Abstract
Ultraviolet A (UVA) irradiation is effectively used to treat patients with atopic dermatitis and other T cell mediated, inflammatory skin diseases. In the present study, successful phototherapy of atopic dermatitis was found to result from UVA radiation-induced apoptosis in skin-infiltrating T helper cells, leading to T cell depletion from eczematous skin. In vitro, UVA radiation-induced human T helper cell apoptosis was mediated through the FAS/FAS-ligand system, which was activated in irradiated T cells as a consequence of singlet oxygen generation. These studies demonstrate that singlet oxygen is a potent trigger for the induction of human T cell apoptosis. They also identify singlet oxygen generation as a fundamental mechanism of action operative in phototherapy.
The therapeutic use of ultraviolet (UV) radiation is of fundamental importance in the treatment of atopic dermatitis (1). Atopic dermatitis is a chronic inflammatory skin disease with an estimated prevalence of 10% in children and 0.5–1% in adults and increasing in incidence by about twofold in 10 yr (2). The pathogenesis of atopic dermatitis is at least in part immunologic in nature and involves a T cell mediated immune response directed against inhalant allergens and other atopens (3). Eczematous skin lesions are thought to result from cytokines which are produced by skin-infiltrating T helper cells present in the dermis (4).
The mechanism of action underlying the effectiveness of UV phototherapy of atopic dermatitis patients is not well understood. Recent observations indicate that T helper cells present in lesional skin of atopic dermatitis patients are important targets for UV phototherapy. Phototherapy of atopic dermatitis using longwave UVA radiation (340–400 nm), which effectively penetrates the dermal layers of human skin and thus has the potential to directly affect intradermal T cells (5), has been shown to be superior to short wavelength UVB radiation (6), which is almost exclusively absorbed by the epidermis (5). Accordingly, successful UVA phototherapy of atopic dermatitis was associated with downregulation of the in situ expression of T helper cell derived cytokines as well as a significant reduction in the number of intradermal CD4+ T cells (6, 7). These observations led us to speculate that UVA phototherapy acts through depletion of skin-infiltrating T helper cells.
Therefore, it has been of interest to learn that UVA radiation can induce apoptosis (8). In murine lymphoma cells, in vitro UVA irradiation induced apoptosis 4 h after exposure by a process which did not require macromolecular synthesis, and also 24–48 h after irradiation through a mechanism depending on de novo protein synthesis. In the present study we demonstrate that UVA phototherapy induced apoptosis in T helper cells present in eczematous skin of atopic dermatitis patients.
Materials and Methods
UVA Phototherapy.
Five patients with atopic dermatitis as defined by Hanifin and Rajka (9) were enrolled after informed consent was obtained. All patients had extensive atopic dermatitis (total clinical score greater than 40; reference 10). Patients were hospitalized for UVA phototherapy. Patients had not been treated with any systemic or topical agent 4 wk before start of UVA phototherapy. For phototherapy, the patient's whole body was exposed to 130 J/cm2 UVA1 radiation from UVASUN 30,000 BIOMED (Mutzhas, Munich, Germany), as previously described (11). UVA phototherapy was conducted as a monotherapy with daily exposures for 10 consecutive days. Sequential biopsies were taken in each patient from chronic, lichenified eczematous skin lesions present in the flexural creases of their elbows before and after the 1st, 2nd, 3rd, 4th, and 10th UVA radiation exposure.
In Situ Detection of Apoptosis in CD4+ T Cells.
Cryostat sections were prepared and fixed in chilled acetone for 10 min. After permeabilization with 0.1% sodium citrate and 0.1% Triton X-100 (Boehringer Mannheim, Mannheim, Germany), free DNA 3′-OH termini were labeled with fluorescein-labeled nucleotides in the presence of a terminal deoxynucleotidyl transferase (TdT) for 1 h at 37°C. Sections were subsequently stained with RPE-conjugated mouse anti–human CD4 monoclonal antibody MT310 (mIgG1; Dako Diagnostika, Hamburg, Germany) for 10 min, washed three times with PBS, and then immediately examined by fluorescence microscopy using an Axioplan microscope (Zeiss, Jena, Germany). For semiquantitative assessment of apoptotic, CD4+ cells, three serial sections per specimen were analyzed and the number of double positive cells in three high-power (×200) view fields was counted.
Atopen-specific Human T Helper Cells from Atopic Dermatitis.
Human atopen-specific T helper cell lines were used for in vitro studies. These T cell lines are specific for Dermatophagoides pteronyssinus (Dp) antigen and have been generated from lesional atopic skin as previously described (12). The T helper cell lines employed in this study exhibited either a Th0 or a Th1 cytokine profile (12).
In Vitro Ultraviolet A Irradiation.
