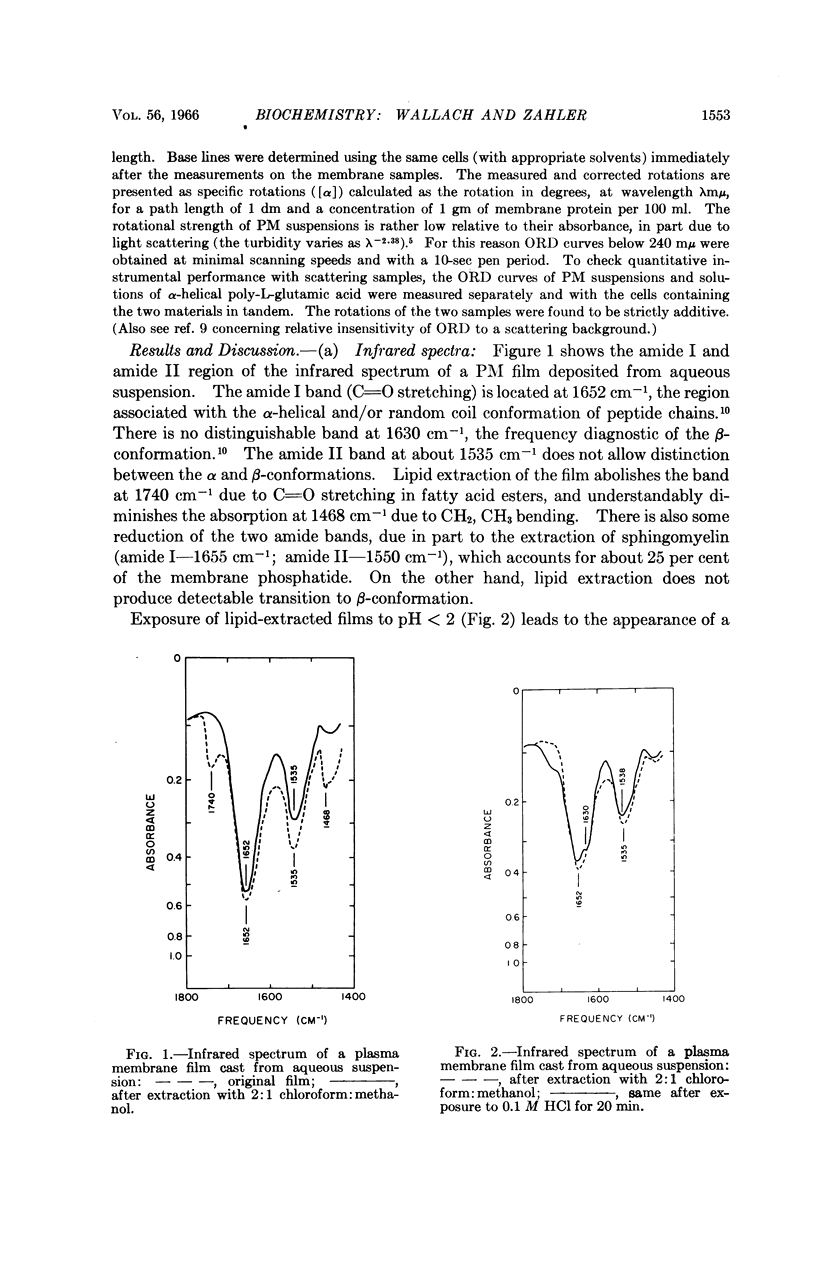
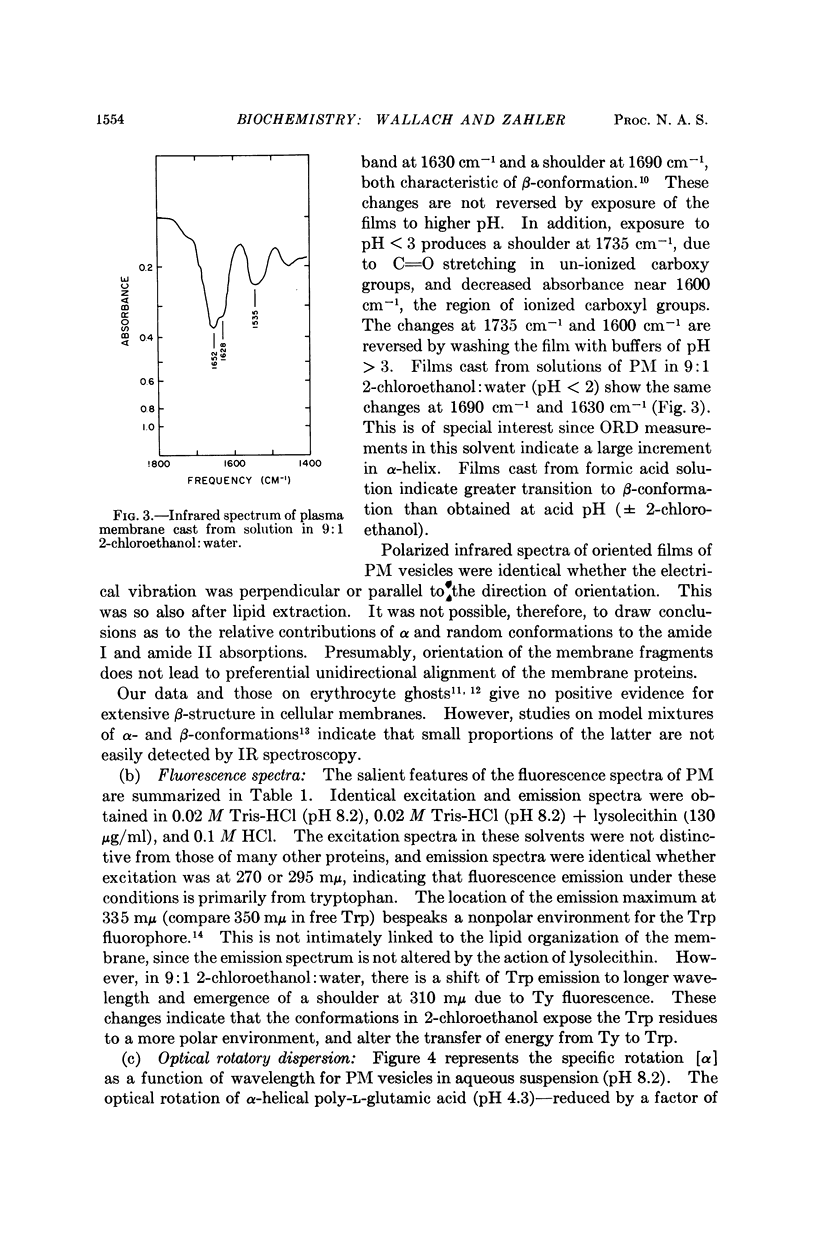
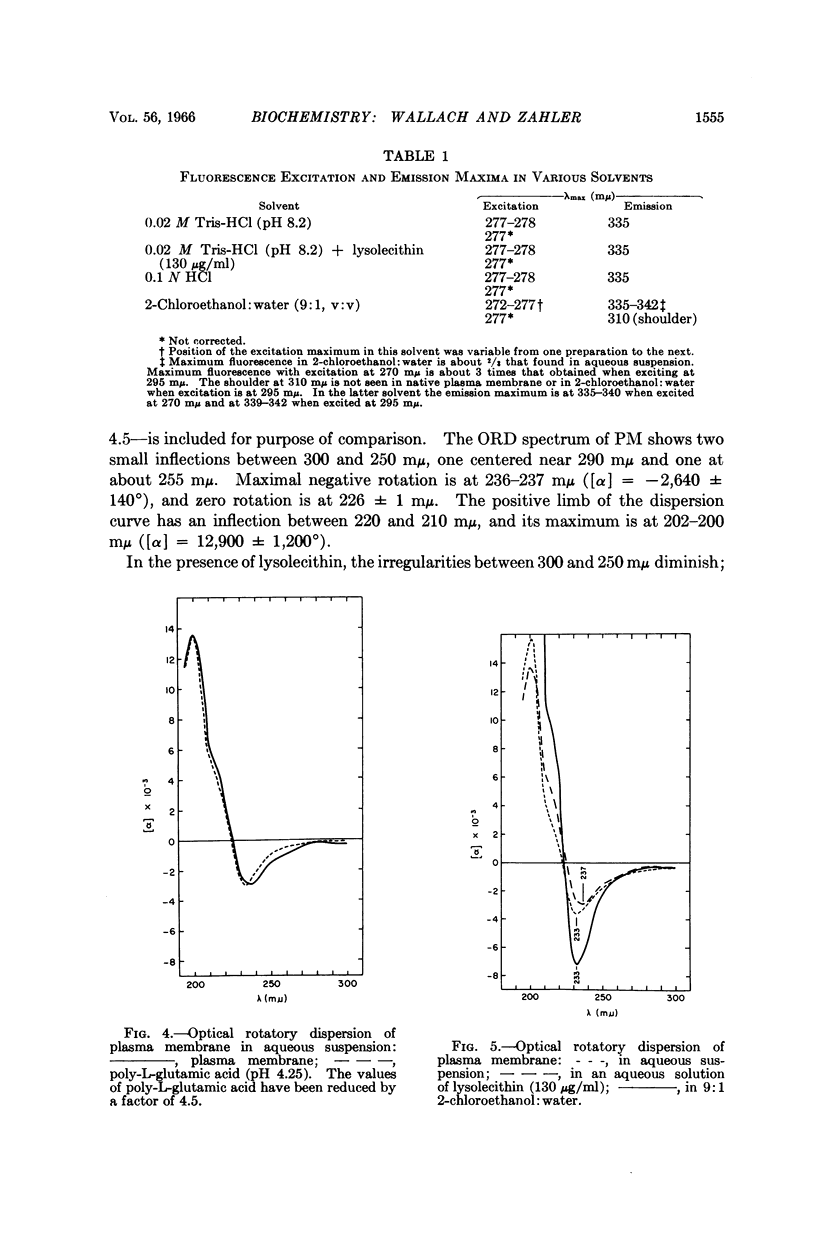
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auer H. E., Doty P. The synthesis, structure, and optical properties of some copolypeptides containing nonpolar amino acid residues. Biochemistry. 1966 May;5(5):1708–1715. doi: 10.1021/bi00869a037. [DOI] [PubMed] [Google Scholar]
- BEYCHOK S., FASMAN G. D. CIRCULAR DICHROISM OF POLY-L-TYROSINE. Biochemistry. 1964 Nov;3:1675–1678. doi: 10.1021/bi00899a012. [DOI] [PubMed] [Google Scholar]
- BROWN A. D. HYDROGEN ION TITRATIONS OF INTACT AND DISSOLVED LIPOPROTEIN MEMBRANES. J Mol Biol. 1965 Jun;12:491–508. doi: 10.1016/s0022-2836(65)80272-8. [DOI] [PubMed] [Google Scholar]
- Beychok S., Kabat E. A. Optical activity and conformation of carbohydrates. I. Optical rotatory dispersion studies on immunochemically reactive amino sugars and their glycosides, milk oligosaccharides, oligosaccharides of glucose, and blood group substances. Biochemistry. 1965 Dec;4(12):2565–2574. doi: 10.1021/bi00888a004. [DOI] [PubMed] [Google Scholar]
- Beychok S. Side-chain optical activity in cystine-containing proteins: circular dichroism studies. Proc Natl Acad Sci U S A. 1965 May;53(5):999–1006. doi: 10.1073/pnas.53.5.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassim J. Y., Taylor E. W. The effects of solvent environment on the optical rotatory dispersion parameters of polypeptides. I. Studies on poly-gamma-benzyl-L-glutamate. Biophys J. 1965 Jul;5(4):553–571. doi: 