Abstract
Many studies have positioned Notch signaling at various critical junctions during T-cell development. There is, however, debate regarding the role of Notch in the CD4 versus CD8 lineage commitment. Because there are 4 Notch receptors and RBP-Jκ–independent Notch signaling has been reported, we decided to eliminate γ-secretase activity once its activity is required for all forms of Notch signaling. T-cell–specific elimination of γ-secretase was carried out by crossing presenilin-1 (PS1) floxed mice with CD4-Cre mice and PS2 KO mice, generating PS KO mice. Thymic CD4+CD8+ double-positive (DP) cells from these mice were strikingly resistant to apoptosis by anti-CD3 treatment in vivo and expressed more Bcl-XL than control thymocytes, and deletion of only one allele of Bcl-XL gene restored wild-type levels of sensitivity to apoptosis. In addition, these PS KO animals displayed a significant decrease in the number of CD8+ T cells in the periphery, and these cells had higher level of phosphorylated p38 than cells from control littermates. Our results show that ablation of presenilins results in deficiency of CD8 cells in the periphery and a dramatic change in the physiology of thymocytes, bringing to our attention the potential side effects of presenilin inhibitors in ongoing clinical trials.
Introduction
Notch signaling was first identified in strains of Drosophila flies that exhibit Notching at their wing edges, and subsequent analyses showed that Notch signals regulate cell fate, cell numbers via effects on proliferation and survival, and cell position, all of them dependent of dose, timing, and context of the Notch signal.1 Nearly all aspects of invertebrate Notch signaling are recapitulated in mammals in which this pathway plays multiple roles in normal development and disease.1,2
Engagement of Notch receptor by its ligands results in a series of proteolytic cleavage events that cause the release of the Notch intracellular domain (NIC). The final processing step of Notch is achieved by a γ-secretase complex where presenilins (PSs) play a major role.3 Indeed, the absence of PS function through genetic ablation or using γ-secretase inhibitors induces a phenocopy of Notch loss of function.4–9 Once NIC is in the nucleus, it interacts with RBP-Jκ resulting in the transcription of several Notch targets such as hes, hrt, and deltex family members, or pre-Tα, to name a few.10,11 It is important to note that Notch activities independent of RBP-Jκ but linked to other proteins such as deltex have been described.2,12–15 Despite the crucial role of presenilins in the Notch pathway, PSs could also be directly involved in MAPK activation/suppression, so it is also possible that any PS effect on the differentiation, activation, cell growth, and death of T cells could be independent of Notch signaling.
Many studies have positioned Notch receptor-ligand interactions at various critical junctions in T-cell development, most notably in the T versus B lineage choice, a Notch role for which there is complete agreement.16,17 In other set of studies, Notch1 signals have also been shown to promote the αβ over the γδ T lineage.18 However, the effect of the Notch pathway in CD4 versus CD8 T-cell lineage commitment is controversial because overexpression of NIC1 or NIC2, but not NIC3, decreases the CD4/CD8 ratio in the thymus,19–23 although conditional ablation of Notch1 or Notch2 does not affect in obvious way the CD8 or CD4 lineage commitment.24,25 Moreover, removal of the canonical Notch pathway by eliminating RBP-Jκ does not have an effect on the CD4/CD8 cell ratio in the thymus.26 Another observation in these conditional KO mice was the absence of a defect in the number of CD4+ or CD8+ cells in secondary lymphoid organs.24–26 Interestingly, 2 groups used γ-secretase inhibitors in fetal thymus organ culture (FTOC) experiments, and they reported a decrease in CD8+ single-positive cells, suggesting that elimination of Notch signaling could indeed have an effect in CD4/CD8 lineage commitment or survival,27,28 although other explanations are possible.
To shed light on these issues, we decided to analyze conditional knockout mice with presenilin-deficient T cells (PS KOs). We found that thymocytes from PS KOs displayed high resistance to in vivo apoptosis upon TCR engagement, with a concomitant elevation of Bcl-XL expression. Interestingly, a striking decrease in CD8+ T cells was found in secondary lymphoid organs, but not in the thymus of PS KO mice.
Materials and methods
Mice and in vivo injections
PS1flox/flox mice,29 PS2 KO mice,30,31 CD4-Cre mice,25,32 and Bcl-XLflox/flox mice33 have been described. All controls for the experiments were littermates. Animals used in the experiments were between 4 and 8 weeks old. All procedures were approved by New York University's Institutional and Animal Care Use Committee (IACUC).
Antibodies and other reagents
Unlabeled anti-CD3 mAb, as well as fluorochrome-labeled mAbs anti-CD3, anti-CD4, anti-CD8, anti-CD11c, anti-CD19, anti-TCRγδ, anti-NK1.1, and anti-F4/80, and all anti-Vβ antibodies were purchased from BD Pharmingen (San Diego, CA). Anti-NIC (catalog no. 2421), anti–phospho-ERK (catalog no. 9101), anti–phospho JNK (catalog no. 9251), anti–Bcl-XL (catalog no. 2762), and anti–phospho-p38α,β,γ,δ antibodies (catalog no. 9216) were purchased from Cell Signaling (Danvers, MA). Anti–total ERK (catalog no. sc-93), anti–total JNK (catalog no. sc-56929), anti–total p38 (catalog no. sc-571), and anti-Bax (catalog no. sc-526) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-PS1 was purchased from Calbiochem (catalog no. 529592). Anti–Bcl-2 was purchased from BD Transduction laboratories (catalog no. 610539). Anti-ROR gamma was a kind gift from Dr Dan Littman (NYU-SoM) and its use was described.34
PCR and real-time PCR analysis
To check the deletion efficiency of PS1 locus by cre recombinase, thymocytes or splenocytes were sorted in a MoFlo apparatus (Cytomation, Fort Collins, CO). Sorted cells were treated with proteinase K, and after heat inactivation all samples were ready for polymerase chain reaction (PCR). Primers to test deletion efficiency were described.29 PCRs were run in a Perkin Elmer 9600 thermal cycler (Perkin Elmer, Waltham, MA), with 1.5 mM MgCl2 and 0.5 U Taq polymerase per tube. PCR conditions were as follows: 94°C for 3 minutes, 55°C for 2 minutes, 72°C for 5 minutes, 36 cycles at 94°C for 1 minute, 55°C for 1 minute, and 72°C for 2 minutes, and final extension at 72°C for 15 minutes. PCR products were separated and visualized by electrophoresis on 2% agarose gels containing ethidium bromide.
For real-time PCR, total RNA was extracted using TRIzol (Invitrogen Life Technologies, San Diego, CA). The RNA was used to perform a cDNA synthesis using the superscript II reverse transcriptase (Invitrogen Life Technologies) following the instructions of the manufacturers. This cDNA was used at a concentration of 10 ng input RNA per microliter of reaction volume. Real-time PCR was performed with the TaqMan sequence detection system (Applied Biosystems, Foster City, CA). TaqMan primers and probes were designed using PrimerExpress software (Applied Biosystems, Foster City, CA) to span intron/exon boundaries to prevent amplification of genomic DNA. Primers and probes are as follows: Deltex-1: forward, GAG GTC CAC CAG CGT CAG; reverse, GCC AGT GCC ATT CAA GTT CT; probe, CAC GAG GTC CAT GGCCTG CTCA; Deltex-2: forward, CCA CTG CTA CCT ACC CAA CAA;reverse, TGA AGA TGA GTC TGC GTT CC; probe, TGC AGACGC GGA CGA TCA CTC CTT. Samples are quantified using relative standard curves for each amplification reaction and results were normalized to the internal control β-actin.