T cells were harvested and resuspended in RPMI1640 medium without phenol red (Biochrom, Berlin, Germany) in 12 well flat-bottom tissue culture plates (Becton-Dickinson, Heidelberg, Germany). Lids were removed and cells (5 × 105/ml) were exposed to UVA radiation from a UVASUN 5000 BIOMED irradiation device (Mutzhas) as previously described. Subsequently, cells were collected by centrifugation, resuspended in complete medium, and cultured in the presence or absence of mouse anti–human FAS mAb ZB4 (mIgG1; Coulter-Immunotech Diagnostics, Hamburg, Germany) or an isotype control antibody (mIgG1; Sigma Chemicals, Deisenhofen, Germany). The ZB4 antibody was previously shown to prevent FAS/FASL-induced T cell apoptosis by binding to FAS molecules (13). Maximal effects were achieved if the antibodies were added to cells 1 hour before UVA irradiation.
In Vitro Detection of Apoptosis.
For the TUNEL assay, the in situ cell death detection kit from Boehringer Mannheim (Mannheim, Germany) was used. Cells were washed and analyzed by flow cytometry using a FACScan® (Becton-Dickinson) counting 1 × 104 cells per sample. Incubation of dUTP without TdT served as the negative control population and data are given as % positive (= apoptotic) cells.
To examine DNA fragmentation, cells were collected and resuspended in 10 mM Tris HCL (pH 7.5), 1 mM EDTA, 0.15 M NaCl, 1% SDS, and 0.2 mg/ml proteinase K and 0.5 mg/ml of RNAse (Sigma). After 1 h incubation at 65°C DNA was extracted with phenol and chloroform, precipitated with ethanol, and dissolved in 10 mM Tris HCL (pH 7.5) containing 1 mM EDTA. Extracted DNA was loaded in a 2% agarose gel and visualized by staining with ethidium bromide.
Immunofluorescence Flow Cytometry.
FAS and FASL surface expression was assessed by immunofluorescence flow cytometry using anti-FAS mAb DX2 (mIgG1; Pharmingen, Hamburg, Germany) or anti-FASL mAb 33 (mIgG1; Dianova, Hamburg, Germany) as described (16).
Chemical Treatments and Singlet Oxygen Generation.
All chemicals were purchased from Sigma except for sodium azide (Merck, Darmstadt, Germany). Sodium azide (50 mM in PBS) was only present during irradiation of cells. For irradiation in the presence of heavy water, deuterium oxide (99.9 atom % D) was used in a final concentration of 90% in PBS (14–16).
Singlet oxygen was generated by thermal decomposition of the endoperoxide of the disodium salt of 3,3′-(1,4-naphthylidene) dipropionate (NDPO2), 1 mM in PBS, for 1-h in the dark at 37°C yielding excited singlet molecular oxygen and 3,3′-(1,4-naphthylidene)dipropionate (NDP), as described previously (17).
Results
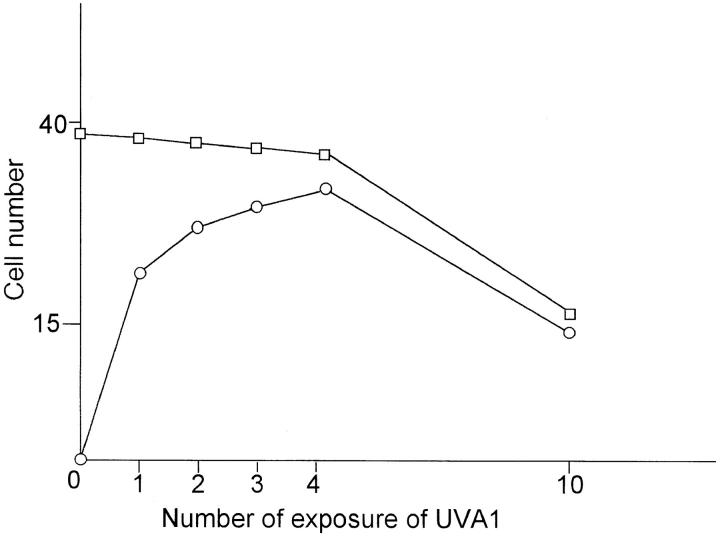
In all patients, UVA phototherapy led to a significant improvement of skin symptoms, as assessed by a clinical scoring system (total score before UVA phototherapy: 65.4 ± 6.2; total score after UVA phototherapy: 18.2 ± 3.4; P <0.001) (10). Skin specimens were analyzed for apoptotic cells using the TdT-mediated dUTP labeling (TUNEL) assay, followed by a anti-CD4 staining to detect T helper cells. Before therapy, numerous CD4+ cells were present intradermally in eczematous skin (3) (Fig. 1 A). This cell population did not contain a significant number of apoptotic cells, and this was in sharp contrast to specimens obtained from the same skin areas after UVA radiation therapy had been started. Already after the 1st UVA radiation exposure, CD4+, apoptotic cells were detected (Fig. 1 B). During subsequent UVA treatments, the number of double positive cells was further increased, whereas the total number of CD4+ cells decreased (Fig. 1, C and D and Fig. 2). After 10 exposures, the total number of intradermally located, CD4+ T cells had been significantly diminished, and most of the remaining cells showed signs of apoptosis (Fig. 2). No apoptotic cells were detected in the epidermal compartment (data not shown).
