (when given by the appropriate route and amount) to induce a potent Th1-type response. There was preferential induction of LACK-specific production of IFN-γ before infection (Fig. 7), and this response was sustained at least 6 wk after infection. The role of IL-12 in mediating both the IFN-γ response and the protective effect induced by LACK DNA vaccination was demonstrated by the fact that neutralizing IL-12 in vivo decreased the LACK-specific IFN-γ production by LN cells, and completely abrogated protection.
Remarkably, the data also show that antigen-specific IL-4 production from lymph cells stimulated in vitro 6 wk after infection was similar in all the groups. These observations were confirmed in vivo by studying antibody isotypes. Although protected mice had an increase in IgG2a (consistent with increased IFN-γ), they also had similar levels of IgG1 and IgE (data not shown), as the unprotected mice, suggesting that IL-4 was present as well. The fact that IgG1 was increased in healing mice in which IFN-γ was enhanced is consistent with previous work showing that IL-12 treatment could induce an increase in both IgG1 and IgG2a (49). In addition, an assessment of cytokine mRNA from draining LN cells showed similar levels of IL-4 mRNA as assessed by semiquantitative PCR, further substantiating that IL-4 was similar in all the groups (data not shown). It is possible that the significant levels of IL-4 detected 6 wk after infection in the protected groups was because LACK DNA vaccination was unable to influence all LACK-reactive T cells that have been recently shown to be highly enriched in their capacity to produce IL-4 (30, 31). Taken together, these data suggest that in our DNA vaccine model, the relative ratios of IL-4 and IFN-γ predict disease outcome and the ability to control progressive infection may not be dependent on the development of a true polarized response with the complete absence of IL-4, consistent with previous studies (50–52). In this regard, it is important to note that despite control of footpad swelling and a reduction in parasite growth in both the footpad and draining node, there was still a significant number of viable parasites present in these sites, even 10 wk after infection. Additional studies are in progress to determine whether neutralization of IL-4 at the time of LACK DNA vaccination leads to more efficient reduction in parasitic burden with more complete polarization of the immune response.
One final observation relates to the role of CD8+ T cells in the immunity observed. Depletion of CD8+ T cells in LACK DNA vaccinated mice at the time of infection abrogated immunity. In this regard, while previous work has provided overwhelming evidence that CD4+ T cells play a critical role in determining disease outcome in this model (20–23, 53, 54), there is also evidence that CD8+ T cells can mediate an anti–leishmanial effect in secondary responses (36–40). Moreover, although it is not yet clear how CD8+ T cells are mediating their effects in vivo, it has been shown that parasite-specific CD8+ T cells can contribute to the IFN-γ response in a secondary infection, suggesting this as a possible mechanism (38). In the present study, the fact that mice vaccinated with LACK DNA and depleted of CD8+ T cells at the time of infection were no longer protected provides evidence that CD8+ T cells can also mediate protective immunity in a primary infection. Because DNA vaccination is a potent inducer of IFN-γ production as well as MHC class I– and class II–restricted T cell responses, we cannot be certain as to the relative contribution of each of these mechanisms in mediating the protective response. However, as noted above, because neutralizing IL-12 in vivo substantially diminished IFN-γ production and completely abrogated protection conferred by LACK DNA, it is likely that contribution of CD8+ T cells in mediating protection is through an IL-12–dependent enhancement of IFN-γ production, although an IL-12–dependent cytolytic mechanism cannot be excluded at this time. In ongoing experiments, in assessing in vitro production of IFN-γ from LN cells of LACK DNA vaccinated mice after infection, it was noted that addition of either anti-CD4 or anti-CD8 antibodies to the cultures caused a significant decrease in IFN-γ production (data not shown). Based on these data, we would speculate that a critical threshold of IFN-γ is required for control of infection and that DNA vaccination elicits IFN-γ from both CD4+ T cells and CD8+ T cells in an IL-12–dependent manner. By contrast, we would speculate that protective immunity achieved by treating mice with LACK protein plus IL-12 protein is likely to result from IFN-γ produced primarily by CD4+ T cells. Thus, it is possible that vaccination with DNA in BALB/c mice may induce protection to L. major by a somewhat different mechanism than vaccination with protein plus adjuvant. Nevertheless, these data indicate that DNA vaccination can substitute for conventional protein vaccination requiring a cytokine adjuvant in providing effective immunity against an intracellular parasite.
Acknowledgments
We thank Dr. Ethan Shevach and Dr. Richard Locksley for helpful discussions.