In vivo antibody injections
Anti-CD3 mAb (50 μg) was injected intraperitoneally to each animal, and 40 hours later the apoptosis levels and total cell numbers were determined by fluorescence-activated cell sorting (FACS) analysis and hemocytometer counting, and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick-end labeling (TUNEL) staining of thymus sections was performed, as described in situ apoptosis.
FACS analysis
Thymi and spleens from were made into single-cell suspensions in staining buffer (PBS containing 2% FCS and 0.1% NaNB3). Cells were stained for 30 minutes at 4°C with the antibody cocktails. To measure the amount of phospho-p38 cells, splenocytes were labeled as described above but in the presence of sodium orthovanadate (Sigma, St Louis, MO). After that, cells were permeabilized with cytofix/cytoperm (BD Pharmingen) for 30 minutes at room temperature (RT). After wash, cells were fixed with PFA 1% and Tween-20 0.5% for 30 minutes at RT; anti–phospho-p38 antibody was added after washing the cells and incubated for 1 hour at RT. The excess of antibody was removed by washing the cells, and FITC-antirabbit antibody (BD Pharmingen) was added for 30 minutes at RT. After wash in PBS, cells were ready to be analyzed. To measure cell death, freshly isolated cells were labeled with annexin-V PE (BD Pharmingen) for 10 minutes in annexin-V buffer (Hepes 10 mM, NaCl 150 mM, KCl 5 mM, Mg26H2O 1 mM, CaCl22H2O 1.8 mM). After this first incubation, 1 μL 7-AAD (BD Pharmingen) was added and cells were immediately washed. BrdU (Caltag, Carlsbad, CA) was used in vivo (one single intraperitoneal dose of 50 mg/kg) and incorporation was assessed by FACS 4 hours after injection. All samples were analyzed in a FACScalibur instrument (Becton Dickinson, San Jose, CA).
In situ apoptosis
TUNEL (terminal deoxynucleotidyl transferase dUTP-biotin nick end labeling) assay35 was performed according to the manufacturer's instructions (In Situ Cell Detection Kit, Fluorescein; Roche Diagnostics, Mannheim, Germany). Briefly, slides containing 5-μm cryosections of thymii were fixed in PFA 1%, for 30 minutes at RT. Slides were then washed 3 times with PBS, and a permeabilization buffer (1% vol/vol Triton X-100 and 1% wt/vol sodium citrate, in ddH2O) was added over each slide for 2 minutes, at 4°C. Subsequently, each slide was washed twice and a labeling solution was added. At this point, slides were incubated for 1 hour, at 37°C, in a humidified dark chamber. At the end of this incubation, each slide was washed twice with PBS and mounted with Fluoromont G (Southern Biotechnology, Birmingham, AL). Images were acquired using SlideBook v.3.0.9.0 software (Intelligent Imaging Innovations, Denver, CO) in an Axioplan 2 Zeiss microscope (Carl Zeiss, Heidelberg, Germany).
Western blot analysis
Thymocytes were lysed in TNE buffer (1% Triton X-100, 10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA) containing a mixture of protease inhibitors (Sigma) and sodium orthovanadate (Sigma). Cell lysates were then subjected to Western blot analysis using Abs directed against either Bcl-2, Bax, NIC, PS1, actin, Bcl-XL, tubulin, RORγ, pP38, pJNK, JNK-1, P38, pERK, or ERK. The densitometry of gel bands were performed using NIH Image 1.62 program (National Institutes of Health, Bethesda, MD).
Stimulation of thymocytes and electrophoretic mobility shift assay (EMSA)
Thymocytes were incubated at 37°C either in complete RPMI alone, with PMA (50 ng/mL) and ionomycin (200 ng/mL), with immobilized anti-CD3 (10 μg/mL) for 8 hours, or with mouse recombinant TNF-α 20 μg/mL (R&D Systems) for 15 minutes. Nuclear extracts were then prepared from the cells according to published methods.36 To test NF-κB binding, a 32P-labeled oligonucleotide probe containing the MHC class-I κB site or the NF-Y binding site (Santa Cruz Biotechnology) was incubated with the nuclear extracts. The binding reaction contained 60 000 cpm of the radiolabeled probe, 4 μg nuclear protein, 500 ng poly(dI-dC) (Amersham Biosciences, Pittsburgh, PA), 10 μg BSA, 20 mM HEPES (pH 7.9), 1 mM EDTA, 1% NoNICet P-40, 5% glycerol, and 5 mM DTT in a final volume of 20 μL. Reactions were incubated at room temperature for 15 minutes and subjected to electrophoresis on a 5% polyacrylamide gel in 0.5 × Tris-buffered EDTA buffer. For supershift assays, 5 μg rabbit polyclonal Ab directed against p50 (sc-7178), obtained from Santa Cruz Biotechnology, was added to the binding reactions and incubated for 10 minutes on ice before the samples were subjected to gel electrophoresis. The gels were dried and exposed to x-ray film and quantified by phosphor imager analysis.
Obtention of deltex1-expressing TK1 cells
The retroviral vector pMIGR-DELTEX1 that encodes the murine deltex 1 gene37 was kindly provided by Dr Warren Pear (University of Pennsylvania). Stable lines expressing deltex and GFP or only GFP from the empty p-MIGR vector of the murine T-cell lymphoma TK1 cells (ATCC, CRL-2396; American Type Culture Collection, Manassas, VA) were obtained as previously described.37 Single-cell purification by MoFlo of GFP-expressing TK-1 cells was performed to obtain homogeneous stable cell lines.
