Abstract
Tissue-derived adenosine, acting via the adenosine A2A receptor (A2AR), is emerging as an important negative regulator of T-cell function. In this report, we demonstrate that A2AR stimulation not only inhibits the generation of adaptive effector T cells but also promotes the induction of adaptive regulatory T cells. In vitro, antigen recognition in the setting of A2AR engagement induces T-cell anergy, even in the presence of costimulation. T cells initially stimulated in the presence of an A2AR agonist fail to proliferate and produce interleukin-2 and interferon (IFN)-γ when rechallenged in the absence of A2AR stimulation. Likewise, in an in vivo model of autoimmunity, tissue-derived adenosine promotes anergy and abrogates tissue destruction. Indeed, A2AR stimulation inhibits interleukin-6 expression while enhancing the production of transforming growth factor-β. Accordingly, treating mice with A2AR agonists not only inhibits Th1 and Th17 effector cell generation but also promotes the generation of Foxp3+ and LAG-3+ regulatory T cells. In this regard, A2AR agonists fail to prevent autoimmunity by LAG-3−/− clonotypic T cells, implicating an important role for LAG-3 in adenosine-mediated peripheral tolerance. Overall, our findings demonstrate that extracellular adenosine stimulates the A2AR to promote long-term T-cell anergy and the generation of adaptive regulatory T cells.
Introduction
Under normal physiologic conditions, the level of adenosine in the tissue microenvironment is relatively low.1 At the height of inflammation, the destruction of host tissue by a vigorous immune response combined with damaged microcirculation and hypoxia leads to increases in extracellular adenosine. The presence of adenosine in the microenvironment prevents further tissue destruction by inhibiting inflammation.2,3 The importance of the adenosine A2A receptor (A2AR) in mediating this negative feedback loop has been dramatically demonstrated in A2AR null mice.4 These knockout mice are unable to control inflammation, which leads to extensive, often fatal, tissue destruction. For example, when immune-mediated liver inflammation was induced in mice by injecting Con A, the A2AR null mice died because of massive immune–mediated liver destruction.
The inability of the thymus to eliminate all self-reactive T cells necessitates peripheral mechanisms of T cell tolerance to prevent tissue damage from autoreactive T cells. The role of clonal deletion and T-cell anergy in maintaining peripheral tolerance has been demonstrated in several in vivo models.5,6 More recently, the roles of Foxp3+ and LAG-3+ regulatory T cells in peripheral tolerance has been better defined.7,8 Likewise, cell-surface receptors such as CTLA-4 and PD-1 and cytokines such as interleukin (IL)-10 and transforming growth factor (TGF)-β are thought not only to inhibit T-cell activation but also to promote peripheral T-cell tolerance.9–12 In this report, we identify extracellular adenosine as a novel inducer of peripheral T-cell tolerance. We propose that tissue-derived adenosine acts through the A2AR to generate peripheral T-cell tolerance by inducing T-cell anergy and adaptive regulatory T cells.
Methods
Reagents
Cyclosporine A (Calbiochem, Cambridge, MA) was dissolved in ethanol and used at 1 μM. CGS-21680 (Sigma, St Louis, MO) was dissolved in phosphate-buffered saline (PBS) and used at indicated concentrations. Anti-CD3 (2C11, BD PharMingen, San Diego, CA) was diluted in PBS and used at 1 μg/mL, as indicated. Soluble anti-CD28 (a kind gift from Dr J. Allison, University of California at Berkeley, Berkeley, CA) was used at a 1/1000 dilution. Hemagglutinin (HA) class II is an I-Ed specific peptide (SFERFEIFPKE), which was manufactured by the Johns Hopkins School of Medicine Oncology Department Peptide Synthesis Facility. Antiphospho-ERK (Santa Cruz Biotechnology, Santa Cruz, CA) was used at 1:400 in 5% nonfat dry milk (NFDM)/TBS-Tween (0.1%). Anti-p42/p44 MAPK (Cell Signaling Technology, Danvers, MA) was used at 1:500 in 5% BSA/TBS-Tween (0.1%). Anti–jun B (Santa Cruz) and anti-actin (Sigma) were both used at 1:1000 in 5% NFDM/TBS-Tween (0.1%) mAbs used for staining: biotinylated anticlonotypic 6.5 T-cell receptor (TCR) (generously provided by H. Von Boehmer); avidin-PE (Caltag, Burlingame, CA); FITC-conjugated anti-CD4 (RM4-4); Cy-chrome-conjugated anti-CD4 (RM4-5); PE-conjugated antiThy-1.1 (OX-7); FITC-conjugated anti-CD44 (1 M7); APC-conjugated anti-interferon (IFN)-γ (XMG1.2); PE-conjugated anti-IL-17 (TC11–18H10); biotinylated anti-Thy1.2 (53-2.1) (all purchased from BD PharMingen). Anti–IFN-γ and anti–IL-4 BD PharMingen) were used at 10 μg/mL. TGF-β (Sigma) was used at 5 ng/mL. IL-6 (Peprotech, Rocky Hill, NJ), was used at 20 ng/mL. FACSCalibur was used for flow cytometry event collection, and events were analyzed using FlowJo software (Ashland, OR). Sorting was done with FACSAria (BD Biosciences, San Jose, CA).
Cell culture
A.E7 is a clonotypic CD4+, Th1 T-cell clone specific for pigeon cytochrome c (PCC) and is maintained as previously described.13 A.E7s were rendered anergic with plate-bound anti-CD3 (1 μg/mL) or by incubation with APC, PCC (10 μM), and CGS (1 μM).
Splenocytes and lymphocytes from A2AR wt or null 6.5+ mice (see “Transgenic mice”) were harvested and enriched for CD4+ T cells via negative selection with the CD4+ T cell isolation kit and LS MACs column (all from Miltenyi Biotech, Auburn, CA). The T cells were then cultured in the presence or absence of 10 μg/mL HA and 1 μM CGS. The T cells were rechallenged for 3 to 4 hours with 100 μg/mL HA, GolgiStop (BD PharMingen), and irradiated APCs.
cAMP production
Total cAMP of naive or previously activated cells were assayed with the Biotrak EIA system (Amersham Biosciences, Amersham, United Kingdom).
Microarray
In vitro microarray was performed as previously described.14 In vivo microarray was performed as previously described.8
Transgenic mice
The C3HA expressing transgenic (recipient) mice express HA under the rat C3 promoter and has been previously described.15 The HA-specific TCR-transgenic mouse line 6.5 (donor mice) has been previously described.8 A2AR−/− mice were bred to this 6.5 mouse line. LAG-3 knockout mice on a C57/B6 were a generous gift of Dr Dario Vignali. The LAG-3 KO genotype was bred onto a B10.D2 6.5+ TCR background. All experiments involving the mice were performed in accordance with protocols approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
5C.C7 TCR-transgenic Rag2−/− mice on a B10.A background were purchased from Taconic (Petersburgh, NY). T cells from 5C.C7 mice are specific for pigeon cytochrome c.