Figure 1.
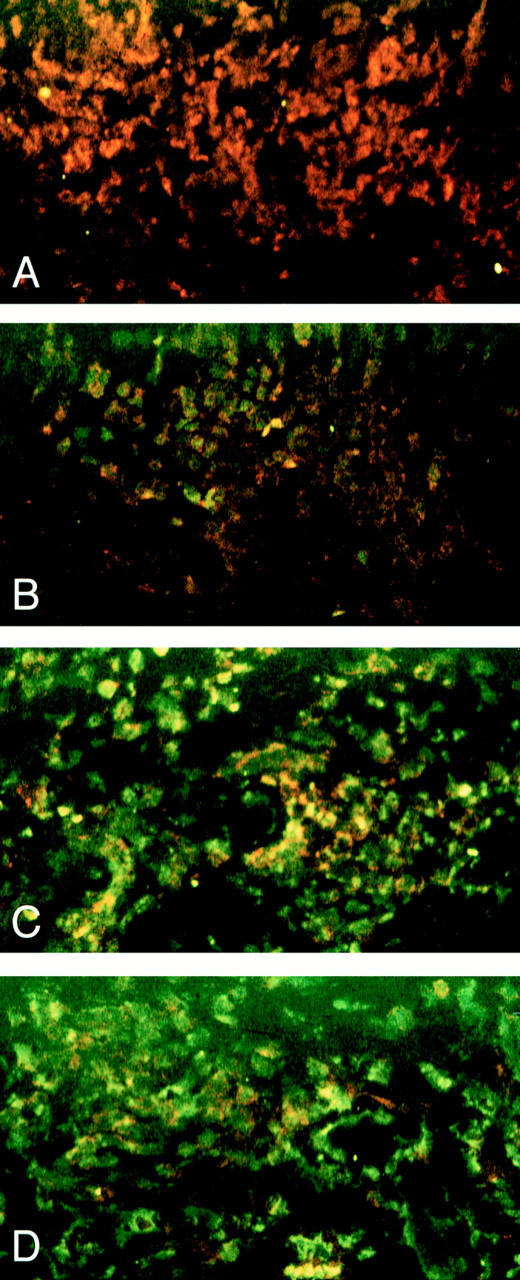
Qualitative analysis of UVA phototherapy induced apoptosis in CD4+ cells present in lesional skin of a patient with atopic dermatitis. Biopsy specimens were obtained from lesional skin (flexural creases of the left elbow) of a patient with atopic dermatitis before (A) and after one (B), two (C) and three (D) exposures to UVA radiation and analyzed for apoptotic (green fluorescence) and CD4+ (red fluorescence) cells as described in Materials and Methods. All photographs show dermis.
Figure 2.
Semiquantitative assessment of apoptotic (○) and CD4+ (□) cells in lesional skin of five patients with atopic dermatitis before, during and after UVA phototherapy. Each time point was taken from each patient and the standard deviation for all time points was <15%.
In atopen-specific human T helper cells, in vitro UVA irradiation induced apoptosis (Fig. 3, see also Figs. 5, 6). Significant apoptosis was already detectable 4 h after exposure, reaching a maximum 24 h after irradiation with 30 J/cm2 UVA radiation (Fig. 3 and data not shown).