Results
Deletion of presenilins 1 and 2 after the CD4+CD8+ stage increases resistance to in vivo apoptosis by anti-CD3 injection
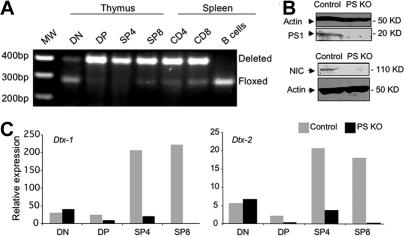
Mice deficient in the expression of PS1 (PS1−/− mice) die shortly after birth.9 To circumvent this problem, we studied mice in which deletion of PS1 occurred only in the T-cell compartment by crossing CD4-Cre animals with PS1flox/flox mice.29 Because PS2 KO animals have a mild phenotype, we crossed the conditional CD4-Cre PS1 KO animals with PS2 KO mice30 and generated PS1/PS2 KO mice, referred hereafter as PS KO mice. We used our PS KO animals to study the effect of ablation of γ-secretase activity in T lymphocytes. Although PS1 gene deletion started at the CD4−CD8− double-negative (DN) stage, it became prominent at the CD4+CD8+ double-positive (DP) stage (Figure 1A). As specificity control, B cells were also analyzed and displayed no detectable deletion of the floxed PS1 locus. We also checked the expression of PS1 gene at the protein level in total thymocytes. As expected from the pattern of gene deletion, the expression of PS1 was almost abolished (Figure 1B). Notch signaling is completely dependent on γ-secretase activity. To confirm that Notch signaling was eliminated in PS KO T cells, we assessed the expression of Notch1 intracellular domain (NIC). As anticipated, we could not detect NIC in PS KO thymocytes by Western blot (Figure 1B). Once the PS1 gene deletion was demonstrated to occur in thymocytes at the DP stage, we sought to analyze the biologic impact of this deletion in the expression of 2 known target genes in the Notch pathway, Deltex 1 and 2. The expression of both genes was dramatically reduced in DP (3- to 4-fold) and SP thymocytes (more than 10-fold) compared with control animals (Figure 1C). As expected given the timing of PS1 deletion, the expression of deltex 1 and 2 at the DN stage was equivalent in PS KO and control animals. In this and all other experiments, PS1flox/+ PS2+/+ animals (lacking Cre) were used as controls; additional controls such as CD4-Cre–only mice will be indicated as appropriate.
Figure 1.
Deletion of PS1 expression starts at DP stage and reduces activity of gamma-secretase as well as expression of Deltex-1/2 genes. DNA from DN, DP, SP4, or SP8 thymocytes as well as from CD4, CD8, or B splenocytes from control or PS KO animals was used to evaluate the deletion of presenelin-1 gene by PCR with specific primers (A). Protein extracts from control or PS KO thymocytes were used to measure the amount of PS1 and NIC proteins by Western blot (B). cDNA from DN, DP, SP4, or SP8 thymocytes was analyzed by real-time PCR for Deltex-1/2 gene expression (C). Representative of 3 independent experiments.
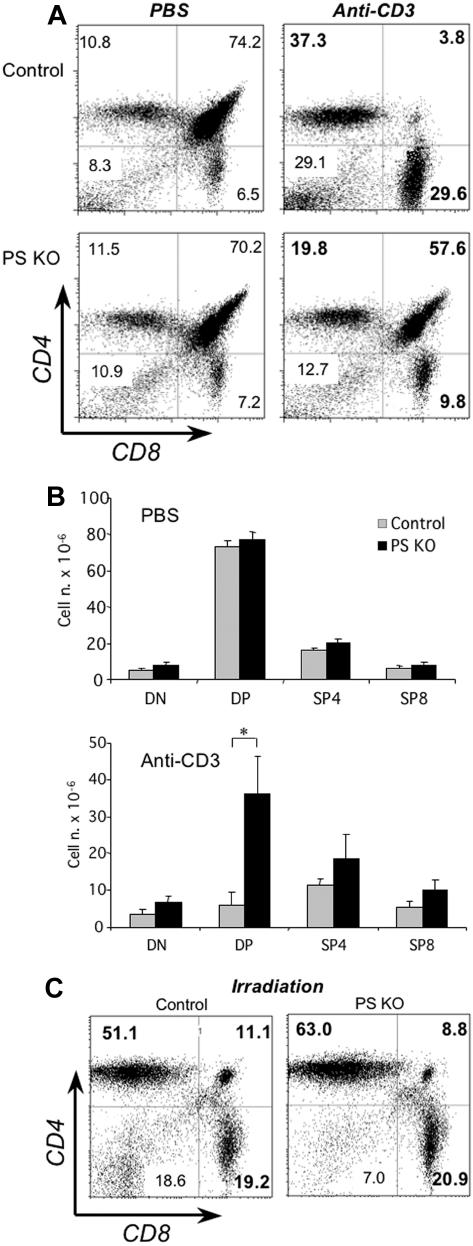
When we analyzed the number and proportion of the different thymocyte subsets we found it to be similar in PS KO or control mice (Figure 2A left panels and Figure 2B top). Because the 4 Notch receptors are dependent on gamma-secretase activity, this result confirms that the Notch pathway does not play a significant role in the CD4-CD8 lineage commitment. Next, we sought to determine the biologic impact of impaired Notch signaling on the physiology of thymocytes. We investigated whether DP thymocytes from PS KO animals had a different behavior after in vivo treatment with anti-CD3 antibodies, a procedure known to generate the deletion of DP cells. As expected, thymi from control animals harvested 40 hours after injection of anti-CD3 mAb had a major decrease in DP cells (Figure 2A top panels and Figure 2B). In stark contrast, the number and percentage of DP thymocytes from PS KO animals was reduced much less (Figure 2A bottom panels and Figure 2B). However, both control and PS KO thymocytes were equally susceptible to die after in vivo γ-irradiation (Figure 2C), indicating that the mechanism responsible for this resistance was specific for TCR-triggered apoptosis. We confirmed the striking difference in anti-CD3–induced apoptosis in PS KO and control mice by in situ TUNEL staining (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The dramatically different response to anti-CD3 stimulation of DP thymocytes was not related to any noticeable difference in thymocyte maturation at steady state, as assessed by CD24 and CD3 expression (Figure S2). From our analysis, we conclude that PS KO DP thymocytes have a dramatically enhanced survival rate upon stimulation via the TCR.
Figure 2.
In vivo treatment with anti-CD3 mAb and resistance against apoptosis of PS KO thymocytes. Control (A, top panels; C, left panel) or PS KO thymocytes (A, bottom panels; C, right panel) were obtained 40 hours after intraperitoneal injection of PBS or 50 μg/mouse anti-CD3 mAb. Total thymocyte numbers were counted and cell numbers for DN, DP, SP4, or SP8 cells estimated by percentages of FACS analysis with anti-CD4 and anti-CD8 fluorescent mAb (B). The γ-irradiation dose was 5Gy. *P < .05. Representative of 5 animals. (A,C) Value in each quadrant represents the percentage of each cell population inside the thymus.
Thymocytes from PS KO mice have increased expression of Bcl-XL
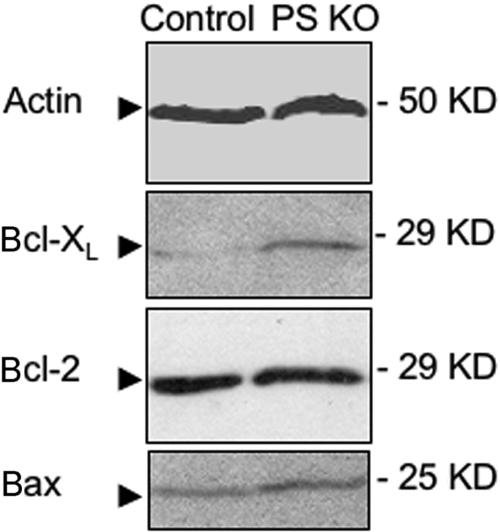
It is well known that Bcl-XL is the key antiapoptotic gene affecting DP cell survival.38–41 We therefore analyzed Bcl-XL expression in thymocytes from PS KO animals, and we found that they had a clearly increased expression of Bcl-XL compared with thymocytes from control animals (Figure 3). We also assessed the expression of another antiapoptotic protein, Bcl-2, as well as the proapoptotic protein Bax, because it is the balance of proapoptotic and antiapoptotic molecules that determines the apoptosis outcome. We did not find any clear difference in the expression of these 2 proteins (Figure 3), suggesting that the increase in Bcl-XL expression could explain the resistance of PS KO DP thymocytes to TCR-induced apoptosis.