Adoptive transfer
Clonotypic CD4+ T cells were harvested from 6.5+ transgenic mice. The unfractionated population was resuspended to contain 1.2 × 106 6.5+ T cells in 200 μL HBSS for intravenous injection into C3HA mice. Recipient mice were given twice-daily intraperitoneal injections of vehicle (PBS alone) or CGS (2.5 mg/kg) on days 1 to 4 after the transfer. No CGS was administered after day 4.
The numbers of IFN-γ and IL-17 cells were determined by multiplying the number of infiltrating cells recovered from the entire lung by the percentage of donor (Thy1.1+) cells and the percentage of clonotypic cells that produce the cytokine.
Western blot
For phospho-ERK and total ERK Western blots, splenocytes from 6.5+ mice were stimulated overnight with 10 μg/mL HA class II peptide then harvested and enriched for CD4+ T cells. CD4+ purified T cells were then stimulated in 500 μL with soluble anti-CD3 (10 μg/mL) and soluble anti-CD28 (1:20 dilution).
For the junB and actin Western blots, CD4+ purified, 6.5+ T cells were stimulated overnight in the presence or absence of 1 μM CGS. Nuclear extracts were then probed for junB and actin.
Electrophoretic mobility shift assay
Nuclear extracts from CD4+ purified 6.5+ T cells that were prepared. The AP-1 probe was: 5′-CGC TTG ATG ACT CAG CCG GAA-3′. The NFκB probe was: 5′-AGT TGA GGG GAC TTT CCC AGG C-3′.
Reverse-transcribed polymerase chain reaction
For in vitro studies, 5C.C7 splenocytes were stimulated overnight with 10 μM PCC in the presence or absence of 1 μM CGS. mRNA was isolated with Trizol and cDNA was generated. Reverse-transcribed polymerase chain reaction (RT-PCR) was performed as previously described.14
For in vivo studies, recipient C3HA mice were given 1.2 × 106 6.5+ donor T cells as described above. On day 3, donor T cells were sorted and cDNA generated as above. RT-PCR was performed as previously described.14
LAG-3 primers and probe sets used were: primer 5′-ACATCAACCAGACAGTGGCCA-3′/primer 5′-GCATCCCCTGGTGAAGGTC-3′/probe 5′-6FAM-CCCACTCCCATCCCGGCCC-TAMRA-3′.
FoxP3 primers and probe sets used were: primer 5′-GGC CCT TCT CCA GGA CAG A-3′/primer 5′-GCT GAT CAT GGC TGG GTT GT-3′/probe 5′-6FAM-ACT TCA TGC ATC AGC TCT CCA CTG TGG ATT AT-TAMRA-3′ IL-6 and TGF-β primers and probe sets were purchased from Applied Biosystems (Foster City, CA).
Th17 driving conditions
4 × 106 5C.C7 splenocytes were stimulated as previously described.16 Briefly, cells were stimulated in media containing TGF-β (5 ng/mL), IL-6 (20 ng/mL), and anti–IL-4 and anti–IFN-γ blocking antibodies (10 μg/mL).
Statistics
Quantitative data were expressed as mean plus or minus standard deviation and compared using paired Student t tests. Values of P less than .05 were considered significant.
Results
A2AR expression is induced by signal 1 alone in an NF-AT–dependent fashion
If cyclosporine A (CSA), which inhibits the translocation of NF-AT to the nucleus, is added during priming, the induction of anergy is inhibited (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article). We established a microarray database of T cells stimulated under conditions that promote or inhibit the induction of anergy and devised a novel statistical algorithm (hypothesis-based analysis of microarray) to analyze these data.14,17 The adenosine A2AR was among the NF-AT-dependent genes that showed increased expression on TCR engagement and was inhibited by CSA (Figure S1B). For comparison, the adenosine A1 receptor expression was not regulated by TCR signaling. The adenosine A2B receptor displayed a similar, although less robust, expression profile to the A2AR.
To confirm these microarray results, we assayed the CD4+ Th1 T-cell clone, A.E7, for their expression of functional A2AR. We took advantage of the fact that the A2AR is a G-protein coupled receptor and directly stimulates adenylyl cyclase. At a given dose of an A2AR-specific agonist, differences in intracellular cAMP levels indicate the relative abundance of the A2AR on the cell surface.18 We stimulated A.E7s with PBS alone (mock), plate-bound anti-CD3 or anti-CD3 + CSA overnight, rested the cells in fresh media for 24 hours, treated the cells with the A2AR-specific agonist, CGS-21680 (CGS), and measured intracellular cAMP. Consistent with the microarray results, T cells treated with anti-CD3 alone produced more cAMP than mock-stimulated cells, indicating that the A2AR is more highly expressed on activated T cells than resting ones (Figure 1A). Moreover, the presence of CSA during TCR stimulation prevented the cAMP increase, indicating that there is no up-regulation of the A2AR when TCR-induced NF-AT activation is inhibited.
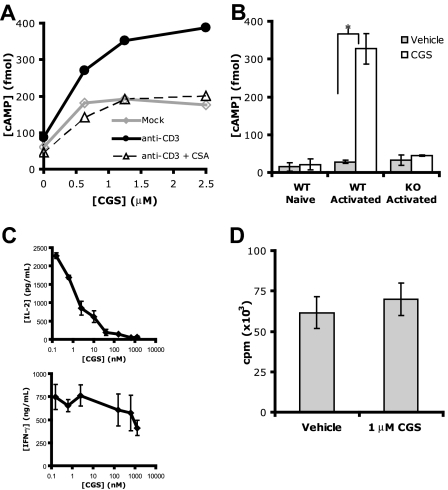
Figure 1.
A2AR expression is up-regulated on T-cell activation and preferentially inhibits IL-2 production. (A) cAMP levels after CGS incubation of A.E7s previously mock stimulated (◇) or stimulated with anti-CD3 in the presence or absence of CSA (▵ or •, respectively). (B) cAMP levels of naive or previously activated primary T cells from A2AR Wt or null mice after incubation with 0 or 1 μM CGS ( and
and  , respectively, *P < 0.05). (C) IL-2 (top) and IFN-γ (bottom) production of A.E7 T cells during activation with increasing doses of CGS. ID50 for IL-2 is 8 nM, and for IFN-γ 750 nM. (D) Proliferation of A.E7s with 0 or 1 μM of CGS. Data are representative of 3 separate experiments.
, respectively, *P < 0.05). (C) IL-2 (top) and IFN-γ (bottom) production of A.E7 T cells during activation with increasing doses of CGS. ID50 for IL-2 is 8 nM, and for IFN-γ 750 nM. (D) Proliferation of A.E7s with 0 or 1 μM of CGS. Data are representative of 3 separate experiments.