Figure 3.
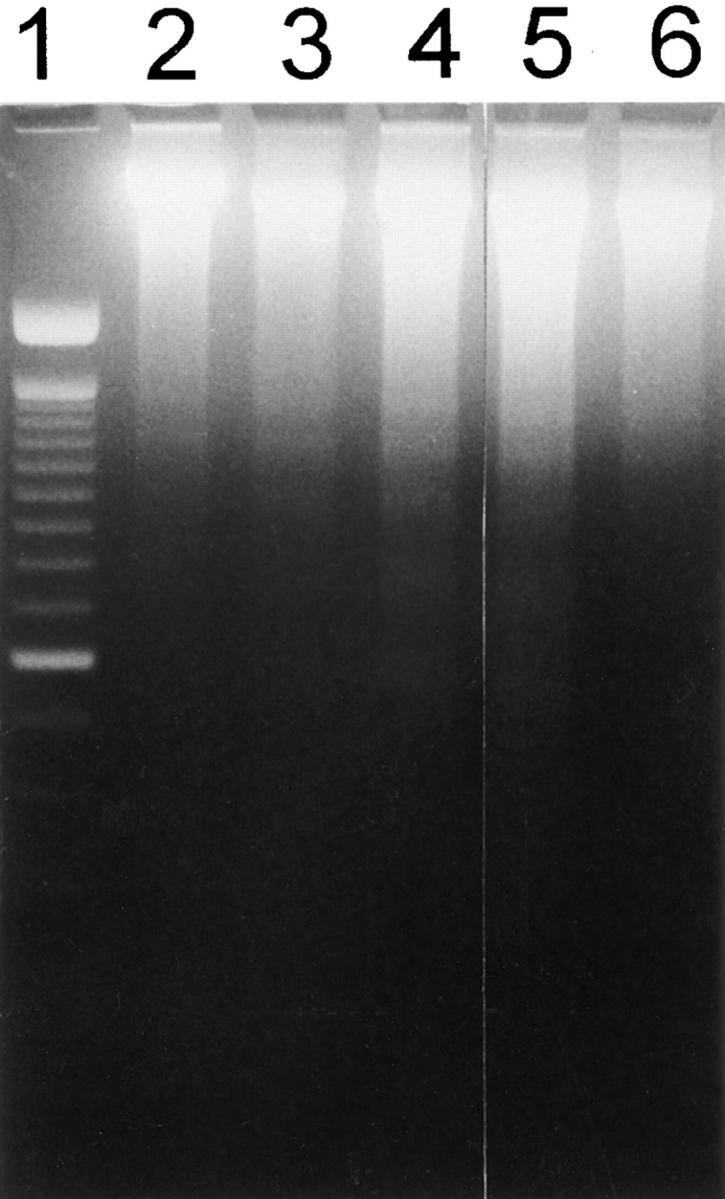
Ultraviolet A radiation-induced apoptosis in atopen-specific, human T helper cells. UVA-irradiated T cells were analyzed for induction of apoptosis by assessing DNA laddering 2 h (lane 3), 4 h (lane 4), 8 h (lane 5), 24 h (lane 6) after UVA (30 J/cm2) irradiation; lane 1, 100 bps marker; lane 2, 4 h after sham irradiation.
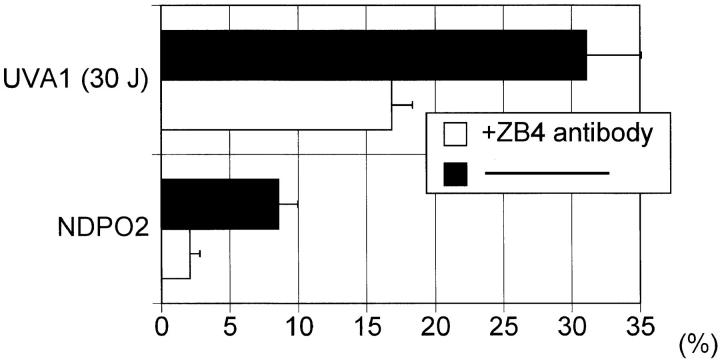
Figure 5.
UVA radiation and NDPO2 induced T cell apoptosis. T cells were preincubated in the presence of anti-Fas antibody ZB4 (1 μg/ml) (open bar) or an isotype control antibody (solid bar) for 1 h at 37°C. Cells were then exposed to UVA radiation (30 J/cm2) or NDPO2 (15 mM). After 4 h, the percentage of apoptotic cells was determined using the TUNEL method as described in Materials and Methods. Data represent mean ± SD of six experiments.
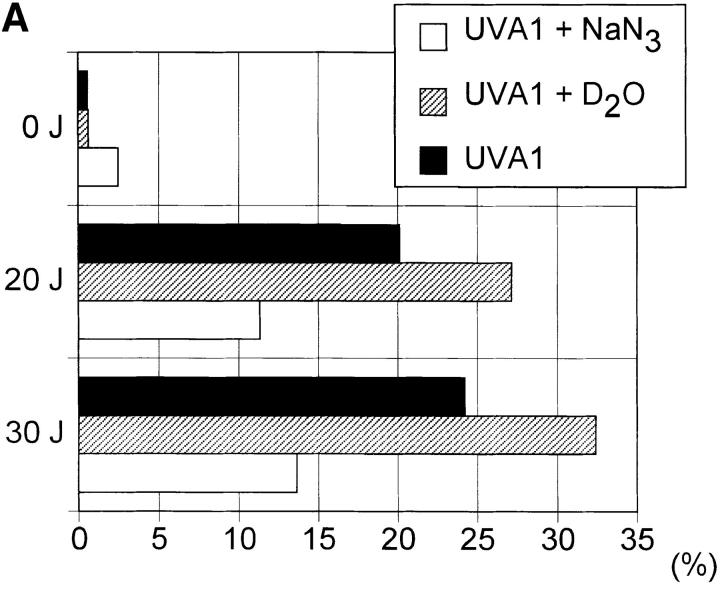
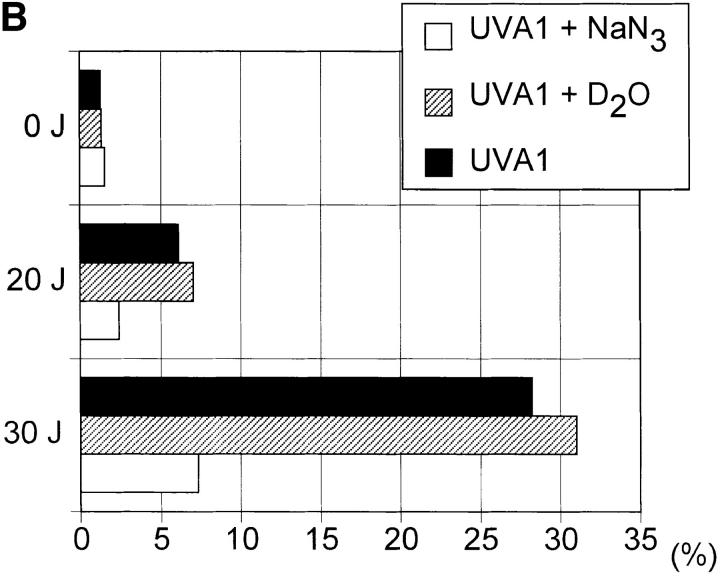
Figure 6.