Figure 3.
Thymocytes from PS KO animals express more Bcl-XL than thymocytes from control animals, without changing the expression of Bcl-2 or Bax. Protein extracts were obtained from thymocytes of control or PS KO animals, and expression of actin, Bcl-2, Bcl-XL, and Bax was visualized by Western blot (WB).
Decrease in Bcl-XL expression restores susceptibility to anti-CD3–induced apoptosis
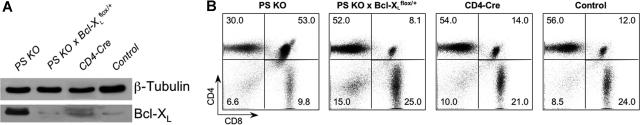
To demonstrate that elevated levels of Bcl-XL expression in PS KO animals were responsible for resistance of DP cells to apoptosis after in vivo treatment with anti-CD3 antibodies, we crossed the PS KO animals with Bcl-XL floxed animals.33 We wished to establish whether the elimination of only one Bcl-XL allele would affect the DP cell survival after antibody treatment. We generated PS1flox/flox, PS2−/−, CD4-cre, Bcl-XLflox/+ animals, referred to as PS KO × Bcl-XLflox/+ mice. As expected, expression of Bcl-XL in PS KO-Bcl-XLflox/+ thymocytes was decreased compared with PS KO animals and actually it was equivalent to control animals (Figure 4A). CD4-Cre control animals have higher Bcl-XL expression than the control PS1flox/+ PS2+/+ (lacking Cre) animals, although much less than the PS KO thymocytes (Figure 4A). Strikingly, PS KO × Bcl-XLflox/+ DP thymocytes were now susceptible to death upon in vivo treatment with anti-CD3, demonstrating that Bcl-XL overexpression was indeed responsible for the resistance to apoptosis observed in PS KO animals (Figure 4B).
Figure 4.
Bcl-XL expression and DP apoptosis in PS KO × Bcl-XLflox/+ animals. Thymocytes from PS KO, PS KO × Bcl-XLflox/+, CD4-Cre, or control animals were harvested, protein were extracts collected, and Bcl-XL level was measured by WB (A). Some animals from these same groups were treated intraperitoneally with anti-CD3, thymocytes were harvested 40 hours later, and the percentage of DP or SP thymocytes was measured by FACS (B). Each graphic is representative of 1 animal of 3 evaluated. (B) Value in each quadrant represents the percentage of each cell population inside the thymus.
It is known that a major inducer of Bcl-XL in DP cells is the orphan receptor RORγ.34 However we could not detect any increase in the expression of RORγ in PS KO thymocytes compared with control thymocytes, indicating that the higher expression of Bcl-XL should be caused by other PS-dependent mechanisms (Figure S3A). Using a transgenic mouse that ectopically overexpresses a dominant-negative form of IκB-α in the thymus, different groups have reported that NF-κB activation is needed to allow DP cells to undergo apoptosis.42 Furthermore, lack of NF-κB activity was associated with an increase in Bcl-XL that could explain the phenotype we observed in PS KO thymocytes. However, we could not find any NF-κB activation defect in PS KO thymocytes (Figure S3B). The 3 main MAPK pathways associated with the TCR (ERK, JNK, and p38 kinases) have been also associated with the development of thymocytes.43,44 Actually, when the JNK pathway is abrogated in thymocytes, the DP compartment acquires resistance to apoptosis by anti-CD3 in vivo injection.44,45 We decided to check the status of activation of the JNK, p38, and ERK pathways in PS KO thymocytes. We observed that there was an increase in JNK and p38 activation (Figure S4A), while there was no change in ERK activation (Figure S4B).
Thus, it is clear that increased expression of Bcl-XL in PS KO thymocytes is responsible for their enhanced survival, although we could not attribute the Bcl-XL increase to a particular pathway.
PS KO animals have fewer CD8 cells in the periphery than control animals
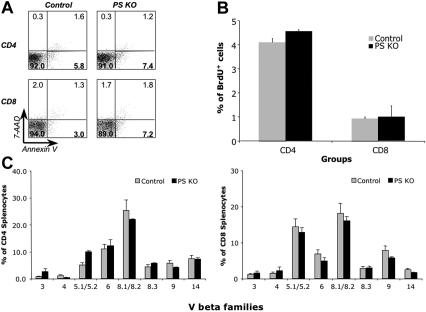
Once we addressed the role of PS ablation on the thymic environment, we focused our studies on the peripheral lymphoid cells in PS KO animals. The results show that the percentages of B cells, dendritic cells (DCs), NK1.1 cells, TCRγδ cells, and macrophages in spleens were similar between PS KO and control animals (Figure 5A and Figure S5A). However, the percentage of peripheral CD8+ cells in PS KO mice both in spleen and LNs was significantly decreased in comparison with control animals (Figure 5B and Figure S5B). This finding was interesting because the percentage and number of thymic CD8 cells were normal (Figure 2A left panels and Figure 2B). To understand the dynamics of cell division and death of peripheral T cells in the absence of presenilins, we determined the percentage of dying cells by annexin-V/7-AAD staining and the percentage of dividing cells by BrdU incorporation. CD8+ T cells from PS KO animals were dying more than T cells from their control littermates (Figure 6A), and our data are likely an underestimate due to the quick removal of apoptotic cells in vivo. By contrast, the levels of BrdU incorporation were similar between PS KO T cells and control T cells (Figure 6B). Despite the reduction in the number of peripheral CD8+ T cells, the majority of CD4+ and CD8+ T cells in PS KO mice displayed a naive phenotype (Figure S6). We also assessed whether the reduction in CD8+ cells was caused by preferential deletion of T cells expressing particular Vβ TCR. However, staining with a panel of anti-Vβ monoclonal antibodies showed equivalent Vβ TCR proportions in PS KO and control splenocytes, both in CD4+ and CD8+ cells (Figure 6C). We conclude that, in contrast to the thymus, in PS KO mice there is a specific reduction of peripheral CD8+ T cells, which can be attributed to an increased cell death of these cells.
Figure 5.