10.1016/S0006-3495(65)86734-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FASMAN G. D., BODENHEIMER E., LINDBLOW C. OPTICAL ROTATORY DISPERSION STUDIES OF POLY-L-TYROSINE AND COPOLYMERS OF L-GLUTAMIC ACID AND L-TYROSINE. SIGNIFICANCE OF THE TYROSYL COTTON EFFECTS WITH RESPECT TO PROTEIN CONFORMATION. Biochemistry. 1964 Nov;3:1665–1674. doi: 10.1021/bi00899a011. [DOI] [PubMed] [Google Scholar]
- GLAZER A. N., ROSENHECK K. Solvent and conformational effects on the ultraviolet spectra of polypeptides and substituted amides. J Biol Chem. 1962 Dec;237:3674–3678. [PubMed] [Google Scholar]
- GREEN D. E., FLEISCHER S. THE ROLE OF LIPIDS IN MITOCHONDRIAL ELECTRON TRANSFER AND OXIDATIVE PHOSPHORYLATION. Biochim Biophys Acta. 1963 Oct 22;70:554–582. doi: 10.1016/0006-3002(63)90793-5. [DOI] [PubMed] [Google Scholar]
- Gratzer W. B., McPhie P. Conformational change in poly-L-lysine on reaction with polyacids. Biopolymers. 1966 Jun;4(5):601–606. doi: 10.1002/bip.1966.360040510. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Taylor J. The stability and properties of bimolecular lipid leaflets in aqueous solutions. J Theor Biol. 1963 May;4(3):281–296. doi: 10.1016/0022-5193(63)90007-9. [DOI] [PubMed] [Google Scholar]
- Huang C., Thompson T. E. Properties of lipid bilayer membranes separating two aqueous phases: determination of membrane thickness. J Mol Biol. 1965 Aug;13(1):183–193. doi: 10.1016/s0022-2836(65)80088-2. [DOI] [PubMed] [Google Scholar]