UVA radiation- induced apoptosis and FASL expression in human T cells. Human T helper cells were exposed to increasing doses of UVA radiation (0–30 J/cm2) in the presence (open bar) or absence (solid bar) of sodium azide (50 mM) or deuterium oxide (90%) (hatched bar). 4 h after irradiation, the percentage of apoptotic cells (A) and FASL+ cells (B) was determined as described in Materials and Methods. Data represent one of three essentially identical experiments.
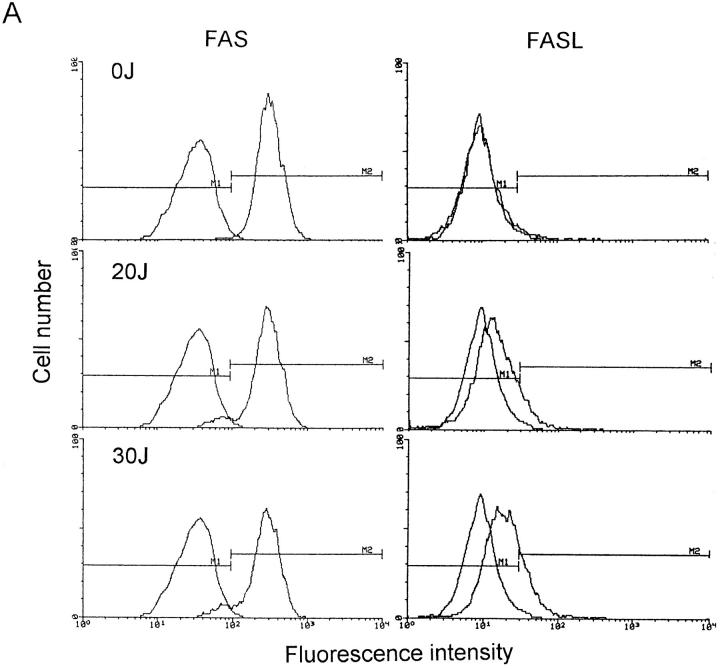
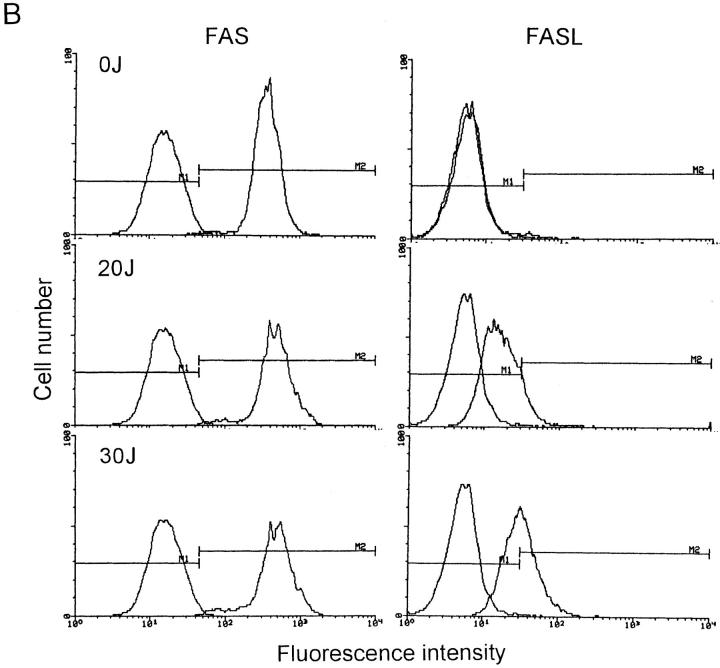
Before UVA radiation exposure, FASL molecules were not present on the cell surface (Fig. 4), but significant FASL surface expression was detected in UVA-irradiated cells already 4 h after exposure. Ultraviolet A radiation-induced surface FASL expression was dose-dependent and maximal upon exposure of cells to 30 J/cm2 UVA. In contrast to FASL, FAS surface expression remained essentially unaltered upon UVA irradiation (Fig. 4).
Figure 4.