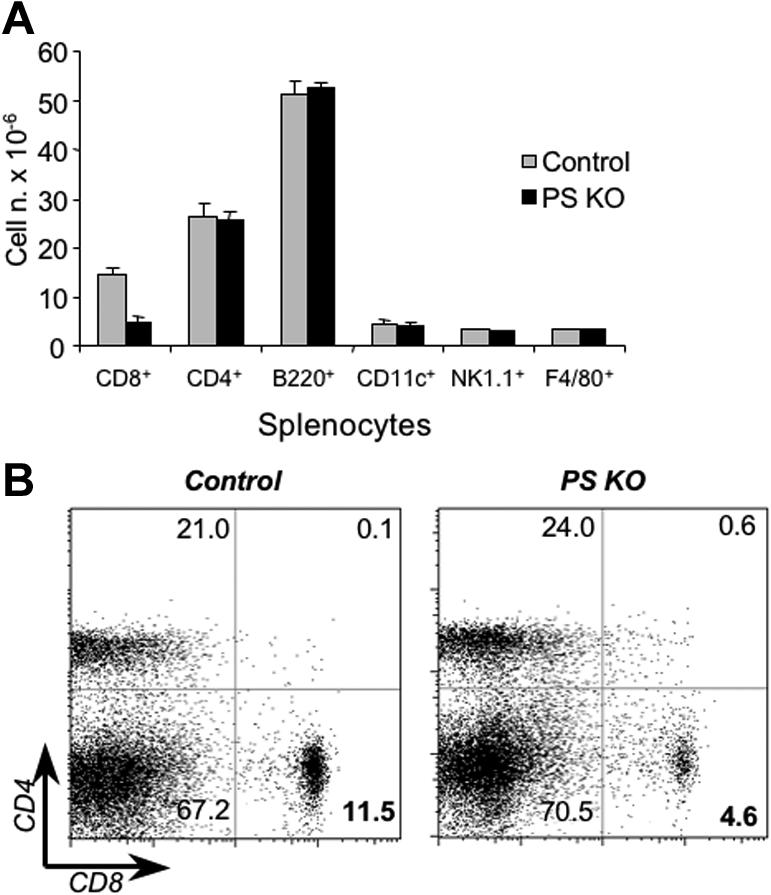
Phenotypic characterization of PS KO animals. Splenocytes (A) or lymph node cells (B) from control (left panels) or PS KO animals (right panels) were staining with fluorescent-labeled mAb against CD4, CD8, CD11c, CD19, NK1.1, and F4/80. Representative of 3 animals. (B) Value in each quadrant represents the percentage of CD4 or CD8 T-cell populations inside the splenocytes of lymph node cells.
Figure 6.
Cell death, T-cell turnover, and Vβ family expression in PS KO animals. Splenocytes from control or PS KO animals were harvested and stained with anti-CD4 APC, anti-CD8α PerCP, annexin-V PE, and 7-AAD (A), or FITC anti-BrdU mAb (B), or a panel of fluorescent-labeled mAbs against Vβ families (C). Only animals used to detect BrdU incorporation were intraperitoneally injected with BrdU (2 doses, 4 hours and 8 hours before splenocyte harvesting). Each bar represents mean and standard deviation of 3 animals. (A) Value in each quadrant represents the percentage of splenocytes labeled with annexin-V or 7-AAD.
Splenic T cells from PS KO animals have higher levels of phosphorylated p38 in steady state.
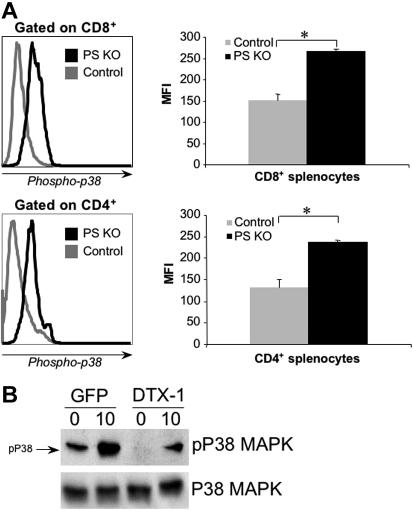
It was described that high levels of p38 phosphorylation had a deleterious effect on CD8+ T-cell survival, without affecting CD8+ T-cell generation in the thymus44,46 or the CD4+ subpopulation in the periphery. Given the similarities between these reports and our findings with PS KO T cells, we assessed whether CD8+ T-cell reduction in the periphery of PS KO mice was due to higher levels of phospho-p38. As shown in Figure 7A, both CD8+ and CD4+ T cells from spleens of PS KO animals had a higher level of phospho-p38 than cells isolated from control animals. These data closely resemble the phenotype of a dominant-active MKK6 transgenic mice,47 where the higher p38 phosphorylation in both CD4+ and CD8+ cells leads to higher CD8+ T-cell death without altering the CD4+ compartment.
Figure 7.
Phospho-p38 expression in PS KO T cells and in TK1 cells transfected with Dtx-1. Splenocytes from control or PS KO animals were harvested and labeled with FITC anti–phospho-p38 mAb in the presence of sodium orthovanadate (A). To check the effect of deltex-1 expression over p38 activation in thymocytes, TK1 cells were transfected with pMIGR-DELTEX1 vector containing this gene or empty vector. Than, stable TK1 cell lines were sorted by GFP expression and stimulated (or not) for 10 minutes with anti-CD3 before protein extraction (B). Results are representative of 3 animals (A); one experiment representative of three (B).
We showed thus far that in PS KO thymocytes expression of Deltex 1 and 2 was strongly decreased and phospho-p38 levels were increased (Figure 1C and Figure S4); likewise, we showed that phospho-p38 levels were also higher in splenic T cells (Figure 7A). As expected, we also found that Deltex 1 and 2 mRNA levels were greatly reduced in peripheral CD8+ T cells from PS KO mice compared with control CD8+ T cells (Figure S7). It has been described that Deltex 1 inhibited p38 phosphorylation by a mechanism that is not well understood.48 We therefore determined whether enforced Deltex 1 expression would inhibit p38 phosphorylation. Toward that end, we generated a stable cell line that expresses Deltex 1 in TK1 thymoma cells, and analyzed p38 phosphorylation by Western blot before or after in vitro stimulation with anti-CD3. TK1 cells expressing Deltex 1 had lower levels of phospho-p38 than the cell line generated with control vector only (Figure 7B). Because enforced expression of Deltex 1 inhibits p38 phosphorylation, it is reasonable to assume that elimination of Deltex expression in PS KO mice could be the cause of higher p38 phosphorylation, which results in higher CD8+ T-cell death.
Discussion
In this paper, we describe the phenotype of mice harboring a T-cell–specific deletion of γ-secretase activity, starting at the CD4+CD8+ (DP) stage. In PS KO mice, Notch signaling is completely abolished in the T-cell lineage, something important to accomplish because there are 4 Notch receptors, and there are some Notch targets that can be induced independently of the canonical transcription factor RBP-Jκ.1,2,12–15,49 In addition to Notch receptors, T cells express other presenilin substrates, such as CD44.50
Using the PS KO mice, we made 3 main observations. First, PS KO thymocytes were remarkably resistant to anti-CD3–induced apoptosis in vivo (Figure 2), caused by increased Bcl-XL expression levels, as reducing Bcl-XL expression brought the susceptibility of PS KO DP thymocytes up to wild-type levels (Figure 4). Second, there was no obvert change in the proportion or number of any of the thymic T-cell populations (Figure 2 and Figure S5A). Third, there was a striking reduction of CD8+ cells in the periphery (Figure 5 and Figure S5B) that was correlated with enhanced levels of phospho-p38 in these cells (Figure 7).