Next, we analyzed A2AR expression in primary T cells from Wt and A2AR−/− mice. In the absence of T-cell stimulation, CGS treatment did not increase intracellular cAMP (“Wt Naive”). On activation, however, CGS treatment dramatically increased cAMP production in Wt T cells, again indicating that A2AR protein expression has increased. Activated T cells from A2AR−/− mice did not produce cAMP in response to CGS treatment, which is expected and confirms the specificity of CGS for the A2AR (Figure 1B). These results directly confirm our findings with the A.E7 T-cell clones and show that in primary T cells the A2AR is expressed on TCR signaling in an NF-AT–dependent manner. Previous studies have clearly demonstrated the ability of the A2AR to inhibit T-cell activation both in vitro and in vivo.19–23 In agreement with these studies, we found that A2AR engagement during A.E7 stimulation markedly inhibited IL-2 production (Figure 1C). By comparison, the inhibition of IFN-γ by A2AR stimulation was less robust. This finding was not secondary to CGS-induced cell death, as determined by trypan blue exclusion (data not shown). Furthermore, we failed to see any inhibition of T-cell proliferation by CGS in this dose-response range (Figure 1D). Thus, the A2AR is induced by signal 1 alone in an NF-AT–dependent fashion and plays a role in inhibiting T-cell function, most specifically IL-2 expression.
A2AR engagement promotes long-term tolerance
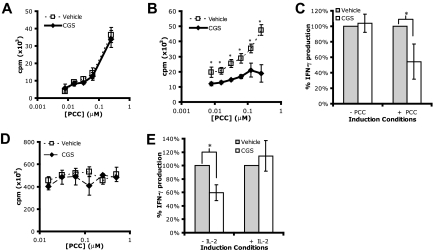
In light of these initial findings, we wanted to determine the effect of A2AR engagement on the induction of T-cell anergy. We examined whether A2AR engagement during the initial antigen encounter could promote tolerance such that the T cells would be unresponsive on rechallenge in the absence of A2AR stimulation. To this end, A.E7 T cells were stimulated for 4 days with irradiated APCs (signal 1 + 2) in the presence or absence of PCC (the cognate antigen for A.E7s) and in the presence or absence of CGS. The cells were then harvested, washed extensively, and rechallenged with irradiated APCs and PCC in the absence of CGS. Incubating A.E7s with CGS in the absence of peptide had no consequence on rechallenge with PCC (Figure 2A,C left bars). These results were consistent with the fact that A2AR expression requires TCR engagement. However, if the T cells were activated by PCC in the presence of CGS, they failed to proliferate or produce IFN-γ on subsequent rechallenge (Figure 2B,C). Exogenous IL-2 can prevent and reverse clonal T-cell anergy.24,25 Therefore, we explored the effect of exogenous IL-2 on A2AR stimulation. The addition of exogenous IL-2 during the induction mitigated the effect of A2AR engagement (Figure 2D,E). When IL-2 was present in the induction cultures, CGS had no effect on proliferation and IFN-γ production on rechallenge. Overall, our data indicate that A2AR stimulation even in the presence of costimulation can induce a hyporesponsive state on rechallenge that resembles T-cell anergy.
Figure 2.
A2AR engagement during activation promotes T cell tolerance. (A,B) Proliferation on rechallenge of A.E7 T cells after 4-day incubation without (A) or with (B) peptide in the absence (□) or presence (♦) of 1 μM CGS. (C) IFN-γ production on rechallenge of A.E7 T cells after priming without or with peptide (left or right side, respectively) in the absence ( ) or presence (
) or presence ( ) of 1 μM CGS. (D,E) Proliferation and IFN-γ production on rechallenge of A.E7s incubated with peptide and exogenous IL-2 in the absence or presence of 1 μM CGS. All rechallenges are done in the absence of CGS or exogenous IL-2. Data are representative of at least 3 independent experiments (*P < .05).
) of 1 μM CGS. (D,E) Proliferation and IFN-γ production on rechallenge of A.E7s incubated with peptide and exogenous IL-2 in the absence or presence of 1 μM CGS. All rechallenges are done in the absence of CGS or exogenous IL-2. Data are representative of at least 3 independent experiments (*P < .05).
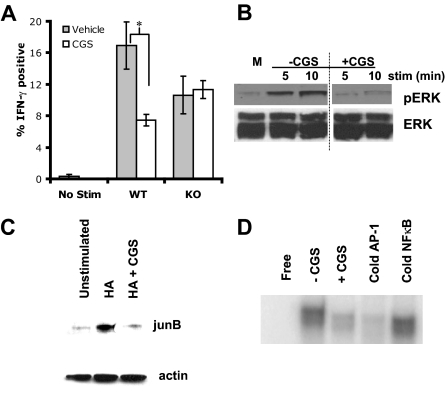
Having determined that A2AR engagement can promote anergy in A.E7 T-cell clones, we next wanted to examine whether A2AR signaling can induce tolerance in primary T cells. We isolated CD4+ T cells from Wt or A2AR−/− 6.5+ mice and cultured them with irradiated APCs and HA peptide in the presence or absence of CGS. The T cells were harvested, extensively washed, and rechallenged with HA peptide in the absence of CGS. On rechallenge with HA, Wt T cells produced IFN-γ. In contrast, T cells that were initially incubated with HA peptide and CGS were hyporesponsive on rechallenge. Because no CGS was present during the rechallenge, the decreased cytokine production reflects the induction of anergy. This effect was A2AR specific as T cells from A2AR−/− mice cultured initially in the presence or absence of CGS have equivalent numbers of IFN-γ+ T cells (Figure 3A). A2AR signaling during an initial antigen encounter can induce a state of anergy in primary T cells such that they are hyporesponsive on rechallenge.
Figure 3.
A2AR signaling promotes decreases signaling of the Ras-MAP-Kinase pathway. (A) Percentage of A2AR Wt or KO T cells that were IFN-γ positive on rechallenge after incubation with peptide in the absence ( ) or presence (
) or presence ( ) of 1 μM CGS during induction (*P < 0.05). (B) Representative Western blots for phospho-ERK and total ERK (top and bottom, respectively). Activated CD4+, 6.5+ primary T cells were stimulated with anti-CD3+ anti-CD28 in the absence or presence of 1 μM CGS. A vertical line has been inserted to indicate a repositioning of gel lanes from the same experiment. (C) Representative Western blots for junB, and actin (top and bottom, respectively). CD4+, 6.5+ primary T cells were stimulated with HA and irradiated APCs overnight in the absence or presence of 1 μM CGS. (D) Representative EMSA for AP-1. CD4+, 6.5+ primary T cells were stimulated with HA and irradiated APCs overnight in the absence or presence of 1 μM CGS. Data are representative of 3 independent experiments.
