Abstract
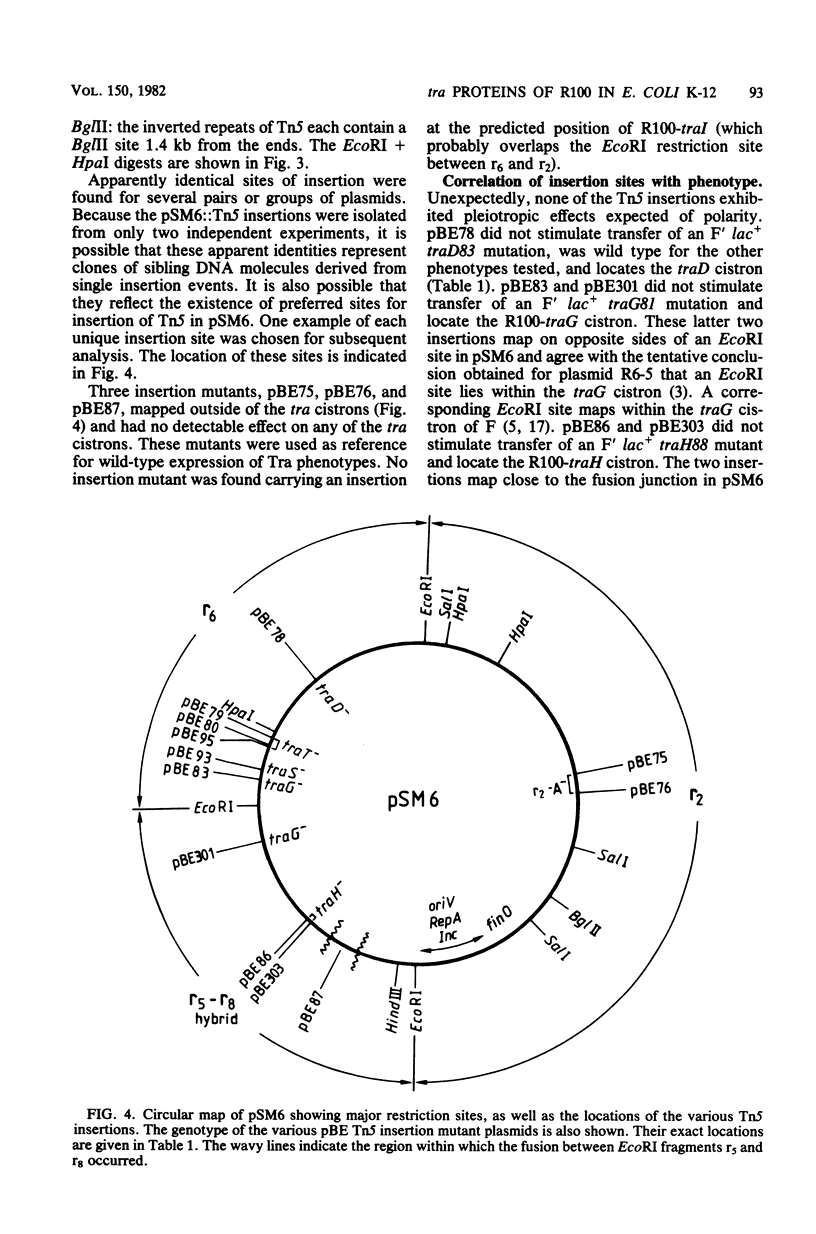
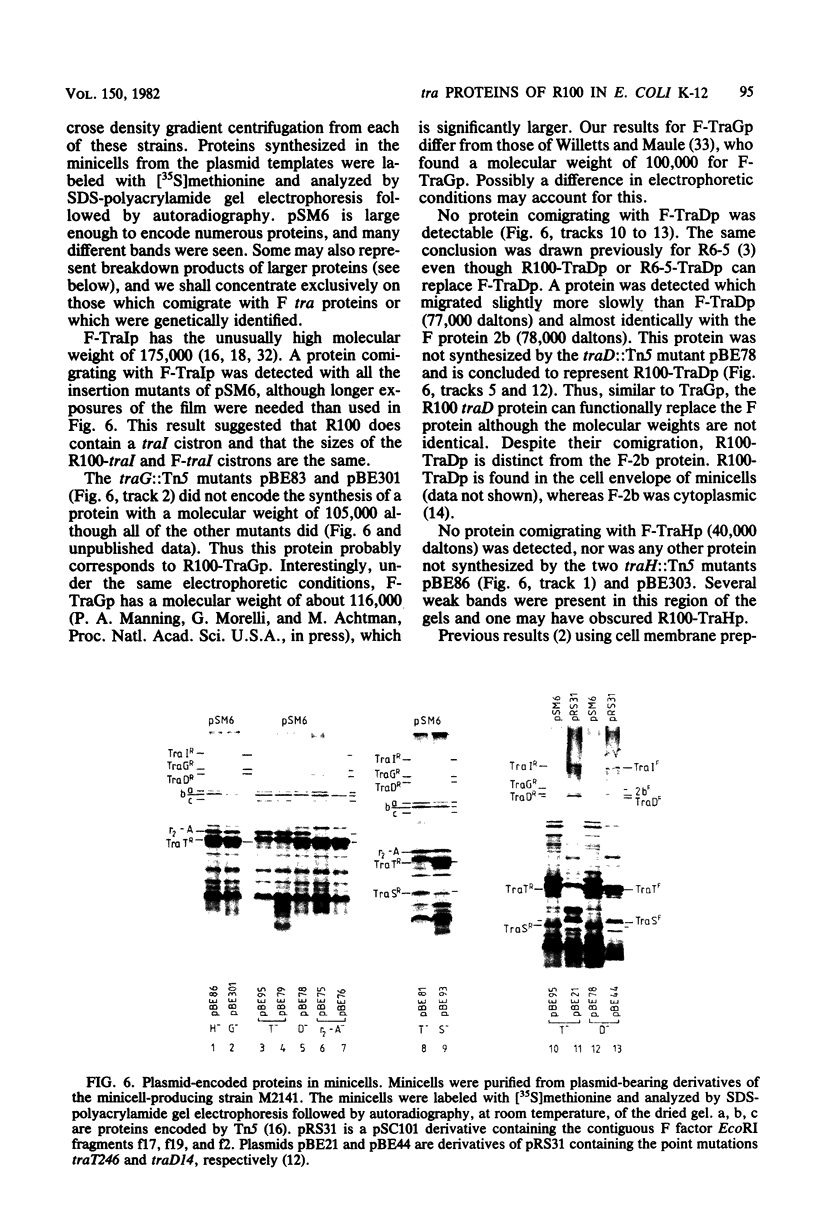
The distal region of the tra (transfer) operon of F-like plasmid R100 was investigated, using small plasmids derived from R100, primarily the plasmid pSM6. The transposon Tn5 (which confers kanamycin resistance) was inserted at different positions into pSM6, and the transposition derivatives were tested for ability to complement defined tra mutants of the F sex factor. Thus, the tra genes traH, G, T, and D were localized on the plasmid R100. A restriction map of pSM6 was constructed, and the locations of the insertions were mapped, using restriction endonuclease digestion of the plasmid DNA and exploiting the fact that several restriction sites are localized in the inverted repeat regions of the transposon. The gene products of the genes traG, S, T, and D were identified by radioactive labeling of proteins synthesized in minicells carrying the various insertion plasmids followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The presence of another transfer gene, traI, was inferred from these data. Another protein, the r2-A protein, was also identified, and its gene was mapped. On the basis of the data, a best-fit physical map of this region of the tra operon of R100 was constructed. The results confirmed that the general order and size of the distal transfer genes is as in the F sex factor, but showed that differences exist with respect to all of the gene products. The significance of these differences are discussed in the light of the genetic and physical homology (Manning et al., J. Bacteriol. 150:76-88) of the transfer regions.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Kennedy N., Skurray R. Cell--cell interactions in conjugating Escherichia coli: role of traT protein in surface exclusion. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5104–5108. doi: 10.1073/pnas.74.11.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Kusećek B., Timmis K. N. Tra cistrons and proteins encoded by the Escherichia coli antibiotic resistance plasmid R6-5. Mol Gen Genet. 1978 Jul 11;163(2):169–179. doi: 10.1007/BF00267407. [DOI] [PubMed] [Google Scholar]