PS KO mice display a higher resistance to apoptosis of DP thymocytes after injection of anti-CD3 antibodies in vivo in comparison with control animals (Figure 2). We attribute the higher resistance to TCR-mediated apoptosis in PS KO animals to the increased expression of Bcl-XL found in PS KO thymocytes (Figure 3). Importantly, when wild-type levels of Bcl-XL expression were restored in PS KO mice, apoptosis susceptibility was restored. It has been reported that increased Bcl-XL expression promotes survival of DP cells to anti-CD3 treatment.51,52 At this time, we do not know whether the higher Bcl-XL expression is directly attributable to the absence of Notch signaling on T cells. First, while in the absence of PS there is no Notch activation, Notch is by no means the only substrate of PS cleavage on T cells.3 In support of a role for Notch, in vivo overexpression of the Notch ligand dll4 in all the cells of the hematopoietic system resulted in decreased Bcl-XL expression.53 On the other hand, it has been shown that ectopic expression of the intracellular domain of Notch (NIC) could protect DP thymocytes from apoptosis by inducing increased expression of IAP-2, Bcl-XL, and FLIP in in vitro cultures.54 It therefore seems that Notch activity can either induce or reduce Bcl-XL expression depending on the cellular context and the system of study. Because Deltex expression is greatly reduced in PS KO mice (Figure 1 and Figure S7), we sought to determine if overexpression of deltex in TK1 thymoma cells would decrease the level of Bcl-XL. However the expression of Bcl-XL was already too low in TK1 cells infected with the empty retrovirus to allow an evaluation of a further decrease of Bcl-XL due to enforced deltex expression (data not shown). Importantly, both PS1 and PS2 have been found to interact directly with Bcl-2 and Bcl-XL, suggesting a direct role of PS proteins in Bcl-XL protein activity.55,56 Lastly, the proapoptotic effect of PS2 overexpression in cultured neurons is associated with down modulation of Bcl-2.57 Thus, the increased Bcl-XL expression in PS KO thymocytes could be caused directly by lack of PS (Notch independent) or it could be mediated by the absence of Notch signaling. These doubts notwithstanding, we can conclude with certainty that the overexpression of Bcl-XL in PS KO animals was indeed responsible for the increased survival of DP thymocytes in PS KO animals (Figure 4). We attempted to link the up-regulation of Bcl-XL to 3 different mechanisms known to induce Bcl-XL expression or confer protection against anti-CD3–induced death in vivo, namely RORγ, MAPK, and NF-κB.34,42,44,45 We found that none of the 3 pathways was responsible for the phenotype we described here (Figures S3,S4).
There is a discrepancy in the literature regarding the role of Notch in the CD4/CD8 lineage determination in the thymus. Overexpression of both NIC1 and NIC2 decreases the CD4/CD8 ratio, indicating that Notch could enhance or promote the CD8 SP cell development.19–22 In contrast, there is no change in total numbers of either CD4 or CD8 cells in the thymus after conditional elimination of the Notch receptors 1 or 2, or the canonical transcription factor RBP-Jκ. Because there is more than one Notch receptor and some Notch targets could act independently of RBP-Jκ,1,2,12–15,49 we sought to reassess this important question by completely abrogating the Notch pathway, which was achieved by eliminating PS proteins. The results that we obtained, shown in Figure 2, should remove any doubts about the possible role of the Notch pathway in the CD4/CD8 lineage commitment because even after elimination of the γ-secretase activity at the DP stage there were no changes in the percentage or number of CD4 and CD8 SP thymocytes. A possible explanation for the controversy is discussed below.
The genetic ablation of both PS1 and PS2 in the T-cell lineage caused a decrease in CD8+ cells in secondary lymphoid organs, an effect that was not described either in Notch1 or -2, or RBP-Jκ conditional deletion.24–26 This decrease was correlated with increased death of CD8+ cells (Figure 6A) with similar levels of proliferation (Figure 6B) compared with control cells. These results indicate that CD8+ cells die at a higher rate than control cells in the periphery. Why is this happening? Three important observations could help answer this question. First, both SP thymocytes and mature T cells from PS KO animals had decreased expression of Deltex 1 and 2 (Figures 1 and S7). Second, both thymocytes and mature T cells from PS KO animals had increased phospho-p38 compared with control cells (Figure S4A and Figure 7A); and third, a thymoma cell line transfected with Deltex 1 had decreased phospho-p38 levels (Figure 7B). It was demonstrated by others that enhanced activation of p38 in T cells affects survival of CD8+ T cells without affecting CD4+ cells or generation of SP cells in the thymus.46 Taken together, the data suggest that absence of Deltex in PS KO animals overactivates the p38 pathway, thus affecting the viability of CD8+ cells. It is also noteworthy that elimination of Deltex 1 and 2 in combination with a siRNA for Deltex 4 did not have any obvious phenotype in the immune system, including CD8 T cells.58 This controversy can be understood because the authors also mentioned that in Drosophila the Deltex phenotype is mild unless combined with other Notch mutations.58 Because PS KO animals lack other Notch pathways besides Deltex, it could be possible that this CD8 T-cell phenotype is the combination of loss of different Notch signals, a possibility to be addressed by future work.
The fact that absence of PS activity affects particularly CD8+ cells gives us a hint regarding the phenotype observed with overexpression of both NIC1 and NIC2 in terms of promoting CD8+ T-cell generation. It is tempting to speculate that although Notch is not important for the generation of CD8 cells (24–26 and this report), enforced Notch signaling drives Deltex expression, which in turn could hamper p38 activation, conferring specifically more viability to the CD8 compartment.
In conclusion, our results clearly demonstrate that animals lacking presenilin activity in the T-cell lineage after the DP stage had higher resistance to anti-TCR–induced apoptosis in vivo, which was acquired by overexpression of Bcl-XL, and fewer CD8+ T cells in the periphery. These data are significant in the evaluation of therapies that consider presenilin inhibition59,60 because one could, on one hand, control β-amyloid peptide accumulation or cancer, but at the same time one could promote a decrease of CD8+ T cells that are important to control the tumor.
Supplementary Material
Acknowledgments
This work was partially supported by National Institutes of Health (NIH) grant AI41647 and the National Multiple Sclerosis Society. C.E.T. had a postdoctoral fellowship from the National Multiple Sclerosis Society (FG 1733-A-1).