- Iizuka E., Yang J. T. Optical rotatory dispersion and circular dichroism of the beta-form of silk fibroin in solution. Proc Natl Acad Sci U S A. 1966 May;55(5):1175–1182. doi: 10.1073/pnas.55.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMAT V. B., WALLACH D. F. SEPARATION AND PARTIAL PURIFICATION OF PLASMA-MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA MICROSOMES. Science. 1965 Jun 4;148(3675):1343–1345. doi: 10.1126/science.148.3675.1343. [DOI] [PubMed] [Google Scholar]
- Ke B. Optical rotatory dispersion of chloroplast-lamellae fragments. Arch Biochem Biophys. 1965 Dec;112(3):554–561. doi: 10.1016/0003-9861(65)90095-0. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Structure of biological membranes. Science. 1966 Sep 23;153(3743):1491–1498. doi: 10.1126/science.153.3743.1491. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MYERS D. V., EDSALL J. T. OPTICAL ROTATORY DISPERSION OF HUMAN CARBONIC ANHYDRASES: COTTON EFFECTS AND AROMATIC ABSORPTION BANDS. Proc Natl Acad Sci U S A. 1965 Jan;53:169–177. doi: 10.1073/pnas.53.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddy A. H., Malcolm B. R. Protein conformations in the plasma membrane. Science. 1965 Dec 17;150(3703):1616–1618. doi: 10.1126/science.150.3703.1616. [DOI] [PubMed] [Google Scholar]
- McPhie P., Gratzer W. B. The optical rotatory dispersion of ribosomes and their constituents. Biochemistry. 1966 Apr;5(4):1310–1315. doi: 10.1021/bi00868a026. [DOI] [PubMed] [Google Scholar]
- RICHARDSON S. H., HULTIN H. O., FLEISCHER S. INTERACTIONS OF MITOCHONDRIAL STRUCTURAL PROTEIN WITH PHOSPHOLIPIDS. Arch Biochem Biophys. 1964 May;105:254–260. doi: 10.1016/0003-9861(64)90006-2. [DOI] [PubMed] [Google Scholar]
