Abstract
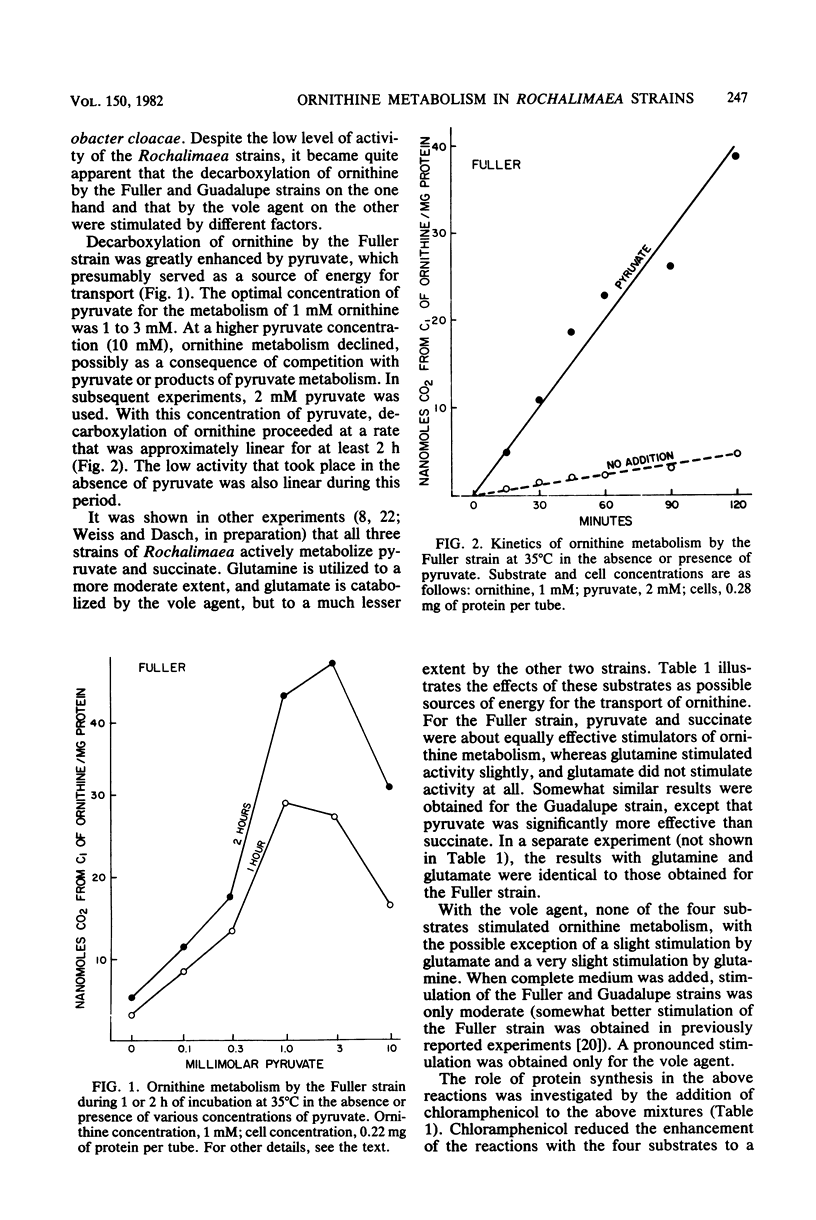
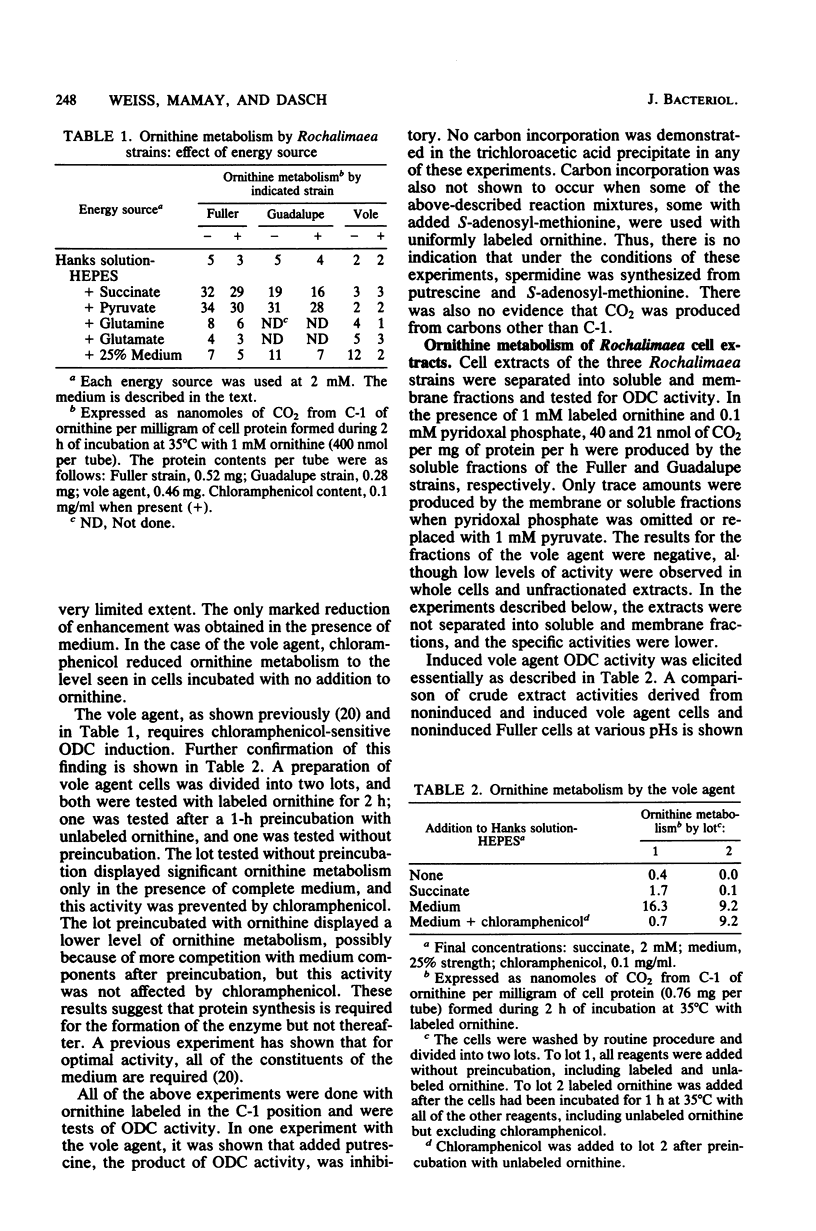
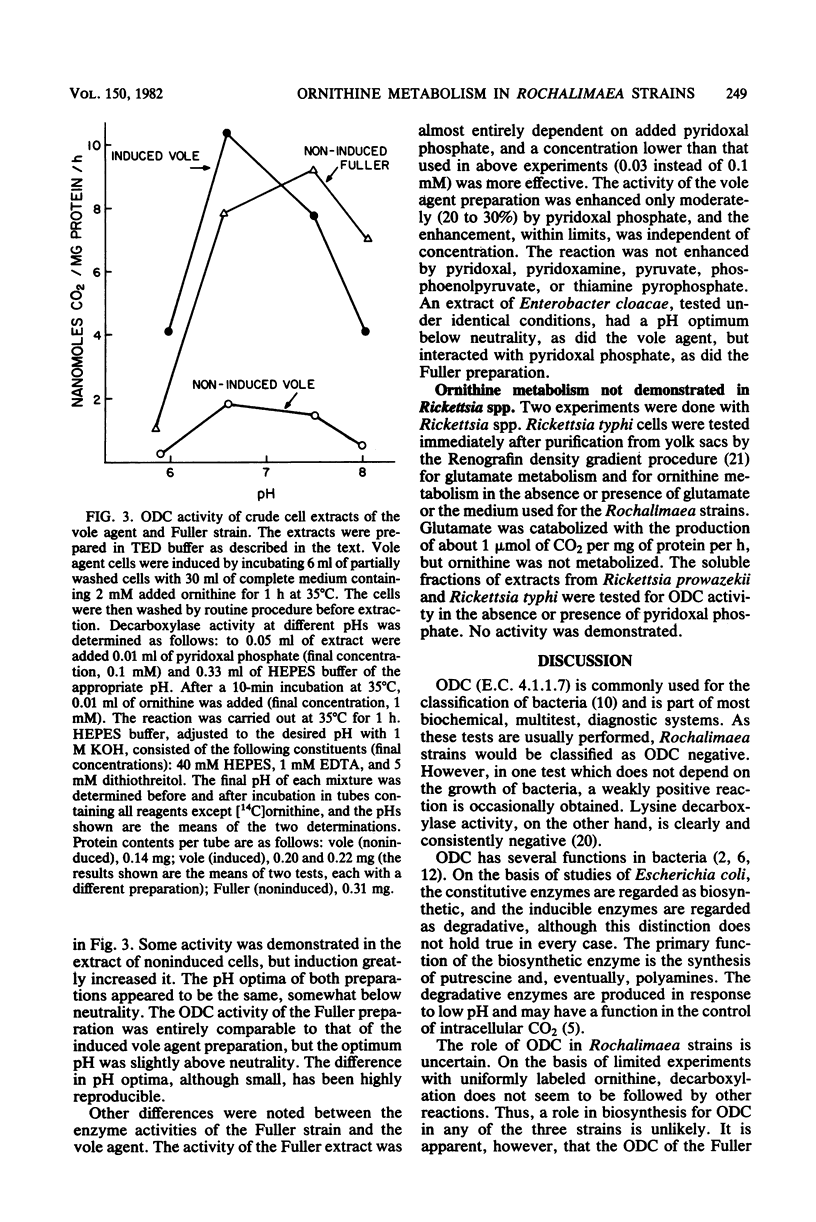
Ornithine metabolism was studied in two strains of the trench fever rickettsia Rochalimaea quintana, Fuller and Guadalupe, and in the vole agent, a strain of Rochalimaea but not necessarily of Rochalimaea quintana. The metabolic activity of intact cells and cell-free extracts was measured by monitoring the evolution of 14CO2 from [1-14C]ornithine. Low levels of activity were obtained with all three strains, but requirements for the demonstration of this activity differed. With the cells of the Fuller and Guadalupe strains, the decarboxylation of ornithine was almost completely dependent on added pyruvate or succinate, presumably as sources of energy for transport. This enhancement was not prevented by the presence of chloramphenicol. The activity of the vole agent, on the other hand, required the complete medium. This activity was prevented by chloramphenicol added at the same time as the medium but not by chloramphenicol added after 1 h of incubation. In cell-free extracts, the demonstration of ornithine decarboxylase activity in the vole agent required prior induction with medium containing ornithine, whereas in the other two strains, the activity was constitutive. The activities of the extracts of the Fuller strain and the vole agent differed also in pH optimum, which was somewhat lower for the vole agent, and