Abstract
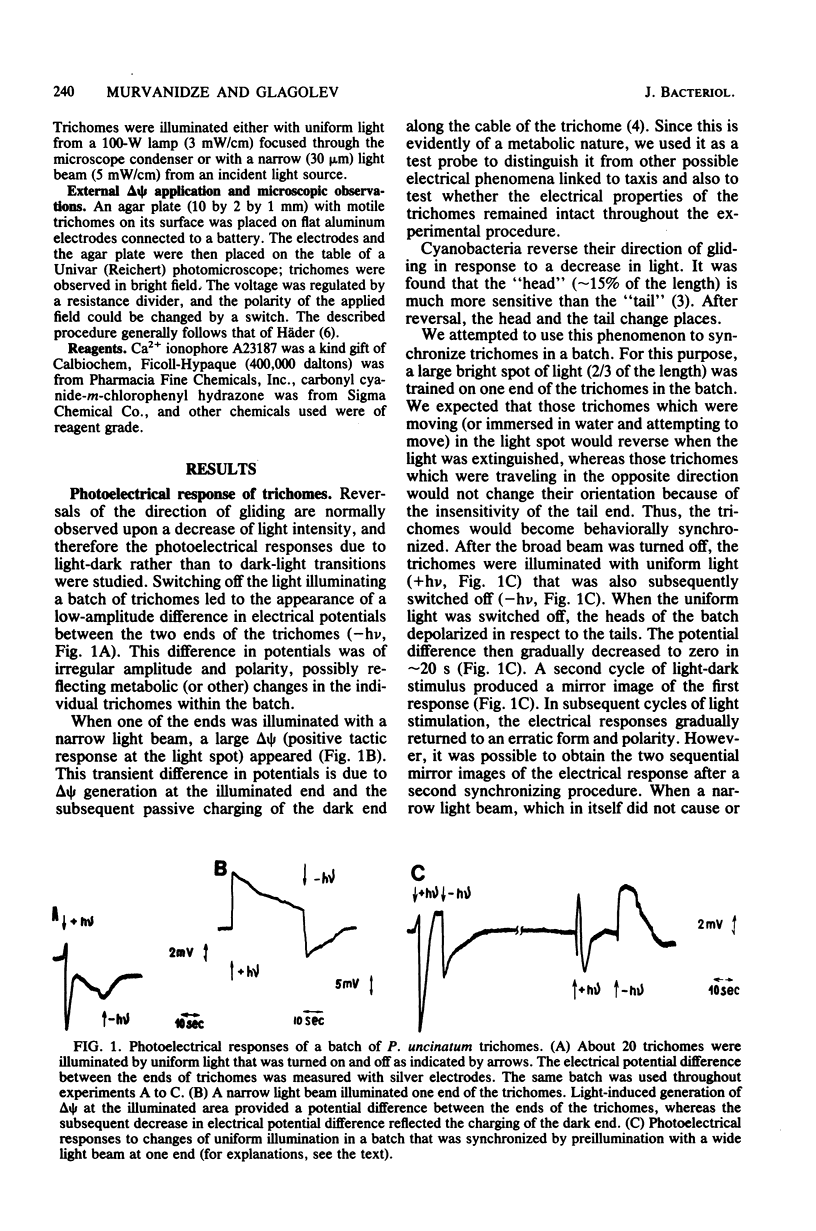
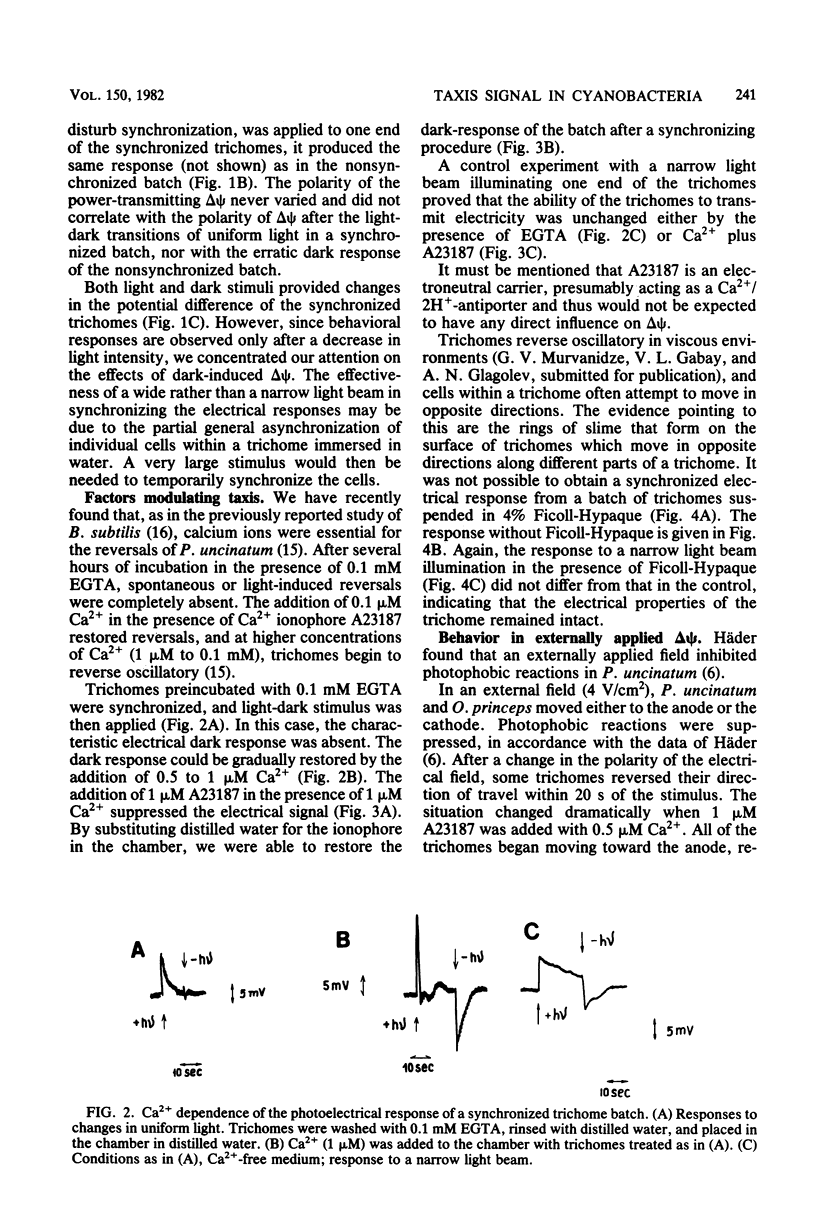
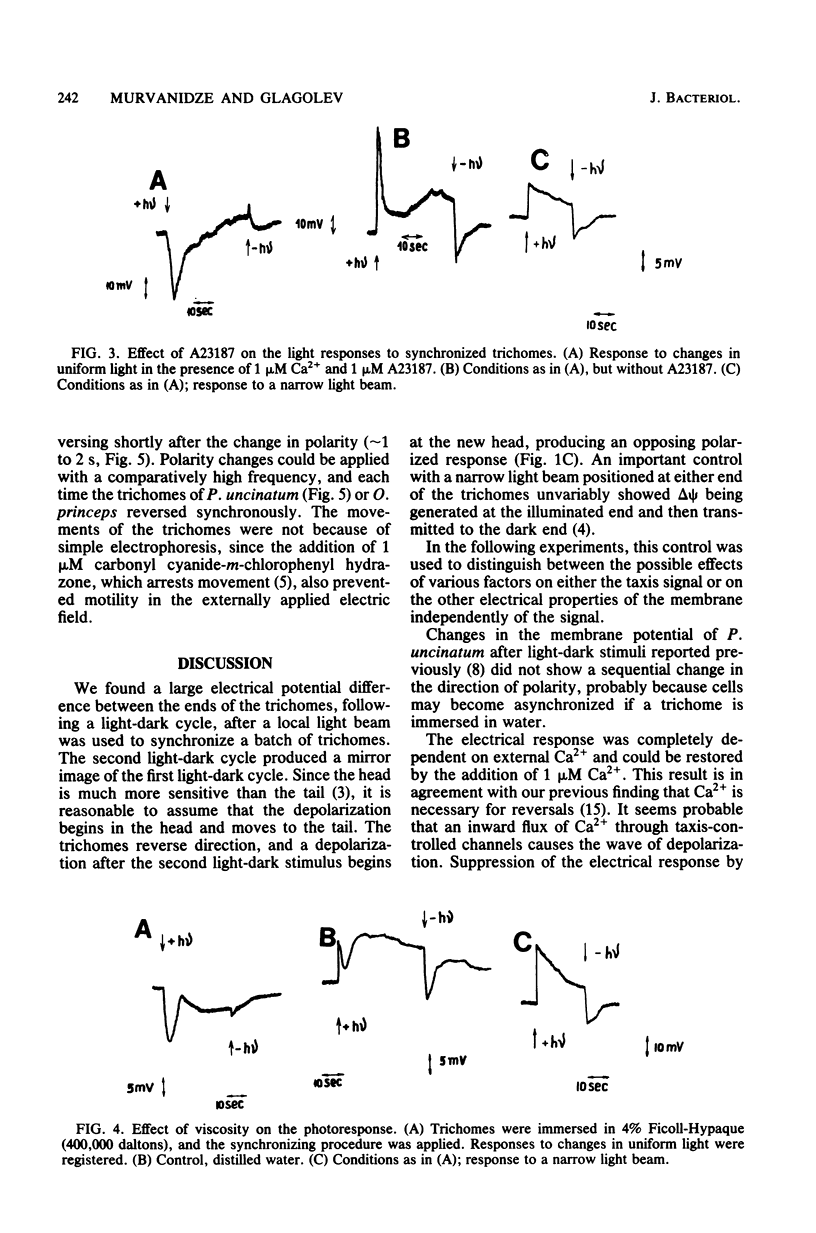
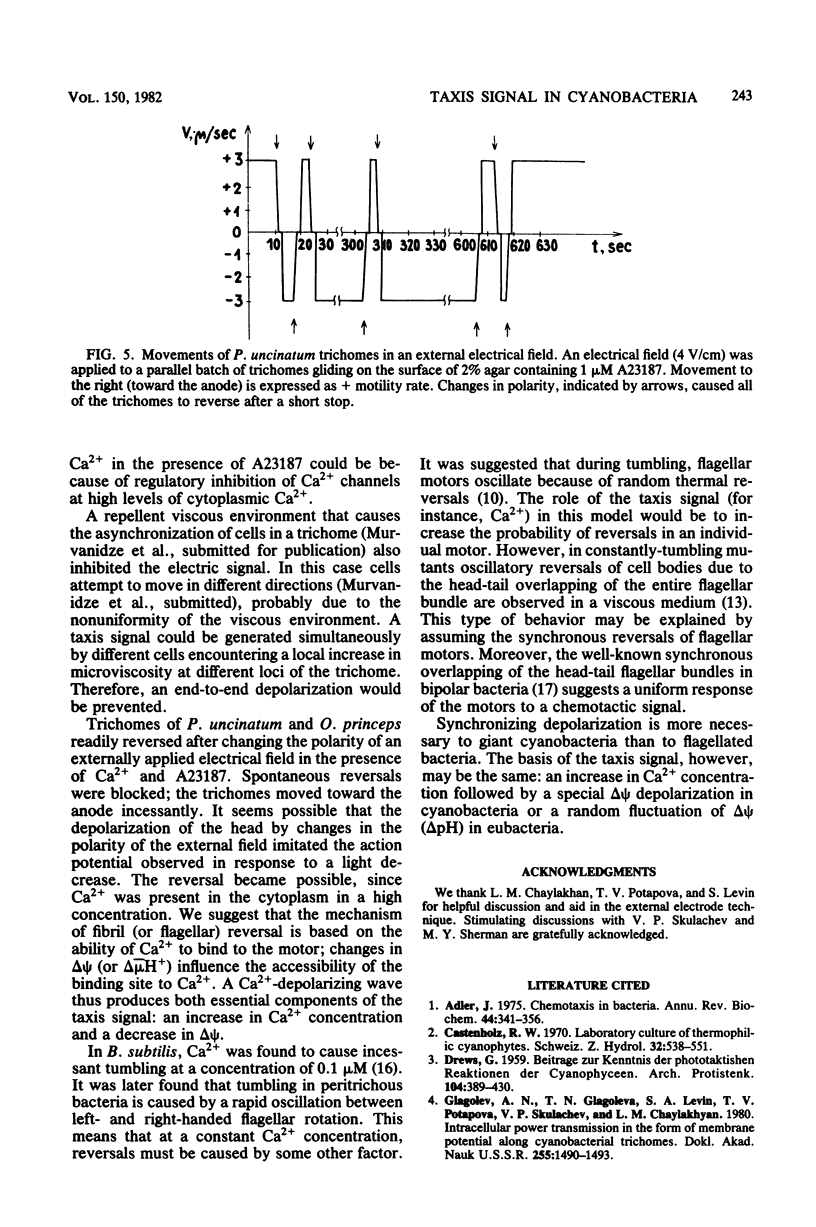
Electrical events after a light-dark stimulus were studied in the multicellular organism Phormidium uncinatum. Normally, such a stimulus causes the gliding trichome to reverse direction. By directing a large light spot on the end of a batch of trichomes and then switching it off, we achieved synchronization of the trichomes, since the "head" is much more sensitive than the "tail." The abrupt disappearance of a uniform light produced a depolarization wave which initiated at the head, as registered by externally applied electrodes. The second stimulus produced a depolarization of the opposite