) of 1 μM CGS during induction (*P < 0.05). (B) Representative Western blots for phospho-ERK and total ERK (top and bottom, respectively). Activated CD4+, 6.5+ primary T cells were stimulated with anti-CD3+ anti-CD28 in the absence or presence of 1 μM CGS. A vertical line has been inserted to indicate a repositioning of gel lanes from the same experiment. (C) Representative Western blots for junB, and actin (top and bottom, respectively). CD4+, 6.5+ primary T cells were stimulated with HA and irradiated APCs overnight in the absence or presence of 1 μM CGS. (D) Representative EMSA for AP-1. CD4+, 6.5+ primary T cells were stimulated with HA and irradiated APCs overnight in the absence or presence of 1 μM CGS. Data are representative of 3 independent experiments.
A2AR signaling inhibits activation of the Ras-MAP-kinase pathway
Next, we tested the hypothesis that A2AR signaling might promote anergy by inhibiting TCR-induced AP-1 activation. A series of experiments was performed to determine the effect of A2AR signaling on the Ras-MAP-kinase pathway. CD4+ 6.5+ T cells were stimulated with HA overnight to induce expression of the A2AR. The cells were then harvested and washed before being stimulated with anti-CD3 and anti-CD28 plus or minus CGS. In the absence of CGS, ERK was rapidly phosphorylated. However, in the presence of CGS, this phosphorylation was prevented. Total ERK levels remain constant (Figure 3B). We then looked at the effect of A2AR signaling on the expression of junB, a component of AP-1. In the absence of stimulation, CD4+ 6.5+ T cells had low levels of junB expression. On stimulation, junB levels drastically increased. But if the CD4+ 6.5+ T cells were stimulated in the presence of the A2AR-agonist, junB levels remained similar to the unstimulated cells (Figure 3C). Finally, EMSA was performed to examine AP-1 activity in the nucleus. In the absence of stimulation, minimal AP-1 binding was observed. On stimulation, there was an increase in AP-1 in the vehicle-treated cells; however, in the presence of A2AR signaling, AP-1 binding was inhibited (Figure 3D).
Extracellular adenosine promotes long-term tolerance in vivo
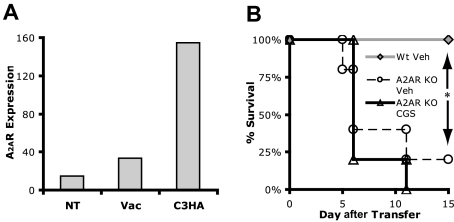
Using T-cell clones and TCR transgenic primary T cells, we have shown that A2AR signaling promotes a state of hyporesponsiveness similar to T-cell anergy, even in the presence of costimulation. We then sought to determine whether endogenous, tissue-derived adenosine in vivo could promote T-cell tolerance. A series of experiments used a previously established model of T-cell tolerance induction and autoimmunity in vivo.8,15,26,27 C3HA mice express HA as a self-antigen, with especially high HA expression in the lung and prostate. When HA-specific 6.5+ T cells are adoptively transferred into C3HA mice, the donor 6.5+ T cells are initially activated by HA, which is presented as a “self” antigen. But by day 4, they have been rendered anergic. If a sufficient number of 6.5+ T cells are transferred, the initial activation of the clonotypic cells dominates and the recipient mice die of autoimmune-induced pneumonitis. We wanted to determine whether the A2AR is up-regulated on the HA-specific T cells in vivo. Clonotypic T cells were adoptively transferred into C3HA mice, isolated 4 days later and assessed by microarray analysis for A2AR expression. Compared with the T cells transferred into nontransgenic mice or mice infected with vaccinia virus expressing HA, the A2AR was markedly up-regulated on the in vivo tolerized clonotypic T cells (Figure 4A).
Figure 4.
Endogenous adenosine is required to prevent death by autoimmunity. (A) In vivo A2AR mRNA expression as determined by Affymetrix microarray analysis for 6.5+ TCR transgenic clonotypic T cells specific for hemagglutinin (HA) that had been transferred into B10.D2 mice (NT), vaccinated B10.D2 mice (Vac), or C3HA mice (C3HA). (B) Survival curve of C3HA mice given Wt ( ) or A2AR−/− 6.5+ T cells (○) (n = 5 each condition). Data are representative of 2 independent experiments.
) or A2AR−/− 6.5+ T cells (○) (n = 5 each condition). Data are representative of 2 independent experiments.
Having determined that the A2AR is up-regulated on the adoptively transferred T cells in vivo, we investigated the role that endogenous adenosine might play in promoting tolerance. Wt and A2aR−/− clonotypic T cells were adoptively transferred into C3HA mice. As seen in Figure 4B, the dose of Wt 6.5+ T cells was insufficient to induce lethal autoimmunity. The clonotypic T cells become tolerant and 100% of the mice survive. On the other hand, transfer of the A2AR−/− 6.5+ T cells led to 80% death because of autoimmune pneumonitis. Thus, in the absence of A2AR signaling on the adoptively transferred T cells, a tolerizing interaction (A2AR engagement) is removed leading to tissue destruction and death. Because only the adoptively transferred cells are A2AR−/−, our observations reflect the direct effect of endogenous tissue-derived adenosine on T cells and not an increase in the general inflammatory milieu (for example, the host APCs are Wt and thus are responsive to the tissue-released adenosine).
We then asked whether we could pharmacologically induce tolerance in vivo by administering CGS to hosts that receive A2AR competent 6.5+ T cells. We transferred a lethal dose of T cells followed by a 4-day course of CGS (2.5 mg/kg, intraperitoneally, twice daily). CGS treatment resulted in long-term survival (30+ days) of 76% of the host mice, whereas only 24% of the vehicle-treated hosts survived past day 9 (Figure 5A). We wanted to rule out that the ability of CGS to induce tolerance was simply because of the deletion of the clonotypic cells. To test this, we transferred 6.5+ T cells into C3HA mice and harvested the donor 6.5+ T cells 2 or 3 days after transfer. CGS did not affect the frequency of donor 6.5+ T cells (Figure S2). The relatively equivalent numbers of clonotypic cells is consistent with our in vitro finding that CGS is a poor inhibitor of T-cell proliferation (Figure 1D). It also suggests that deletion is not the mechanism by which A2AR signaling promotes tolerance in vivo. We next tested the clonotypic T cells from the treated and untreated mice for their function in response to rechallenge with HA antigen in vitro. Donor 6.5+ T cells harvested from vehicle- or CGS-treated mice on day 2 showed no differences in proliferation or IFN-γ and IL-2 production (Figure 5B-D gray bars). By day 3, however, donor 6.5+ T cells from CGS-treated hosts demonstrated greatly reduced proliferation and IFN-γ and IL-2 production compared with those from vehicle-treated hosts (Figure 5B-D open bars). A2AR signaling, in vivo, promotes T-cell tolerance, resulting in reduced lung damage and increased survival.