We thank Drs Iannis Aifantis, Philip Ashton-Rickardt, and Fabio R. Santori for critical reading of this paper; Dr Lothar Hennighausen (NIH) for the use of Bcl-XLflox/flox mice; Dr Dan R. Littman for the CD4-Cre mice; and Dr Warren Pear for the MIGR-Deltex1 retroviral vector.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.M. and C.E.T. were the principal participants and designed and performed research, analyzed data, and contributed to the writing of the paper; M.L.B. performed research and contributed to the preparation of the paper; J.S. generated the PS1-floxed animals and contributed to the preparation of the paper; M.S. provided reagents and contributed to the preparation of the paper; J.J.L. was responsible for the overall study, designed research, and wrote the paper. A.M. and C.E.T. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Juan J. Lafaille, New York University School of Medicine–Skirball Institute of Biomolecular Medicine, 540 1st Ave, 2nd Fl, Lab 07, New York, NY 10016; e-mail: lafaille@saturn.med.nyu.edu.
References
- 1.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–974. doi: 10.1146/annurev.immunol.23.021704.115747. [DOI] [PubMed] [Google Scholar]
- 2.Weng AP, Aster JC. Multiple niches for Notch in cancer: context is everything. Curr Opin Genet Dev. 2004;14:48–54. doi: 10.1016/j.gde.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Iwatsubo T. The gamma-secretase complex: machinery for intramembrane proteolysis. Curr Opin Neurobiol. 2004;14:379–383. doi: 10.1016/j.conb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Geling A, Steiner H, Willem M, Bally-Cuif L, Haass C. A gamma-secretase inhibitor blocks Notch signaling in vivo and causes a severe neurogenic phenotype in zebrafish. EMBO Rep. 2002;3:688–694. doi: 10.1093/embo-reports/kvf124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ye Y, Lukinova N, Fortini ME. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature. 1999;398:525–529. doi: 10.1038/19096. [DOI] [PubMed] [Google Scholar]
- 6.Struhl G, Greenwald I. Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature. 1999;398:522–525. doi: 10.1038/19091. [DOI] [PubMed] [Google Scholar]
- 7.Wittenburg N, Eimer S, Lakowski B, Rohrig S, Rudolph C, Baumeister R. Presenilin is required for proper morphology and function of neurons in C. elegans. Nature. 2000;406:306–309. doi: 10.1038/35018575. [DOI] [PubMed] [Google Scholar]
- 8.Wong PC, Zheng H, Chen H, et al. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 9.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 10.Iso T, Kedes L, Hamamori Y. HES and HERP families: multiple effectors of the Notch signaling pathway. J Cell Physiol. 2003;194:237–255. doi: 10.1002/jcp.10208. [DOI] [PubMed] [Google Scholar]
- 11.Warren LA, Rothenberg EV. Regulatory coding of lymphoid lineage choice by hematopoietic transcription factors. Curr Opin Immunol. 2003;15:166–175. doi: 10.1016/s0952-7915(03)00011-6. [DOI] [PubMed] [Google Scholar]
- 12.Hori K, Fostier M, Ito M, et al. Drosophila deltex mediates suppressor of Hairless-independent and late-endosomal activation of Notch signaling. Development. 2004;131:5527–5537. doi: 10.1242/dev.01448. [DOI] [PubMed] [Google Scholar]
- 13.Ordentlich P, Lin A, Shen CP, et al. Notch inhibition of E47 supports the existence of a novel signaling pathway. Mol Cell Biol. 1998;18:2230–2239. doi: 10.1128/mcb.18.4.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramain P, Khechumian K, Seugnet L, Arbogast N, Ackermann C, Heitzler P. Novel Notch alleles reveal a Deltex-dependent pathway repressing neural fate. Curr Biol. 2001;11:1729–1738. doi: 10.1016/s0960-9822(01)00562-0. [DOI] [PubMed] [Google Scholar]
- 15.Martinez Arias A, Zecchini V, Brennan K. CSL-independent Notch signalling: a checkpoint in cell fate decisions during development? Curr Opin Genet Dev. 2002;12:524–533. doi: 10.1016/s0959-437x(02)00336-2. [DOI] [PubMed] [Google Scholar]
- 16.Radtke F, Wilson A, Stark G, et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity. 1999;10:547–558. doi: 10.1016/s1074-7613(00)80054-0. [DOI] [PubMed] [Google Scholar]
- 17.Pui JC, Allman D, Xu L, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11:299–308. doi: 10.1016/s1074-7613(00)80105-3. [DOI] [PubMed] [Google Scholar]
- 18.Washburn T, Schweighoffer E, Gridley T, et al. Notch activity influences the alphabeta versus gammadelta T cell lineage decision. Cell. 1997;88:833–843. doi: 10.1016/s0092-8674(00)81929-7. [DOI] [PubMed] [Google Scholar]
- 19.Fowlkes BJ, Robey EA. A reassessment of the effect of activated Notch1 on CD4 and CD8 T cell development. J Immunol. 2002;169:1817–1821. doi: 10.4049/jimmunol.169.4.1817. [DOI] [PubMed] [Google Scholar]
- 20.Izon DJ, Punt JA, Xu L, et al. Notch1 regulates maturation of CD4+ and CD8+ thymocytes by modulating TCR signal strength. Immunity. 2001;14:253–264. doi: 10.1016/s1074-7613(01)00107-8. [DOI] [PubMed] [Google Scholar]
- 21.Robey E, Chang D, Itano A, et al. An activated form of Notch influences the choice between CD4 and CD8 T cell lineages. Cell. 1996;87:483–492. doi: 10.1016/s0092-8674(00)81368-9. [DOI] [PubMed] [Google Scholar]
- 22.Witt CM, Hurez V, Swindle CS, Hamada Y, Klug CA. Activated Notch2 potentiates CD8 lineage maturation and promotes the selective development of B1 B cells. Mol Cell Biol. 2003;23:8637–8650. doi: 10.1128/MCB.23.23.8637-8650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellavia D, Campese AF, Alesse E, et al. Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice. EMBO J. 2000;19:3337–3348. doi: 10.1093/emboj/19.13.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saito T, Chiba S, Ichikawa M, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity. 2003;18:675–685. doi: 10.1016/s1074-7613(03)00111-0. [DOI] [PubMed] [Google Scholar]
- 25.Wolfer A, Bakker T, Wilson A, et al. Inactivation of Notch 1 in immature thymocytes does not perturb CD4 or CD8T cell development. Nat Immunol. 2001;2:235–241. doi: 10.1038/85294. [DOI] [PubMed] [Google Scholar]
- 26.Tanigaki K, Tsuji M, Yamamoto N, et al. Regulation of alphabeta/gammadelta T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity. 2004;20:611–622. doi: 10.1016/s1074-7613(04)00109-8. [DOI] [PubMed] [Google Scholar]