FAS and FASL expression in UVA-irradiated T helper cells. T cells were exposed to increasing doses of UVA radiation (0–30 J/cm2). 4 (A) and 16 (B) h after exposure, cells were analyzed for FAS and FASL surface expression by FACS® analysis as described in Materials and Methods. Data are given as histograms of cell number versus fluorescence intensity and represent one of five essentially identical experiments.
Addition of the blocking anti-FAS antibody ZB4 (13), but not of equivalent concentrations of an isotype control antibody (Fig. 5) or an isotype-matched anti-CD4 mAb (data not shown), significantly lowered UVA radiation-induced human T helper cell apoptosis (Fig. 5).
In the following experiments, reagents capable of quenching (sodium azide) or enhancing (deuterium oxide) singlet oxygen effects were assessed for their capacity to modulate UVA radiation-induced human T helper cell apoptosis (14–16). Irradiation of cells in the presence of sodium azide significantly inhibited UVA radiation-induced FASL surface expression as well as apoptosis in human T helper cells, whereas irradiation of cells in the presence of deuterium oxide resulted in a slight, but consistent increase in the percentage of FASL expressing as well as apoptotic cells (Fig. 6).
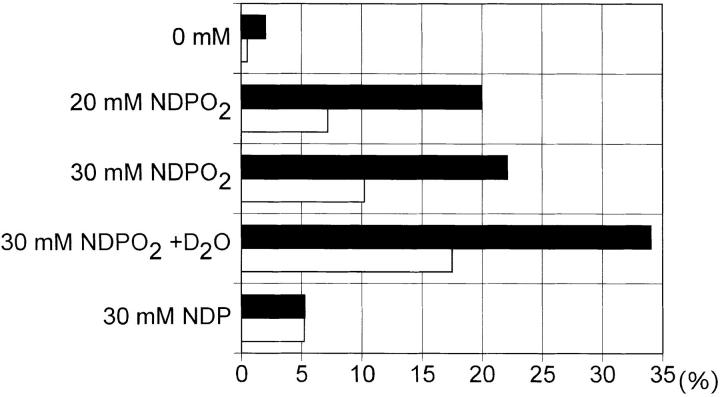
We next assessed whether UVA radiation-induced apoptosis could be mimicked by stimulating unirradiated human T helper cells with singlet oxygen. Singlet oxygen was generated by thermal decomposition of NDPO2 (17). As shown in Fig. 7, singlet oxygen increased FASL surface expression in unirradiated T cells to an extent similar to that observed in UVA-irradiated cells. Similar to FASL surface expression, singlet oxygen generation also induced apoptosis in unirradiated cells (Fig. 7). NDPO2-induced FASL surface expression as well as apoptosis were significantly enhanced, if T cells were stimulated in the presence of deuterium oxide. Treatment of cells with NDP did not induce FASL surface expression or apoptosis. Addition of anti-FAS antibody ZB4 significantly inhibited apoptosis in unirradiated cells, which had been exposed to NDPO2 (Fig. 5).
Figure 7.
Singlet oxygen-induced apoptosis and FASL expression in human T cells. Human T helper cells were stimulated with increasing concentration of NDPO2 (0–30 mM) or NDP (30 mM) in the absence or presence of deuterium oxide (90%). After 4 h, the percentage of apoptotic cells (solid bar) or FASL+ cells (open bar) was determined as described in Materials and Methods. Data represent one of four essentially identical experiments.
Discussion
Successful UVA phototherapy was previously found to downregulate lesional expression of the T helper cell-derived cytokine IFN-γ in atopic dermatitis (17). Interferon-γ production by intradermal T helper cells is thought to be a major cause for the generation and maintenance of eczema in atopic dermatitis patients (4, 7, 18,19). In the present study, phototherapy led to induction of apoptosis in intradermal T helper cells, subsequent depletion of T cells from lesional atopic skin and concomitant improvement of clinical symptoms. Taken together these results indicate that the therapeutic effectiveness of UVA phototherapy for atopic dermatitis results from induction of T helper cell apoptosis. At least under in vitro conditions, human T helper cell apoptosis may be induced by short wavelength UVB radiation as well (20). Depletion of T cells from human skin via induction of apoptosis may thus represent a general mechanism relevant for phototherapy of inflammatory skin diseases.
Because of its physical properties, UVA radiation applied during phototherapy can reach the lower levels of the human dermis (5). Under conditions closely resembling the therapeutic situation, in vitro UVA irradiation induced apoptosis in atopen-specific human T helper cells indicating that phototherapy-induced apoptosis in intradermal T helper cells resulted from direct effects.
Atopen-specific CD4+ T cells employed in the present study constitutively expressed FAS antigen, as has been shown to be the case for activated human T cells (21). Unirradiated T cells did not express significant FASL surface levels, but abundant FASL expression was detected 4 h after UVA radiation and was further increased 16 h after exposure.
FAS/FASL interaction may cause autocrine suicide in FAS-expressing T cells (22–24). Ultraviolet A radiation-induced FASL expression was of functional relevance, because interference with FAS/FASL interaction through addition of a blocking anti-FAS antibody effectively prevented UVA radiation-induced human T cell apoptosis. In this regard, UVA radiation-induced human T helper cell apoptosis resembled T cell apoptosis induced by anti-CD3 antibody, phorbol ester plus calcium ionophore, staphylococcal enterotoxin superantigen and cytotoxic drugs (22–26).
