Abstract
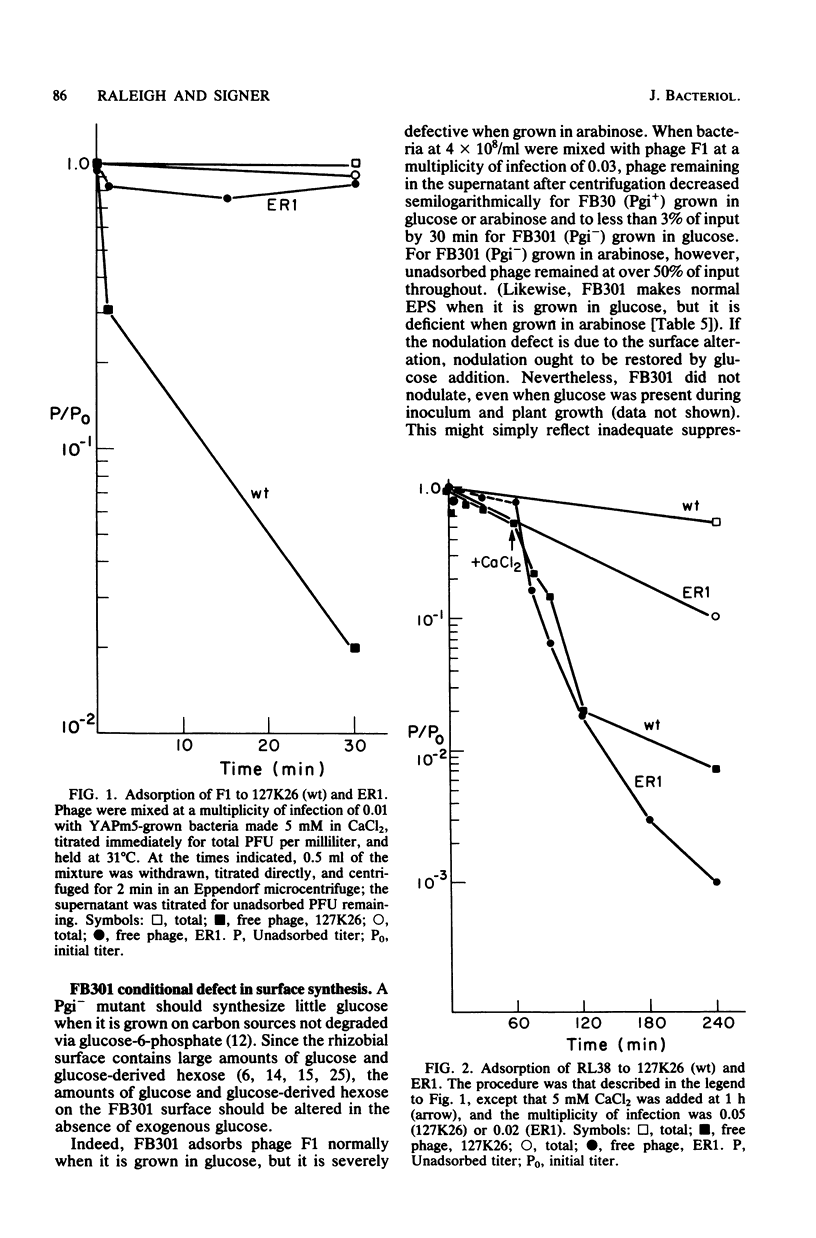
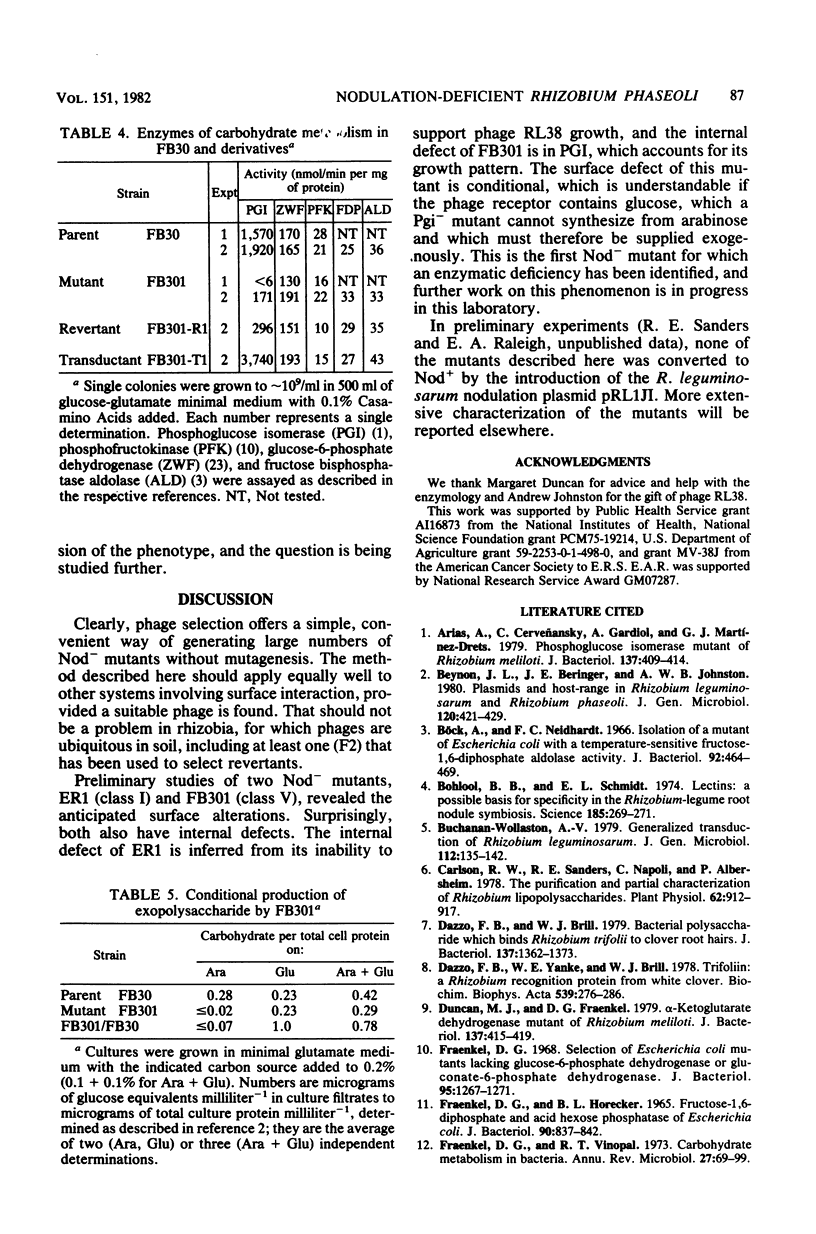
A high proportion of Rhizobium phaseoli mutants that survived infection with phage F1 were found to be nodulation deficient. Two that were examined in detail had internal defects in addition to the expected surface defects. One internal defect was in the enzyme phosphoglucose isomerase. The use of phages to select appropriate mutants should apply generally in any system in which surface components are involved.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias A., Cerveńansky C., Gardiol A., Martínez-Drets G. Phosphoglucose isomerase mutant of Rhizobium meliloti. J Bacteriol. 1979 Jan;137(1):409–414. doi: 10.1128/jb.137.1.409-414.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlool B. B., Schmidt E. L. Lectins: a possible basis for specificity in the Rhizobium--legume root nodule symbiosis. Science. 1974 Jul 19;185(4147):269–271. doi: 10.1126/science.185.4147.269. [DOI] [PubMed] [Google Scholar]
- Böck A., Neidhardt F. C. Isolation of a Mutant of Escherichia coli with a Temperature-sensitive Fructose-1,6-Diphosphate Aldolase Activity. J Bacteriol. 1966 Aug;92(2):464–469. doi: 10.1128/jb.92.2.464-469.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Brill W. J. Bacterial polysaccharide which binds Rhizobium trifolii to clover root hairs. J Bacteriol. 1979 Mar;137(3):1362–1373. doi: 10.1128/jb.137.3.1362-1373.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazzo F. B., Yanke W. E., Brill W. J. Trifolin: a Rhizobium recognition protein from white clover. Biochim Biophys Acta. 1978 Mar 20;539(3):276–286. doi: 10.1016/0304-4165(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Duncan M. J., Fraenkel D. G. alpha-Ketoglutarate dehydrogenase mutant of Rhizobium meliloti. J Bacteriol. 