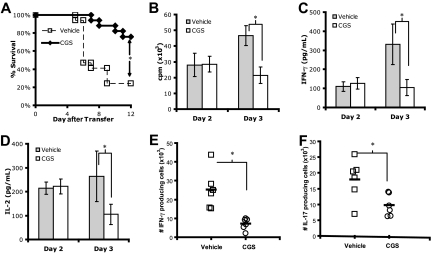
Figure 5.
A2AR stimulation in vivo prevents death by autoimmunity and promotes T-cell tolerance. (A) Survival curve of C3HA mice given 6.5+ T cells and 4 days of vehicle (□) or CGS (♦) (n = 17 mice, each condition). (B-D) In vitro proliferation (B), IFN-γ production (C), and IL-2 (D) of T cells harvested from vehicle- or CGS-treated C3HA mice ( and
and  , respectively). Data are representative of 2 independent experiments, ≥3 mice per group. (E,F) Number of IFN-γ (E) and IL-17 (F) lung-infiltrating 6.5+ T cells from C3HA mice given a lethal dose of autoreactive T cells. Data are a combination of 2 independent experiments, 3 mice per group (*P < .05).
, respectively). Data are representative of 2 independent experiments, ≥3 mice per group. (E,F) Number of IFN-γ (E) and IL-17 (F) lung-infiltrating 6.5+ T cells from C3HA mice given a lethal dose of autoreactive T cells. Data are a combination of 2 independent experiments, 3 mice per group (*P < .05).
Recently, it has been demonstrated that the lethality in the C3HA model is mediated, in part, by lung-infiltrating IL-17–producing cells (Harris and Drake, oral communication, June 2007). Thus, we tested whether A2AR stimulation was able to affect the generation of Th17 effector cells in this model. Recipient C3HA mice were given 10 × 106 6.5+ T cells and treated with vehicle or CGS. The lungs were harvested, and intraparenchymal clonotypic T cells were stimulated with HA peptide in vitro. As seen in Figure 5E,F, there was a decrease in the number of not only IFN-γ producing cells but also IL-17–producing lung-infiltrating T cells in the mice treated with CGS. Thus, treatment with CGS resulted in a decrease in the tissue infiltration of Th1 and Th17 effector T cells.
The generation of IL-17–producing cells requires both IL-6 and TGF-β. In light of the anti-inflammatory properties of A2AR stimulation, we hypothesized that A2AR stimulation might inhibit the generation of IL-17–producing cells by altering the cytokines found in the inflammatory milieu. We tested this hypothesis using a previously defined method of generating Th17 cells in vitro from naive T cells from 5C.C7 TCR-transgenic Rag2−/− mice.16 As seen in Figure 6A, CGS inhibited the expression of IL-6. In the same culture conditions, however, A2AR activation led to a marked up-regulation of TGF-β (Figure 6B). These data suggest that CGS limits the generation of IL-17–producing cells in vivo, in part, by inhibiting IL-6 production. Indeed, CGS did not inhibit the induction of IL-17+ T cells in vitro if exogenous IL-6 was added to the culture conditions (Figure 6C).
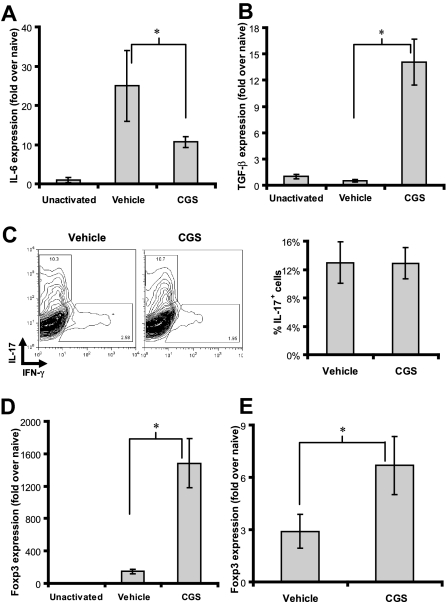
Figure 6.
A2AR engagement during inflammation inhibits IL-6 and promotes Foxp3 expression in vivo. (A,B) Primary T cells from 5C.C7 mice were cultured with PCC plus or minus 1 μM CGS for 1 day. Total RNA was harvested and assayed for abundance of IL-6 (A) and TGF-β (B) transcripts by real-time PCR. Data are representative of 3 independent experiments. (C) Splenocytes from 5C.C7 TCR-transgenic Rag2−/− mice were cultured with antigen (PCC) plus or minus 1 μM CGS for 3 days under Th17-driving conditions (containing TGF-β, IL-6, anti-IFN-γ, and anti-IL-4). The cells were then activated with PMA and ionomycin and assayed for IL-17 by intracellular cytokine staining. Representative FACs plots are shown (left) and bar graph of composite data (right). Data are representative of 3 independent experiments. (D) Primary T cells from 5C.C7 mice were cultured with PCC plus or minus 1 μM CGS for 1 day. Total RNA was harvested and assayed for abundance of Foxp3 transcripts by real-time PCR. Data are representative of 3 independent experiments. (E) 6.5+ donor T cells were harvested 3 days after transfer into C3HA hosts treated with vehicle or CGS. Total RNA was harvested and assayed for abundance of Foxp3 by real-time PCR. Data are representative of 2 independent experiments, 3 mice per group. All PCR samples were done in triplicate and evaluated for significance (*P < .05).
Inducible Foxp3+ regulatory cells are generated when antigen recognition occurs in the presence of TFG-β.28 Because TCR engagement in the presence of CGS led to an increase in TGF-β expression, we wanted to know if CGS could promote the expression of Foxp3. Indeed, CGS-induced inhibition of IL-6 and enhancement TGF-β was accompanied by an increase in Foxp3 expression (Figure 6D). This finding, in turn, prompted us to examine Foxp3 expression in the clonotypic cells derived from the CGS-treated mice. As seen in Figure 6E, there is increased expression of Foxp3 in the clonotypic T cells isolated 3 days after adoptive transfer from the CGS-treated mice compared with the untreated mice. Thus, under immune activating conditions, A2AR activation not only inhibits the expression of proinflammatory cytokines but also enhances the transcription of inhibitory molecules such as TGF-β and Foxp3.