Footnotes
S.L. Reiner is supported by the Burroughs Wellcome Fund and the National Institutes of Health (AI-01309). N. Glaichenhaus is supported by grants from the Conseil Régional de la Région PACA (N. Glaichenhaus) and from the Ministère de l'Education Nationale, de la Recherche et de l'Enseignement Supérieur (N. Glaichenhaus).
Abbreviations used in this paper: ISS, immunostimulatory sequences; mRNA, messenger RNA; SLA, soluble leishmanial antigens.
REFERENCES
- 1.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, De Witt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science (Wash DC) 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 2.Wang B, Ugen KE, Srikantan V, Agadjanyan MG, Dang K, Refaeli Y, Sato AI, Boyer J, Williams WV, Weiner DB. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiang ZQ, Spitalnik S, Tran M, Wunner WH, Cheng J, Ertl HC. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology. 1994;199:132–140. doi: 10.1006/viro.1994.1105. [DOI] [PubMed] [Google Scholar]
- 4.Michel ML, Davis HL, Schleef M, Mancini M, Tiollais P, Whalen RG. DNA-mediated immunization to the hepatitis B surface antigen in mice: aspects of the humoral response mimic hepatitis B viral infection in humans. Proc Natl Acad Sci USA. 1995;92:5307–5311. doi: 10.1073/pnas.92.12.5307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lagging LM, Meyer K, Hoft D, Houghton M, Belshe RB, Ray R. Immune responses to plasmid DNA encoding the hepatitis C virus core protein. J Virol. 1995;69:5859–5863. doi: 10.1128/jvi.69.9.5859-5863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luke CJ, Carner K, Liang X, Barbour AG. An OspA-based DNA vaccine protects mice against infection with Borrelia burgdorferi. J Infect Dis. 1997;175:91–97. doi: 10.1093/infdis/175.1.91. [DOI] [PubMed] [Google Scholar]
- 7.Sedegah M, Hedstrom R, Hobart P, Hoffman SL. Protection against malaria by immunization with plasmid DNA encoding circumsporozoite protein. Proc Natl Acad Sci USA. 1994;91:9866–9870. doi: 10.1073/pnas.91.21.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tascon RE, Colston MJ, Ragno S, Stavropoulos E, Gregory D, Lowrie DB. Vaccination against tuberculosis by DNA injection. Nat Med. 1996;2:888–892. doi: 10.1038/nm0896-888. [DOI] [PubMed] [Google Scholar]
- 9.Huygen K, Content J, Denis O, Montgomery DL, Yawman AM, Deck RR, De Witt CM, Orme IM, Baldwin S, D'Souza C, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Macias C, Lopez-Hernandez MA, Gonzalez CR, Isibasi A, Ortiz-Navarrete V. Induction of antibodies against Salmonella typhi OmpC porin by naked DNA immunization. Ann NY Acad Sci. 1995;772:285–288. doi: 10.1111/j.1749-6632.1995.tb44761.x. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez Armas, J.C., C.S. Morello, L.D. Cranmer, and D.H. Spector. DNA immunization confers protection against murine cytomegalovirus infection. J Virol. 1996;70:7921–7928. doi: 10.1128/jvi.70.11.7921-7928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yokoyama M, Zhang J, Whitton JL. DNA immunization confers protection against lethal lymphocytic choriomeningitis virus infection. J Virol. 1995;69:2684–2688. doi: 10.1128/jvi.69.4.2684-2688.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allsopp CE, Plebanski M, Gilbert S, Sinden RE, Harris S, Frankel G, Dougan G, Hioe C, Nixon D, Paoletti E, Layton G, Hill AV. Comparison of numerous delivery systems for the induction of cytotoxic T lymphocytes by immunization. Eur J Immunol. 1996;26:1951–1959. doi: 10.1002/eji.1830260841. [DOI] [PubMed] [Google Scholar]