1979 Jan;137(1):415–419. doi: 10.1128/jb.137.1.415-419.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G., Horecker B. L. Fructose-1, 6-diphosphatase and acid hexose phosphatase of Escherichia coli. J Bacteriol. 1965 Oct;90(4):837–842. doi: 10.1128/jb.90.4.837-842.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel D. G. Selection of Escherichia coli mutants lacking glucose-6-phosphate dehydrogenase or gluconate-6-phosphate dehydrogenase. J Bacteriol. 1968 Apr;95(4):1267–1271. doi: 10.1128/jb.95.4.1267-1271.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblin J., Kent S. P. Possible role of phytohaemagglutinin in Phaseolus vulgaris L. Nat New Biol. 1973 Sep 5;245(140):28–30. doi: 10.1038/newbio245028a0. [DOI] [PubMed] [Google Scholar]
- Jansson P. E., Kenne L., Lindberg B., Ljunggren H., Lönngren J., Rudén U., Svensson S. Demonstration of an octasaccharide repeating unit in the extracellular polysaccharide of Rhizobium meliloti by sequential degradation. J Am Chem Soc. 1977 May 25;99(11):3812–3815. doi: 10.1021/ja00453a049. [DOI] [PubMed] [Google Scholar]
- KLECZKOWSKA J. A study of phage-resistant mutants of Rhizobium trifolii. J Gen Microbiol. 1950 Sep;4(3):298–310. doi: 10.1099/00221287-4-3-298. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Ludwig R. A., Raleigh E. A., Duncan M. J., Signer E. R., Gibson A. H., Dudman W. F., Schwinghamer E. A., Jordan D. C., Schmidt E. L., Tran D. T. Further examination of presumptive Rhizobium trifolii mutants that nodulate Glycine max. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3942–3946. doi: 10.1073/pnas.76.8.3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Ineffective and non-nodulating mutant strains of Rhizobium japonicum. J Bacteriol. 1976 Aug;127(2):763–769. doi: 10.1128/jb.127.2.763-769.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Brill W. J. Involvement of Rhizobium japonicum O antigen in soybean nodulation. J Bacteriol. 1978 Mar;133(3):1295–1299. doi: 10.1128/jb.133.3.1295-1299.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-De Drets G., Arias A. Enzymatic basis for differentiation of Rhizobium into fast- and slow-growing groups. J Bacteriol. 1972 Jan;109(1):467–470. doi: 10.1128/jb.109.1.467-470.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey A. T., Fraenkel D. G. Selection of fructose 6-phosphate kinase mutants in Escherichia coli. Biochem Biophys Res Commun. 1968 Aug 13;32(3):467–473. doi: 10.1016/0006-291x(68)90685-2. [DOI] [PubMed] [Google Scholar]
- Napoli C., Albersheim P. Rhizobium leguminosarum mutants incapable of normal extracellular polysaccharide production. J Bacteriol. 1980 Mar;141(3):1454–1456. doi: 10.1128/jb.141.3.1454-1456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsen B. K., Aman P., Darvill A. G., McNeil M., Albersheim P. Host-Symbiont Interactions : V. THE STRUCTURE OF ACIDIC EXTRACELLULAR POLYSACCHARIDES SECRETED BY RHIZOBIUM LEGUMINOSARUM AND RHIZOBIUM TRIFOLII. Plant Physiol. 1981 Mar;67(3):389–400. doi: 10.1104/pp.67.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpert J. S., Albersheim P. Host-symbiont interactions. I. The lectins of legumes interact with the o-antigen-containing lipopolysaccharides of their symbiont Rhizobia. Biochem Biophys Res Commun. 1976 Jun 7;70(3):729–737. doi: 10.1016/0006-291x(76)90653-7. [DOI] [PubMed] [Google Scholar]