A2AR signaling promotes the induction of LAG-3+ Tregs in vivo
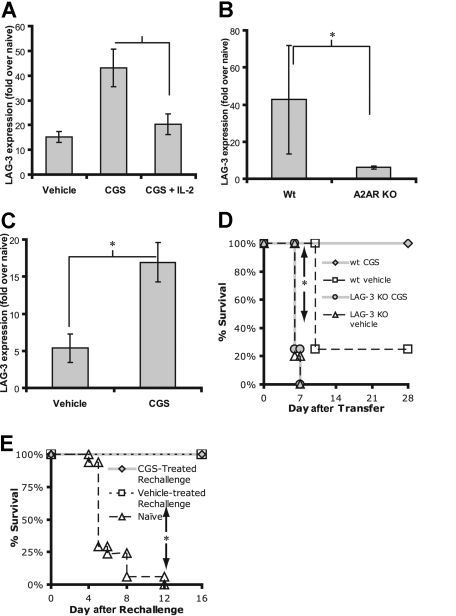
Recently, it has become clear that the CD4 homolog LAG-3 defines a subset of novel inducible regulatory T cells.8,29,30 In the C3HA model, the induction of anergy correlates with increases in LAG-3 expression and the generation of these inducible regulatory T cells. In light of the ability of A2AR stimulation to inhibit the generation of Th17 effector T cells in favor of Foxp3+ T cells, we inquired whether A2AR engagement would also lead to an increase in LAG-3+ regulatory T cells. We stimulated CD4+ 6.5+ T cells in vitro with irradiated APCs and HA in the presence or absence of CGS and IL-2. The cells were harvested and the cDNA was assayed for LAG-3 expression by real-time PCR. T cells stimulated in the presence of CGS showed increased levels of LAG-3 transcript. The presence of exogenous IL-2, however, prevented the CGS-induced up-regulation of LAG-3 (Figure 7A). These results are consistent with our in vitro tolerance data suggesting that IL-2 can overcome A2AR-induced inhibition (Figure 2D,E). We also analyzed LAG-3 expression from 6.5+ T cells that had been stimulated in vivo in C3HA host mice. Donor 6.5+ T cells were harvested from host mice 3 days after transfer and sorted. Wt T cells that were anergized in C3HA hosts displayed a dramatic increase in LAG-3 expression. In contrast, T cells from A2AR−/− 6.5+ mice did not have a robust increase in LAG-3 (Figure 7B). Because only the adoptively transferred cells are A2AR−/− (ie, host-derived APCs are Wt), these data suggest that endogenous adenosine plays a physiologic role in the up-regulation of LAG-3 in vivo. Consistent with this notion is the observation that pharmacologically inducing A2AR signaling by treating hosts with CGS led to enhanced LAG-3 expression on donor T cells compared with donor T cells from vehicle-treated mice (Figure 7C).
Figure 7.
A2AR signaling promotes LAG-3 expression and regulatory T cells. (A) CD4+, 6.5+ primary T cells were cultured with irradiated APCs and HA plus or minus 1 μM CGS and IL-2 for 3 days. Total RNA was harvested and assayed for abundance of LAG-3 transcripts by real-time PCR. (B) A2AR Wt or KO 6.5+ T cells were transferred into C3HA mice, harvested 3 days after adoptive transfer, and sorted to more than 98% purity. LAG-3 expression was determined by real-time PCR. (C) Clonotypic 6.5+ T cells were transferred into C3HA mice, which were treated with vehicle or CGS for 3 days after the adoptive transfer. The donor T cells were harvested and sorted to more than 98% purity. LAG-3 expression was determined by real-time PCR. For panels A-C, data are representative of 3 independent experiments, 3 mice per group. (D) Survival curve of C3HA mice given Wt or LAG-3 KO T cells and a 4-day treatment with CGS. The “Wt Veh” and “LAG-3 KO CGS” both had 4 mice per group. For the “Wt CGS” and “LAG-3 KO Veh,” both had 5 mice per group. Data are representative of 2 independent experiments. (E) In vivo suppression assay in which vehicle- or CGS-treated C3HA mice (the survivors of Figure 5A; □, [n = 4]; or  , [n = 13], respectively) are given a higher dose of 6.5+ T cells. Naive mice (▵, [n = 17]) received only this higher dose of 6.5+ T cells. No drug was administered during this phase of the experiment (*P < .05).
, [n = 13], respectively) are given a higher dose of 6.5+ T cells. Naive mice (▵, [n = 17]) received only this higher dose of 6.5+ T cells. No drug was administered during this phase of the experiment (*P < .05).
Next we wanted to determine whether the increase in LAG-3 corresponded with increased regulatory activity in vivo. First, we transferred LAG-3−/− 6.5+ T cells into C3HA hosts and treated them with PBS or CGS. Consistent with our data in Figure 5A, 75% of the vehicle-treated mice receiving Wt clonotypic T cells died, whereas 100% of the CGS-treated mice survived. On the other hand, eliminating LAG-3 completely abolished the ability of CGS to promote survival, as 100% of the mice that received LAG-3−/− T cells died, regardless of whether they were treated with CGS or not (Figure 7D). These data suggest that the up-regulation of LAG-3 by A2AR signaling plays a critical role in the ability of A2AR signaling to promote tolerance and prevent tissue destruction.
Finally, we examined whether A2AR signaling would lead to an increase in regulatory T cells that could mediate dominant-negative suppression on subsequent rechallenge with naive clonotypic cells. To this end, we designed an in vivo suppression assay. In the C3HA model, the mice that survive the initial transfer of autoreactive T cells develop LAG-3+ regulatory T cells that can suppress a second challenge of autoreactive 6.5+ T cells and prevent lethal tissue destruction.8 We injected the surviving mice of Figure 5A with a dose of T cells that would cause 100% death in naive mice. Indeed, by day 10, all of the naive mice receiving this high dose of clonotypic T cells died of lymphocyte-mediated tissue destruction of the lung (Figure 7E). Alternatively, consistent with previously published results, the 20% of the mice that survived the initial adoptive transfer of clonotypic T cells (Figure 5A) had developed Tregs, which suppressed the activity of the high dose of clonotypic cells and protected the mice from lung inflammation and death. Likewise, in accordance with our hypothesis that A2AR signaling promotes the generation of Tregs, 100% of the CGS-treated hosts that survived the initial challenge of autoreactive T cells also survived the subsequent dose of T cells (Figure 7E). That is, 4 days of CGS treatment 1 month prior led to the generation of regulatory T cells that suppressed the activation of the newly transferred clonotypic T cells, preventing them from inducing tissue destruction and death.
Discussion
A2AR signaling on macrophages, T cells, and dendritic cells has been shown to directly inhibit effector function.19–23,31–34 Our data demonstrate that tissue-derived adenosine acts via the A2AR to promote peripheral T-cell tolerance. Specifically, we demonstrate that A2AR engagement can facilitate the induction of T-cell anergy, skew T-cell differentiation away from adaptive effector cells to adaptive regulatory cells, and promote the generation of LAG-3+ regulatory T cells.