- 27.Doerfler P, Shearman MS, Perlmutter RM. Presenilin-dependent gamma-secretase activity modulates thymocyte development. Proc Natl Acad Sci U S A. 2001;98:9312–9317. doi: 10.1073/pnas.161102498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hadland BK, Manley NR, Su D, et al. Gamma-secretase inhibitors repress thymocyte development. Proc Natl Acad Sci U S A. 2001;98:7487–7491. doi: 10.1073/pnas.131202798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu H, Saura CA, Choi SY, et al. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- 30.Steiner H, Duff K, Capell A, et al. A loss of function mutation of presenilin-2 interferes with amyloid beta-peptide production and Notch signaling. J Biol Chem. 1999;274:28669–28673. doi: 10.1074/jbc.274.40.28669. [DOI] [PubMed] [Google Scholar]
- 31.Saura CA, Choi SY, Beglopoulos V, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- 32.Lee PP, Fitzpatrick DR, Beard C, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 33.Walton KD, Wagner KU, Rucker EB, Shillingford JM, III, Miyoshi K, Hennighausen L. Conditional deletion of the bcl-x gene from mouse mammary epithelium results in accelerated apoptosis during involution but does not compromise cell function during lactation. Mech Dev. 2001;109:281–293. doi: 10.1016/s0925-4773(01)00549-4. [DOI] [PubMed] [Google Scholar]
- 34.Sun Z, Unutmaz D, Zou YR, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 35.Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dyer RB, Herzog NK. Immunodepletion EMSA: a novel method to identify proteins in a protein-DNA complex. Nucleic Acids Res. 1995;23:3345–3346. [PMC free article] [PubMed] [Google Scholar]
- 37.Izon DJ, Aster JC, He Y, et al. Deltex1 redirects lymphoid progenitors to the B cell lineage by antagonizing Notch1. Immunity. 2002;16:231–243. doi: 10.1016/s1074-7613(02)00271-6. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Lin A. Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 2005;15:36–42. doi: 10.1038/sj.cr.7290262. [DOI] [PubMed] [Google Scholar]
- 39.Grillot DA, Merino R, Nunez G. Bcl-XL displays restricted distribution during T cell development and inhibits multiple forms of apoptosis but not clonal deletion in transgenic mice. J Exp Med. 1995;182:1973–1983. doi: 10.1084/jem.182.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma A, Pena JC, Chang B, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci U S A. 1995;92:4763–4767. doi: 10.1073/pnas.92.11.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Motoyama N, Wang F, Roth KA, et al. Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science. 1995;267:1506–1510. doi: 10.1126/science.7878471. [DOI] [PubMed] [Google Scholar]
- 42.Hettmann T, DiDonato J, Karin M, Leiden JM. An essential role for nuclear factor kappaB in promoting double positive thymocyte apoptosis. J Exp Med. 1999;189:145–158. doi: 10.1084/jem.189.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alberola-Ila J, Hernandez-Hoyos G. The Ras/MAPK cascade and the control of positive selection. Immunol Rev. 2003;191:79–96. doi: 10.1034/j.1600-065x.2003.00012.x. [DOI] [PubMed] [Google Scholar]
- 44.Rincon M, Pedraza-Alva G. JNK and p38 MAP kinases in CD4+ and CD8+ T cells. Immunol Rev. 2003;192:131–142. doi: 10.1034/j.1600-065x.2003.00019.x. [DOI] [PubMed] [Google Scholar]
- 45.Rincon M, Whitmarsh A, Yang DD, et al. The JNK pathway regulates the In vivo deletion of immature CD4(+)CD8(+) thymocytes. J Exp Med. 1998;188:1817–1830. doi: 10.1084/jem.188.10.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merritt C, Enslen H, Diehl N, Conze D, Davis RJ, Rincon M. Activation of p38 mitogen-activated protein kinase in vivo selectively induces apoptosis of CD8(+) but not CD4(+) T cells. Mol Cell Biol. 2000;20:936–946. doi: 10.1128/mcb.20.3.936-946.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conze D, Lumsden J, Enslen H, Davis RJ, Le Gros G, Rincon M. Activation of p38 MAP kinase in T cells facilitates the immune response to the influenza virus. Mol Immunol. 2000;37:503–513. doi: 10.1016/s0161-5890(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 48.Liu WH, Lai MZ. Deltex regulates T-cell activation by targeted degradation of active MEKK1. Mol Cell Biol. 2005;25:1367–1378. doi: 10.1128/MCB.25.4.1367-1378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanwar R, Fortini ME. Notch signaling: a different sort makes the cut. Curr Biol. 2004;14:R1043–1045. doi: 10.1016/j.cub.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto I, Kawano Y, Murakami D, et al. Proteolytic release of CD44 intracellular domain and its role in the CD44 signaling pathway. J Cell Biol. 2001;155:755–762. doi: 10.1083/jcb.200108159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khoshnan A, Tindell C, Laux I, Bae D, Bennett B, Nel AE. The NF-kappa B cascade is important in Bcl-xL expression and for the anti-apoptotic effects of the CD28 receptor in primary human CD4+ lymphocytes. J Immunol. 2000;165:1743–1754. doi: 10.4049/jimmunol.165.4.1743. [DOI] [PubMed] [Google Scholar]
- 52.Chao DT, Linette GP, Boise LH, White LS, Thompson CB, Korsmeyer SJ. Bcl-XL and Bcl-2 repress a common pathway of cell death. J Exp Med. 1995;182:821–828. doi: 10.1084/jem.182.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dorsch M, Zheng G, Yowe D, et al. Ectopic expression of Delta4 impairs hematopoietic development and leads to lymphoproliferative disease. Blood. 2002;100:2046–2055. [PubMed] [Google Scholar]
- 54.Sade H, Krishna S, Sarin A. The anti-apoptotic effect of Notch-1 requires p56lck-dependent, Akt/PKB-mediated signaling in T cells. J Biol Chem. 2004;279:2937–2944. doi: 10.1074/jbc.M309924200. [DOI] [PubMed] [Google Scholar]
- 55.Passer BJ, Pellegrini L, Vito P, Ganjei JK, D'Adamio L. Interaction of Alzheimer's presenilin-1 and presenilin-2 with Bcl-X(L): a potential role in modulating the threshold of cell death. J Biol Chem. 1999;274:24007–24013. doi: 10.1074/jbc.274.34.24007. [DOI] [PubMed] [Google Scholar]
- 56.Alberici A, Moratto D, Benussi L, et al. Presenilin 1 protein directly interacts with Bcl-2. J Biol Chem. 1999;274:30764–30769. doi: 10.1074/jbc.274.43.30764. [DOI] [PubMed] [Google Scholar]
- 57.Araki W, Yuasa K, Takeda S, et al. Pro-apoptotic effect of presenilin 2 (PS2) overexpression is associated with down-regulation of Bcl-2 in cultured neurons. J Neurochem. 2001;79:1161–1168. doi: 10.1046/j.1471-4159.2001.00638.x. [DOI] [PubMed] [Google Scholar]
- 58.Lehar SM, Bevan MJ. T cells develop normally in the absence of both Deltex1 and Deltex2. Mol Cell Biol. 2006;26:7358–7371. doi: 10.1128/MCB.00149-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nam Y, Aster JC, Blacklow SC. Notch signaling as a therapeutic target. Curr Opin Chem Biol. 2002;6:501–509. doi: 10.1016/s1367-5931(02)00346-0. [DOI] [PubMed] [Google Scholar]
- 60.Marjaux E, Hartmann D, De Strooper B. Presenilins in memory, Alzheimer's disease, and therapy. Neuron. 2004;42:189–192. doi: 10.1016/s0896-6273(04)00218-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.