direction, reflecting the reorientation of the trichomes. No electrical response was observed at Ca2+ concentrations less than or equal to 10(-8) M. Factors causing oscillatory reversals, i.e., a combination of Ca2+ and A23187, or a viscous environment also abolished the electrical signal. Changes in an externally applied electrical field (4 V/cm2) had little effect on the motile behavior of P. uncinatum or Oscillatoria princeps. However, in the presence of 5 microM Ca2+-1 microM A23187, all the trichomes reversed synchronously to the anode after a change in polarity of an externally applied electrical field. We suggest that an increased Ca2+ concentration together with a change in delta psi (or delta mu H+) represents the taxis signal in cyanobacteria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Häder D. P. Influence of electric fields on photophobic reactions in blue-green algae. Arch Microbiol. 1977 Jul 26;114(1):83–86. doi: 10.1007/BF00429635. [DOI] [PubMed] [Google Scholar]
- Khan S., Macnab R. M. The steady-state counterclockwise/clockwise ratio of bacterial flagellar motors is regulated by protonmotive force. J Mol Biol. 1980 Apr 15;138(3):563–597. doi: 10.1016/s0022-2836(80)80018-0. [DOI] [PubMed] [Google Scholar]
- Krieg N. R., Tomelty J. P., Wells J. S., Jr Inhibitio of flagellar coordination in Spirillum volutans. J Bacteriol. 1967 Nov;94(5):1431–1436. doi: 10.1128/jb.94.5.1431-1436.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M., Ornston M. K. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J Mol Biol. 1977 May 5;112(1):1–30. doi: 10.1016/s0022-2836(77)80153-8. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Koshland D. E., Jr Membrane fluidity and chemotaxis: effects of temperature and membrane lipid composition on the swimming behavior of Salmonella typhimurium and Escherichia coli. J Mol Biol. 1977 Apr;111(2):183–201. doi: 10.1016/s0022-2836(77)80122-8. [DOI] [PubMed] [Google Scholar]
- Ordal G. W. Calcium ion regulates chemotactic behaviour in bacteria. Nature. 1977 Nov 3;270(5632):66–67. doi: 10.1038/270066a0. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Adler J. Change in membrane potential during bacterial chemotaxis. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4387–4391. doi: 10.1073/pnas.73.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]