T-cell anergy occurs when TCR engagement occurs in the absence of costimulation.35 Anergic T cells produce less IL-2 and IFN-γ, and proliferate less on rechallenge to full stimulation (signal 1 + 2). In this report, we demonstrate that A2AR stimulation can promote T-cell anergy even in the presence of costimulation. These observations are consistent with the ability of A2AR stimulation to profoundly inhibit IL-2 production. However, not all inhibitors of IL-2 promote anergy. CSA, for example, potently abrogates IL-2 production but actually inhibits anergy induction by blocking NF-AT–inducible “anergic factors.” The Rao group has proposed that anergy is induced when TCR engagement results in NF-AT activation in the absence of AP-1 stimulation.36 Along these lines, we are able to demonstrate that A2AR stimulation can inhibit TCR-induced Erk phosphorylation and subsequent AP-1 binding (Figure 3B,D). These findings agree with a recent report demonstrating the ability of an A2AR agonist to inhibit TCR-induced ZAP70 phosphorylation.21
Depending on the inflammatory microenvironment, TCR engagement can lead to the development of CD4+ adaptive effector cells, such as Th1, Th2, and Th17 cells.37 Alternatively, in the setting of TGF-β without IL-6, TCR engagement can lead to the generation of “inducible” Foxp3+ regulatory T cells. Our data demonstrate the ability of tissue-derived adenosine, signaling through the A2AR, to drive CD4+ T cells away from Th17 differentiation and toward Foxp3+ T-cell differentiation. Consistent with these observations, A2AR stimulation resulted in increased TGF-β production with a concomitant decrease in the proinflammatory cytokine IL-6. Mechanistically, we propose that A2AR stimulation enhances Foxp3 transcription by decreasing IL-6 expression and enhancing TGF-β expression. These observations are consistent with findings suggesting that A2AR stimulation inhibits proinflammatory cytokine expression while sparing anti-inflammatory cytokine expression.23 Interestingly, there have been several recent reports suggesting that adenosine acting, via the A2AR, might mediate, in part, the suppressive function of regulatory T cells by engaging the A2AR on the suppressed cells. It was found that CD39 and CD73 (both of which are ectoenzymes that promote the generation of extracellular adenosine) appear to be a more specific markers for Foxp3+ regulatory T cells than CD25.38 The authors suggest that the generation of adenosine by regulatory T cells suppresses effector cells via the A2AR. Likewise, in a colitis model, it was found that CD45RBlow or CD25+ T cells from A2AR null mice fail to suppress CD45RBhigh cells and prevent disease and effector cells from A2AR null mice are not suppressed by suppressor cells derived from Wt mice.23
In addition to the ability of A2AR stimulation to promote anergy and the expression of Foxp3, we found that tissue-derived adenosine, engaging the A2AR, promoted the generation of LAG-3+ T regulatory cells. LAG-3 is a CD4 homolog that is up-regulated on T-cell activation and remains elevated on a subset of T cells that possess regulatory cell function.8 Our in vitro and in vivo data demonstrate that A2AR signaling promotes the generation of these regulatory T cells. T cells from LAG-3 null mice have diminished regulatory capacity, and the ectopic expression of LAG-3 confers the ability to suppress.8,29 Recently, it has been shown that on TCR engagement, LAG-3 is cleaved from the cell surface of activated T cells by metalloproteinases.39 This cleavage is necessary for robust T-cell proliferation and cytokine production, suggesting that LAG-3 also possesses cell intrinsic regulatory function. In our model, A2AR agonists promote the generation of LAG-3 expressing T cells that have the capability of inducing tolerance and preventing autoimmunity. LAG-3 expression is required for suppressive function, as A2AR agonists were unable to inhibit autoimmunity from the adoptive transfer of autoreactive LAG-3 null T cells. LAG-3 expression has been noted on tumor-infiltrating lymphocytes that have the capacity to suppress antitumor specific CD8+ T cells.30 The fact that the tumor microenvironment contains relatively high levels of adenosine suggests that tumor-derived adenosine may promote the generation of these regulatory cells.22,40
For T cells, the consequences of TCR engagement are determined by the integration of multiple “accessory” signals.41 Just as IL-10 and TGF-β have been associated with inhibiting immune responses and promoting tolerance induction, we propose that tissue-derived adenosine acts as a “metabokine”42 to perform a similar function. Because uric acid, HMG1-β, and low-molecular- weight species of extracellular matrix (ie, hyaluronic acid) might be considered examples of tissue-derived “danger” signals, we propose that tissue-derived adenosine, acting through the A2AR, is one of many critical accessory signals that promote the induction of peripheral tolerance.43–45 As such, during “noninfectious” tissue injury, adenosine released into the microenvironment may contribute to the maintenance of peripheral tolerance to de novo released self-antigens.
The ability of A2AR agonists to promote T-cell tolerance suggests that such agents could be used in the treatment ofT cell–mediated autoimmune disease and transplantation. In theory, A2AR agonists might promote long-term, peripheral tolerance in the absence of continued immunosuppression. In support of such a hypothesis are studies showing that A2AR agonists can prevent allograft rejection not only by acutely inhibiting T-cell activation but also by promoting the up-regulation of the inhibitory molecules PD-1 and CTLA-4.21 Alternatively, if tumor-derived adenosine inhibits antitumor responses by promoting the generation of anergic and suppressor T cells, A2AR antagonists might prove to be important adjuvants to tumor immunotherapy. Indeed, a role for the A2AR in protecting tumors from antitumor T cells has been described.46
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants P50CA098252 and R01CA098109 and a grant from the Flight Attendants Medical Research Institute. C.G.D. is a Damon Runyon Clinical Investigator supported in part by the Damon Runyon Cancer Research Foundation. P.E.Z. and E.R.L. are doctoral candidates at the Johns Hopkins School of Medicine and this work is submitted in partial fulfillment of the requirement for the doctoral degree.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.E.Z. performed the research and wrote the paper. C.T.H. performed research. E.R.L. performed research. J.K. assisted with microarray work. M.R.H. analyzed the data and assisted with research. J.L. provided the A2AR null mice and helped design experiments. C.G.D. provided the C3HA and 6.5 mouse lines and analyzed the data. J.D.P. conceived of the project, designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan D. Powell, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, CRB 443, 1650 Orleans Street, Baltimore, MD 21231; email: poweljo@jhmi.edu.