The present study indicates that increased FASL surface expression is important for UVA radiation-induced T cell apoptosis. Recently, it has been demonstrated that the generation of singlet oxygen is a primary mediator in UVA radiation-induced biological effects including increased expression of cell surface molecules (14–16). The capacity of sodium azide to suppress, of deuterium oxide to enhance, and of NDPO2 to mimic UVA radiation-induced FASL expression and apoptosis indicated a prominent role for singlet oxygen in this system. Singlet oxygen-induced T cell apoptosis involved the FAS/FASL system, because blocking anti-FAS antibodies effectively inhibited human T helper cell apoptosis which was induced by UVA irradiation or stimulation with a singlet oxygen generating system. These studies demonstrate that singlet oxygen, by virtue of its capacity to activate the FAS/FASL system, serves an important role in control of human T cell apoptosis. This conclusion is supported by previous studies indicating that in human T cells FAS-mediated apoptosis was related to the generation of reactive oxygen species and dependent on the thiol-status of T cells (27). Similarly, the thiol-status of human cells was shown to control UVA radiation-induced, singlet oxygen-mediated gene expression (28, 29).
Singlet oxygen is produced by a variety of biological systems, and is a significant biochemical intermediate in several biological processes (30). The present observation that singlet oxygen can induce human T cell apoptosis has added a previously unrecognized biological activity of great importance to the list of biological effects, which were found to be mediated by singlet oxygen. Our studies also indicate that generation of singlet oxygen within human skin may constitute a therapeutic principle underlying phototherapy of inflammatory skin disease.
Acknowledgments
This study was supported by a grant from the Deutsche Forschungsgemeinschaft, Sonderforschungsbereich 503, Projects B2 and B1 and Sonderforschungsbereich 244, Project C13. A. Morita was supported by a fellowship from the Alexander-von-Humboldt Foundation, Bonn, Germany.
References
- 1.Krutmann, J. 1997. Therapeutic Photomedicine: Phototherapy. In Fitzpatrick's Dermatology in General Medicine. 5th edition. I.M. Freedberg, A.B. Eisen, K. Wolff, K.F. Austen, L.A. Goldsmith, S.I. Katz, T.B. Fitzpatrick, editors. McGraw-Hill, New York. In press.
- 2.Rothe MJ, Grant-Kels JM. Atopic dermatitis: an update. J Am Acad Dermatol. 1996;35:1–13. doi: 10.1016/S0190-9622(96)90486-7. [DOI] [PubMed] [Google Scholar]
- 3.Bos JD, Kapsenberg ML, Sillevis JH, Smitt Pathogenesis of atopic eczema. Lancet. 1994;343:1338–1341. doi: 10.1016/s0140-6736(94)92473-2. [DOI] [PubMed] [Google Scholar]
- 4.Leung DYM. Atopic dermatitis: The skin as a window into the pathogenesis of chronic allergic diseases. J Allergy Clin Immunol. 1995;96:302–319. doi: 10.1016/s0091-6749(95)70049-8. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RR, Parrish JA. The optics of human skin. J Invest Dermatol. 1981;77:13–19. doi: 10.1111/1523-1747.ep12479191. [DOI] [PubMed] [Google Scholar]
- 6.Krutmann J. Phototherapy for atopic dermatitis. Dermatol Therapy. 1996;1:24–31. [Google Scholar]
- 7.Grewe M, Gyufko K, Schöpf E, Krutmann J. Lesional expression of interferon-γ in atopic eczema. Lancet. 1994;343:25–26. doi: 10.1016/s0140-6736(94)90879-6. [DOI] [PubMed] [Google Scholar]
- 8.Godar DE, Miller SA, Thomas DP. Immediate and delayed apoptotic cell death mechanisms: UVA versus UVB and UVC radiation. Cell Death Different. 1994;1:59–66. [PubMed] [Google Scholar]
- 9.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermao-Venereol Suppl. 1980;92:44–47. [Google Scholar]
- 10.Costa C, Rilliet A, Nicolet M, Saurat J-H. Scoring atopic dermatitis: the simpler the better. Acta Dermao-Venereol. 1989;69:41–45. [PubMed] [Google Scholar]
- 11.Krutmann J, Czech W, Diepgen T, Niedner R, Kapp A, Schöpf E. High-dose UVA1 therapy in the treatment of patients with atopic dermatitis. J Am Acad Dermatol. 1992;26:225–230. doi: 10.1016/0190-9622(92)70031-a. [DOI] [PubMed] [Google Scholar]
- 12.Werfel T, Morita A, Grewe M, Renz H, Wahn U, Krutmann J, Kapp A. Allergen specificity of skin-infiltrating T cells is not restricted to a type-2 cytokine pattern in chronic skin lesions of atopic dermatitis. J Invest Dermatol. 1996;107:871–876. doi: 10.1111/1523-1747.ep12331164. [DOI] [PubMed] [Google Scholar]