- 14.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doe B, Selby M, Barnett S, Baenzinger J, Walker CM. Induction of cytotoxic T lymphocytes by intramuscular immunization with plasmid DNA is facilitated by bone marrow–derived cells. Proc Natl Acad Sci USA. 1996;93:8578–8583. doi: 10.1073/pnas.93.16.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raz E, Tighe H, Sato Y, Corr M, Dudler JA, Roman M, Swain SL, Spiegelberg HL, Carson DA. Preferential induction of a Th1 immune response and inhibition of specific IgE antibody formation by plasmid DNA immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen MD, Silverman GJ, Lotz M, Carson DA, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science (Wash DC) 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 18.Pertmer TM, Roberts TR, Haynes JR. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J Virol. 1996;70:6119–6125. doi: 10.1128/jvi.70.9.6119-6125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feltquate DM, Heaney S, Webster RG, Robinson HL. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 20.Scott P, Natovitz P, Coffman RL, Pearce E, Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988;168:1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989;169:59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinzel FP, Sadick MD, Mutha SS, Locksley RM. Production of interferon gamma, interleukin 2, interleukin 4, and interleukin 10 by CD4+ lymphocytes in vivo during healing and progressive murine leishmaniasis. Proc Natl Acad Sci USA. 1991;88:7011–7015. doi: 10.1073/pnas.88.16.7011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boom WH, Liebster L, Abbas AK, Titus RG. Patterns of cytokine secretion in murine leishmaniasis: correlation with disease progression or resolution. Infect Immunol. 1990;58:3863–3870. doi: 10.1128/iai.58.12.3863-3870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177:1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sypek JP, Chung CL, Mayor SE, Subramanyam JM, Goldman SJ, Sieburth DS, Wolf SF, Schaub RG. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177:1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadick MD, Heinzel FP, Holaday BJ, Pu RT, Dawkins RS, Locksley RM. Cure of murine leishmaniasis with anti–interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon γ–independent mechanism. J Exp Med. 1990;171:115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakil AE, Wang ZE, Locksley RM. Leishmania major: targeting IL-4 in successful immunomodulation of murine infection. Exp Parasitol. 1996;84:214–222. doi: 10.1006/expr.1996.0107. [DOI] [PubMed] [Google Scholar]
- 28.Afonso LC, Scharton TM, Vieira LQ, Wysocka M, Trinchieri G, Scott P. The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science (Wash DC) 1994;263:235–237. doi: 10.1126/science.7904381. [DOI] [PubMed] [Google Scholar]
- 29.Mougneau E, Altare F, Wakil AE, Zheng S, Coppola T, Wang ZE, Waldmann R, Locksley RM, Glaichenhaus N. Expression cloning of a protective Leishmania antigen. Science (Wash DC) 1995;268:563–566. doi: 10.1126/science.7725103. [DOI] [PubMed] [Google Scholar]
- 30.Julia V, Rassoulzadegan M, Glaichenhaus N. Resistance to Leishmania major induced by tolerance to a single antigen. Science (Wash DC) 1996;274:421–423. doi: 10.1126/science.274.5286.421. [DOI] [PubMed] [Google Scholar]
- 31.Launois P, Maillard I, Pingel S, Swihart KG, Xenarios I, Acha-Orbea H, Diggelmann H, Locksley RM, MacDonald H, Louis JA. IL-4 rapidly produced by Vβ4Vα8 CD4+T cells instructs Th2 development and susceptibility to Leishmania major in BALB/c mice. Immunity. 1997;6:541–549. doi: 10.1016/s1074-7613(00)80342-8. [DOI] [PubMed] [Google Scholar]
- 32.Seder RA, Gazzinelli R, Sher A, Paul WE. Interleukin 12 acts directly on CD4+ T cells to enhance priming for interferon γ production and diminishes interleukin 4 inhibition of such priming. Proc Natl Acad Sci USA. 1993;90:10188–10192. doi: 10.1073/pnas.90.21.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reiner SL, Zheng S, Wang ZE, Stowring L, Locksley RM. Leishmania promastigotes evade interleukin 12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+T cells during initiation of infection. J Exp Med. 1994;179:447–456. doi: 10.1084/jem.179.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scott P, Eaton A, Gause WC, di Zhou X, Hondowicz B. Early IL-4 production does not predict susceptibility to Leishmania major. Exp Parasitol. 1996;84:178–187. doi: 10.1006/expr.1996.0103. [DOI] [PubMed] [Google Scholar]
- 35.Launois P, Swihart KG, Milon G, Louis JA. Early production of IL-4 in susceptible mice infected with Leishmania major rapidly induces IL-12 unresponsiveness. J Immunol. 1997;158:3317–3324. [PubMed] [Google Scholar]