- Sage H. J., Fasman G. D. Conformational studies on poly-L-glutamic acid and copolymers of L-glutamic acid and L-phenylalanine. Biochemistry. 1966 Jan;5(1):286–296. doi: 10.1021/bi00865a037. [DOI] [PubMed] [Google Scholar]
- Sarkar P. K., Doty P. The optical rotatory properties of the beta-configuration in polypeptides and proteins. Proc Natl Acad Sci U S A. 1966 Apr;55(4):981–989. doi: 10.1073/pnas.55.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDENHEUVEL F. A. STUDY OF BIOLOGICAL STRUCTURE AT THE MOLECULAR LEVEL WITH STEREOMODEL PROJECTIONS. II. THE STRUCTURE OF MYELIN IN RELATION TO OTHER MEMBRANE SYSTEMS. J Am Oil Chem Soc. 1965 Jun;42:481–492. doi: 10.1007/BF02540089. [DOI] [PubMed] [Google Scholar]
- WALLACH D. F., KAMAT V. B. PLASMA AND CYTOPLASMIC MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA. Proc Natl Acad Sci U S A. 1964 Sep;52:721–728. doi: 10.1073/pnas.52.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER G. Fluorescence-polarization spectrum and electronic-energy transfer in proteins. Biochem J. 1960 May;75:345–352. doi: 10.1042/bj0750345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D. F., Kamat V. B., Gail M. H. Physicochemical differences between fragments of plasma membrane and endoplasmic reticulum. J Cell Biol. 1966 Sep;30(3):601–621. doi: 10.1083/jcb.30.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANARI S., BOVEY F. A. Interpretation of the ultraviolet spectral changes of proteins. J Biol Chem. 1960 Oct;235:2818–2826. [PubMed] [Google Scholar]