- 13.Giordano C G, Stasi, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco M, Testi R, Galluzo A. Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto's thyroiditis. Science (Wash DC) 1997;275:960–963. doi: 10.1126/science.275.5302.960. [DOI] [PubMed] [Google Scholar]
- 14.Basu-Modak S, Tyrrell RM. Singlet oxygen: a primary effector in the ultraviolet A/near-visible light induction of the human heme oxygenase gene. Cancer Res. 1993;53:4505–4510. [PubMed] [Google Scholar]
- 15.Scharffetter-Kochanek K, Wlaschek M, Briviba K, Sies H. Singlet oxygen induces collagenase expression in human skin fibroblasts. FEBS Lett. 1993;331:304–306. doi: 10.1016/0014-5793(93)80357-z. [DOI] [PubMed] [Google Scholar]
- 16.Grether-Beck S, Olaizola-Horn S, Schmitt H, Grewe M, Jahnke A, Johnson JP, Briviba K, Sies H, Krutmann J. Activation of transcription factor AP-2 mediates UVA radiation- and singlet oxygen-induced expression of the human intercellular adhesion molecule 1 gene. Proc Natl Acad Sci USA. 1996;93:14586–14591. doi: 10.1073/pnas.93.25.14586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiMascio P, Sies H. Quantification of singlet oxygen generated by thermolysis of 3,3-(1,4-Naphtylidene) dipropionate. Monomol and dimol photoemission and the effects of 1,4-Diazabicyclo(2.2.2)octane. J Am Chem Soc. 1989;111:2909–2914. [Google Scholar]
- 18.Grewe M, Walter S, Gyufko K, Czech W, Schöpf E, Krutmann J. Analysis of the cytokine pattern expressed in situ in inhalant allergen patch test reactions of atopic dermatitis patients. J Invest Dermatol. 1995;105:407–410. doi: 10.1111/1523-1747.ep12321078. [DOI] [PubMed] [Google Scholar]
- 19.Thepe T, Langeveld-Wildschut EG, Bihari IC, van Wichen CF, van Reisen FC, Mudde GC, Bruijnzeel-Koomen CAFM. Biphasic response against aeroallergen in atopic dermatitis showing a switch from an initial TH2 response to a TH1response in situ: an immunocytochemical study. J Allergy Clin Immunol. 1996;97:828–837. doi: 10.1016/s0091-6749(96)80161-8. [DOI] [PubMed] [Google Scholar]
- 20.Krueger JG, Wolfe JT, Nabeja RT, Vallat VP, Gilleaudau P, Heftler NS, Austin LM, Alice, Gottlieb B. Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cells. J Exp Med. 1995;182:2057–2068. doi: 10.1084/jem.182.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16:569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 22.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhein J, Walczak H, Bäumler C, Debatin K-M, Krammer PH. Autocrine T cell suicide mediated by APO-1/(FAS/CD95) Nature (Lond) 1995;373:438–441. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 24.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR. Cell-autonomous Fas (CD95)/ Fas-ligand interaction mediates activation-induced apoptosis in T cell hybridomas. Nature (Lond) 1995;373:441–444. doi: 10.1038/373441a0. [DOI] [PubMed] [Google Scholar]
- 25.Ju S-T, Panka DJ, Cui H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature (Lond) 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 26.Friesen C, Herr I, Krammer P-H, Debatin K-M. Involvement of the CD95 (APO-1/Fas) receptor/ ligand system in drug-induced apoptosis in leukemia cells. Nature Med. 1996;2:574–577. doi: 10.1038/nm0596-574. [DOI] [PubMed] [Google Scholar]
- 27.Chiba T, Takashi S, Sato N, Ishii S, Kikuchi K. Fas-mediated apoptosis is modulated by intracellular glutathione in human T-cells. Eur J Immunol. 1996;26:1164–1169. doi: 10.1002/eji.1830260530. [DOI] [PubMed] [Google Scholar]
- 28.Krutmann J, Grewe M. Involvement of cytokines, DNA damage, and reactive oxygen intermediates in ultraviolet radiation-induced modulation of intercellular adhesion molecule-1 expression. J Invest Dermatol. 1995;105:67S–70S. doi: 10.1111/1523-1747.ep12316095. [DOI] [PubMed] [Google Scholar]
- 29.Morita A, Grewe M, Grether-Beck S, Olaizola-Horn S, Krutmann J. Induction of proinflammatory cytokines in human epidermoid carcinoma cells by in vitro ultraviolet A1 irradiation. Photochem Photobiol. 1997;65:630–635. doi: 10.1111/j.1751-1097.1997.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 30.Kanofsky JR. Singlet oxygen production by biological systems. Chem Biol Interact. 1989;70:1–28. doi: 10.1016/0009-2797(89)90059-8. [DOI] [PubMed] [Google Scholar]