References
- 1.Sitkovsky MV, Lukashev D, Apasov S, et al. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 2.Sitkovsky MV. Use of the A(2A) adenosine receptor as a physiological immunosuppressor and to engineer inflammation in vivo. Biochem Pharmacol. 2003;65:493–501. doi: 10.1016/s0006-2952(02)01548-4. [DOI] [PubMed] [Google Scholar]
- 3.Lappas CM, Sullivan GW, Linden J. Adenosine A2A agonists in development for the treatment of inflammation. Expert Opin Invest Drugs. 2005;14:797–806. doi: 10.1517/13543784.14.7.797. [DOI] [PubMed] [Google Scholar]
- 4.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 5.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Hammerling GJ, Schonrich G, Momburg F, et al. Non-deletional mechanisms of peripheral and central tolerance: studies with transgenic mice with tissue-specific expression of a foreign MHC class I antigen. Immunol Rev. 1991;122:47–67. doi: 10.1111/j.1600-065x.1991.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 7.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 8.Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Chambers CA, Kuhns MS, Egen JG, Allison JP. CTLA-4-mediated inhibition in regulation of T cell responses: mechanisms and manipulation in tumor immunotherapy. Annu Rev Immunol. 2001;19:565–594. doi: 10.1146/annurev.immunol.19.1.565. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27:195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 12.Kriegel MA, Li MO, Sanjabi S, Wan YY, Flavell RA. Transforming growth factor-beta: recent advances on its role in immune tolerance. Curr Rheumatol Rep. 2006;8:138–144. doi: 10.1007/s11926-006-0054-y. [DOI] [PubMed] [Google Scholar]
- 13.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–2784. [PubMed] [Google Scholar]
- 14.Safford M, Collins S, Lutz MA, et al. Egr-2 and Egr-3 are negative regulators of T cell activation. Nat Immunol. 2005;6:472–480. doi: 10.1038/ni1193. [DOI] [PubMed] [Google Scholar]
- 15.Huang CT, Huso DL, Lu Z, et al. CD4+ T cells pass through an effector phase during the process of in vivo tolerance induction. J Immunol. 2003;170:3945–3953. doi: 10.4049/jimmunol.170.8.3945. [DOI] [PubMed] [Google Scholar]
- 16.Laurence A, Tato CM, Davidson TS, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 17.Kowalski J, Drake C, Schwartz RH, Powell J. Non-parametric, hypothesis-based analysis of microarrays for comparison of several phenotypes. Bioinformatics. 2004;20:364–373. doi: 10.1093/bioinformatics/btg418. [DOI] [PubMed] [Google Scholar]
- 18.Armstrong JM, Chen JF, Schwarzschild MA, et al. Gene dose effect reveals no Gs-coupled A2A adenosine receptor reserve in murine T-lymphocytes: studies of cells from A2A-receptor-gene-deficient mice. Biochem J. 2001;354:123–130. doi: 10.1042/0264-6021:3540123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 20.Erdmann AA, Gao ZG, Jung U, et al. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105:4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sevigny CP, Li L, Awad AS, et al. Activation of adenosine 2A receptors attenuates allograft rejection and alloantigen recognition. J Immunol. 2007;178:4240–4249. doi: 10.4049/jimmunol.178.7.4240. [DOI] [PubMed] [Google Scholar]
- 22.Ohta A, Gorelik E, Prasad SJ, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci USA. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB. Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol. 2006;177:2765–2769. doi: 10.4049/jimmunol.177.5.2765. [DOI] [PubMed] [Google Scholar]
- 24.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 25.Essery G, Feldmann M, Lamb JR. Interleukin-2 can prevent and reverse antigen-induced unresponsiveness in cloned human T lymphocytes. Immunology. 1988;64:413–417. [PMC free article] [PubMed] [Google Scholar]
- 26.Adler AJ, Huang CT, Yochum GS, Marsh DW, Pardoll DM. In vivo CD4+ T cell tolerance induction versus priming is independent of the rate and number of cell divisions. J Immunol. 2000;164:649–655. doi: 10.4049/jimmunol.164.2.649. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg MV, Maris CH, Hipkiss EL, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W, Jin W, Hardegen N, et al. Conversion of peripheral CD4+CD25-naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). J Immunol. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- 30.Gandhi MK, Lambley E, Duraiswamy J, et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is co-incident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood. 2006;108:2280–2289. doi: 10.1182/blood-2006-04-015164. [DOI] [PubMed] [Google Scholar]
- 31.Khoa ND, Montesinos MC, Reiss AB, Delano D, Awadallah N, Cronstein BN. Inflammatory cytokines regulate function and expression of adenosine A(2A) receptors in human monocytic THP-1 cells. J Immunol. 2001;167:4026–4032. doi: 10.4049/jimmunol.167.7.4026. [DOI] [PubMed] [Google Scholar]
- 32.Huang S, Apasov S, Koshiba M, Sitkovsky M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood. 1997;90:1600–1610. [PubMed] [Google Scholar]
- 33.Schnurr M, Toy T, Shin A, et al. Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood. 2004;103:1391–1397. doi: 10.1182/blood-2003-06-1959. [DOI] [PubMed] [Google Scholar]
- 34.Panther E, Idzko M, Herouy Y, et al. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 35.Powell JD. The induction and maintenance of T cell anergy. Clin Immunol. 2006;120:239–246. doi: 10.1016/j.clim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Macian F, Garcia-Cozar F, Im SH, Horton HF, Byrne MC, Rao A. Transcriptional mechanisms underlying lymphocyte tolerance. Cell. 2002;109:719–731. doi: 10.1016/s0092-8674(02)00767-5. [DOI] [PubMed] [Google Scholar]
- 37.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Deaglio S, Dwyer KM, Gao W, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li N, Wang Y, Forbes K, et al. Metalloproteases regulate T-cell proliferation and effector function via LAG-3. EMBO J. 2007;26:494–504. doi: 10.1038/sj.emboj.7601520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blay J, White TD, Hoskin DW. The extracellular fluid of solid carcinomas contains immunosuppressive concentrations of adenosine. Cancer Res. 1997;57:2602–2605. [PubMed] [Google Scholar]
- 41.Anand S, Chen L. Control of autoimmune diseases by the B7-CD28 family molecules. Curr Pharm Des. 2004;10:121–128. doi: 10.2174/1381612043453450. [DOI] [PubMed] [Google Scholar]
- 42.Lukashev D, Ohta A, Apasov S, Chen JF, Sit-kovsky M. Cutting edge: physiologic attenuation of proinflammatory transcription by the G(s) protein-coupled A2A adenosine receptor in vivo. J Immunol. 2004;173:21–24. doi: 10.4049/jimmunol.173.1.21. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- 44.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powell JD, Horton MR. Threat matrix: low-molecular-weight hyaluronan (HA) as a danger signal. Immunol Res. 2005;31:207–218. doi: 10.1385/IR:31:3:207. [DOI] [PubMed] [Google Scholar]
- 46.Raskovalova T, Lokshin A, Huang X, et al. Inhibition of cytokine production and cytotoxic activity of human antimelanoma specific CD8+ and CD4+ T lymphocytes by adenosine-protein kinase A type I signaling. Cancer Res. 2007;67:5949–5956. doi: 10.1158/0008-5472.CAN-06-4249. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.