- Achtman M., Manning P. A., Kusecek B., Schwuchow S., Willetts N. A genetic analysis of F sex factor cistrons needed for surface exclusion in Escherichia coli. J Mol Biol. 1980 Apr 25;138(4):779–795. doi: 10.1016/0022-2836(80)90065-0. [DOI] [PubMed] [Google Scholar]
- Achtman M. Mating aggregates in Escherichia coli conjugation. J Bacteriol. 1975 Aug;123(2):505–515. doi: 10.1128/jb.123.2.505-515.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Skurray R. A., Thompson R., Helmuth R., Hall S., Beutin L., Clark A. J. Assignment of tra cistrons to EcoRI fragments of F sex factor DNA. J Bacteriol. 1978 Mar;133(3):1383–1392. doi: 10.1128/jb.133.3.1383-1392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro G., Willetts N. The relationship between the transfer systems of some bacterial plasmids. Genet Res. 1972 Dec;20(3):279–289. doi: 10.1017/s0016672300013811. [DOI] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The site of action of the F transfer inhibitor. Mol Gen Genet. 1973 Dec 31;127(4):307–316. doi: 10.1007/BF00267101. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Willetts N. S. Genetic analysis of deletions of R100-1 that are both transfer-deficient and tetracycline-sensitive. J Gen Microbiol. 1976 Mar;93(1):133–140. doi: 10.1099/00221287-93-1-133. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Meynell E. Serotypes of sex pili. J Hyg (Lond) 1970 Dec;68(4):683–694. doi: 10.1017/s0022172400042625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Beutin L., Achtman M. Outer membrane of Escherichia coli: properties of the F sex factor traT protein which is involved in surface exclusion. J Bacteriol. 1980 Apr;142(1):285–294. doi: 10.1128/jb.142.1.285-294.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Kusecek B., Morelli G., Fisseau C., Achtman M. Analysis of the promoter-distal region of the tra operon of the F sex factor of Escherichia coli K-12 encoded by EcoRI restriction fragments f17, f19, and f2. J Bacteriol. 1982 Apr;150(1):76–88. doi: 10.1128/jb.150.1.76-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickel S., Bauer W. Isolation, by tetracycline selection, of small plasmids derived from R-factor R12 in Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):644–655. doi: 10.1128/jb.127.1.644-655.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Horiuchi T., Willetts N. S. Identification and characterization of four new tra cistrons on the E. coli K12 sex factor F. Plasmid. 1978 Jun;1(3):316–323. doi: 10.1016/0147-619x(78)90048-3. [DOI] [PubMed] [Google Scholar]