- 36.Hill JO, Awwad M, North RJ. Elimination of CD4+ suppressor T cells from susceptible BALB/c mice releases CD8+ T lymphocytes to mediate protective immunity against Leishmania. . J Exp Med. 1989;169:1819–1827. doi: 10.1084/jem.169.5.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller I, Pedrazzini T, Kropf P, Louis J, Milon G. Establishment of resistance to Leishmania major infection in susceptible BALB/c mice requires parasite-specific CD8+ T cells. Int Immunol. 1991;3:587–597. doi: 10.1093/intimm/3.6.587. [DOI] [PubMed] [Google Scholar]
- 38.Muller I, Kropf P, Etges RJ, Louis JA. Gamma interferon response in secondary Leishmania major infection: role of CD8+ T cells. Infect Immunol. 1993;61:3730–3738. doi: 10.1128/iai.61.9.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.da Conceicao-Silva F, Perlaza BL, Louis JA, Romero P. Leishmania major infection in mice primes for specific major histocompatibility complex class I–restricted CD8+ cytotoxic T cell responses. Eur J Immunol. 1994;24:2813–2817. doi: 10.1002/eji.1830241135. [DOI] [PubMed] [Google Scholar]
- 40.Stefani MM, Muller I, Louis JA. Leishmania major–specific CD8+ T cells are inducers and targets of nitric oxide produced by parasitized macrophages. Eur J Immunol. 1994;24:746–752. doi: 10.1002/eji.1830240338. [DOI] [PubMed] [Google Scholar]
- 41.Xu D, Liew FY. Protection against leishmaniasis by injection of DNA encoding a major surface glycoprotein, gp63, of L. major. Immunology. 1995;84:173–176. [PMC free article] [PubMed] [Google Scholar]
- 42.Wolff JA, Ludtke JJ, Acsadi G, Williams P, Jani A. Long-term persistence of plasmid DNA and foreign gene expression in mouse muscle. Human Mol Genet. 1992;1:363–369. doi: 10.1093/hmg/1.6.363. [DOI] [PubMed] [Google Scholar]
- 43.Deck RR, DeWitt CM, Donnelly JJ, Liu MA, Ulmer JB. Characterization of humoral immune responses induced by an influenza hemagglutin DNA vaccine. Vaccine. 1997;15:71–78. doi: 10.1016/s0264-410x(96)00101-6. [DOI] [PubMed] [Google Scholar]
- 44.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature (Lond) 1995;374:546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 45.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halpern MD, Kurlander RJ, Pisetsky DS. Bacterial DNA induces murine interferon-gamma production by stimulation of interleukin-12 and tumor necrosis factor-α. Cell Immunol. 1996;167:72–78. doi: 10.1006/cimm.1996.0009. [DOI] [PubMed] [Google Scholar]
- 47.Sonehara K, Saito H, Kuramoto E, Yamamoto S, Yamamoto T, Tokunaga T. Hexamer palindromic oligonucleotides with 5′-CG-3′ motif(s) induce production of interferon. J Interferon Cytokine Res. 1996;16:799–803. doi: 10.1089/jir.1996.16.799. [DOI] [PubMed] [Google Scholar]
- 48.Stacey KJ, Sweet MJ, Hume DA. Macrophages ingest and are activated by bacterial DNA. J Immunol. 1996;157:2116–2122. [PubMed] [Google Scholar]
- 49.Bliss J, Van Cleave V, Murray K, Wiencis A, Ketchum M, Maylor R, Haire T, Resmini C, Abbas AK, Wolf SF. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J Immunol. 1996;156:887–894. [PubMed] [Google Scholar]
- 50.Morris L, Troutt AB, Handman E, Kelso A. Changes in the precursor frequencies of IL-4 and IFN-γ secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J Immunol. 1992;149:2715–2721. [PubMed] [Google Scholar]
- 51.Morris L, Troutt AB, McLeod KS, Kelso A, Handman E, Aebischer T. Interleukin-4 but not γ interferon production correlates with the severity of murine cutaneous leishmaniasis. Infect Immunol. 1993;61:3459–3465. doi: 10.1128/iai.61.8.3459-3465.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doherty TM, Coffman RL. Leishmania major: effect of infectious dose on T cell subset development in BALB/c mice. Exp Parasitol. 1996;84:124–135. doi: 10.1006/expr.1996.0098. [DOI] [PubMed] [Google Scholar]
- 53.Reiner SL, Wang ZE, Hatam F, Scott P, Locksley RM. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science (Wash DC) 1993;259:1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- 54.Locksley RM, Reiner SL, Hatam F, Littman DR, Killeen N. Helper T cells without CD4: control of leishmaniasis in CD4-deficient mice. Science (Wash DC) 1993;261:1448–1451. doi: 10.1126/science.8367726. [DOI] [PubMed] [Google Scholar]