in the added pyridoxal phosphate requirement, which was greater for the Fuller strain. Comparable experiments with Rickettsia typhi and Rickettsia prowazekii failed to reveal evidence of ornithine metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Christensen R. L., Schmit J. C. Regulation and glutamic acid decarboxylase during Neurospora crassa conidial germination. J Bacteriol. 1980 Dec;144(3):983–990. doi: 10.1128/jb.144.3.983-990.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasch G. A., Samms J. R., Weiss E. Biochemical characteristics of typhus group rickettsiae with special attention to the Rickettsia prowazekii strains isolated from flying squirrels. Infect Immun. 1978 Feb;19(2):676–685. doi: 10.1128/iai.19.2.676-685.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin C. S., Tyrrell D. A., Head B., Rees R. J. Inhibition of haemaggregation by lepromin and other mycobacterial substances. Nature. 1967 Dec 9;216(5119):1019–1020. doi: 10.1038/2161019a0. [DOI] [PubMed] [Google Scholar]
- Guirard B. M., Snell E. E. Purification and properties of ornithine decarboxylase from Lactobacillus sp. 30a. J Biol Chem. 1980 Jun 25;255(12):5960–5964. [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Merrell B. R., Weiss E., Dasch G. A. Morphological and cell association characteristics of Rochalimaea quintana: comparison of the Vole and Fuller strains. J Bacteriol. 