- Morris C. F., Hashimoto H., Mickel S., Rownd R. Round of replication mutant of a drug resistance factor. J Bacteriol. 1974 Jun;118(3):855–866. doi: 10.1128/jb.118.3.855-866.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsubo E. Transfer-defective mutants of sex factors in Escherichia coli. II. Deletion mutants of an F-prime and deletion mapping of cistrons involved in genetic transfer. Genetics. 1970 Feb;64(2):189–197. doi: 10.1093/genetics/64.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff S. W., Luh J., Ganesan A. T., Behrens B., Thompson R., Montenegro M. A., Morelli G., Trautner T. A. The genome of Bacillus subtilis phage SPP1: the arrangement of restriction endonuclease generated fragments. Mol Gen Genet. 1979 Jan 10;168(2):165–172. doi: 10.1007/BF00431442. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: restriction mapping and DNA cloning. Mol Gen Genet. 1978 Oct 24;165(3):295–304. doi: 10.1007/BF00332530. [DOI] [PubMed] [Google Scholar]
- Timmis K. N., Andrés I., Achtman M. Fertility repression of F-like conjugative plasmids: physical mapping of the R6--5 finO and finP cistrons and identification of the finO protein. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5836–5840. doi: 10.1073/pnas.75.12.5836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Maule J. Characterisation of a lambda transducing phage carrying the F conjugation gene traG. Mol Gen Genet. 1980;178(3):675–680. doi: 10.1007/BF00337878. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J. Interactions between the surface exclusion systems of some F-like plasmids. Genet Res. 1974 Aug;24(1):81–89. doi: 10.1017/s0016672300015093. [DOI] [PubMed] [Google Scholar]
- Willetts N., Maule J. Investigations of the F conjugation gene traI:traI mutants and lambdatraI transducing phages. Mol Gen Genet. 1979 Feb 1;169(3):325–336. doi: 10.1007/BF00382278. [DOI] [PubMed] [Google Scholar]
- Willetts N. The transcriptional control of fertility in F-like plasmids. J Mol Biol. 1977 May 5;112(1):141–148. doi: 10.1016/s0022-2836(77)80161-7. [DOI] [PubMed] [Google Scholar]