1978 Aug;135(2):633–640. doi: 10.1128/jb.135.2.633-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Myers W. F., Wisseman C. L., Jr, Fiset P., Oaks E. V., Smith J. F. Taxonomic relationship of vole agent to Rochalimaea quintana. Infect Immun. 1979 Dec;26(3):976–983. doi: 10.1128/iai.26.3.976-983.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., McGill S. Decarboxylation of ornithine and lysine in rat tissues. Biochim Biophys Acta. 1979 Jun 6;568(2):416–427. doi: 10.1016/0005-2744(79)90310-3. [DOI] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Weiss E., Millar D. B., Bozeman F. M., Ormsbee R. A. DNA base composition of rickettsiae. Science. 1973 Apr 27;180(4084):415–417. doi: 10.1126/science.180.4084.415. [DOI] [PubMed] [Google Scholar]
- Varela G., Vinson J. W., Molina-Pasquel C. Trench fever. II. Propagation of Rickettsia quintana on cell-free medium from the blood of two patients. Am J Trop Med Hyg. 1969 Sep;18(5):708–712. [PubMed] [Google Scholar]
- Weiss E., Coolbaugh J. C., Williams J. C. Separation of viable Rickettsia typhi from yolk sac and L cell host components by renografin density gradient centrifugation. Appl Microbiol. 1975 Sep;30(3):456–463. doi: 10.1128/am.30.3.456-463.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Dasch G. A., Woodman D. R., Williams J. C. Vole agent identified as a strain of the trench fever rickettsia, Rochalimaea quintana. Infect Immun. 1978 Mar;19(3):1013–1020. doi: 10.1128/iai.19.3.1013-1020.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]


