Abstract
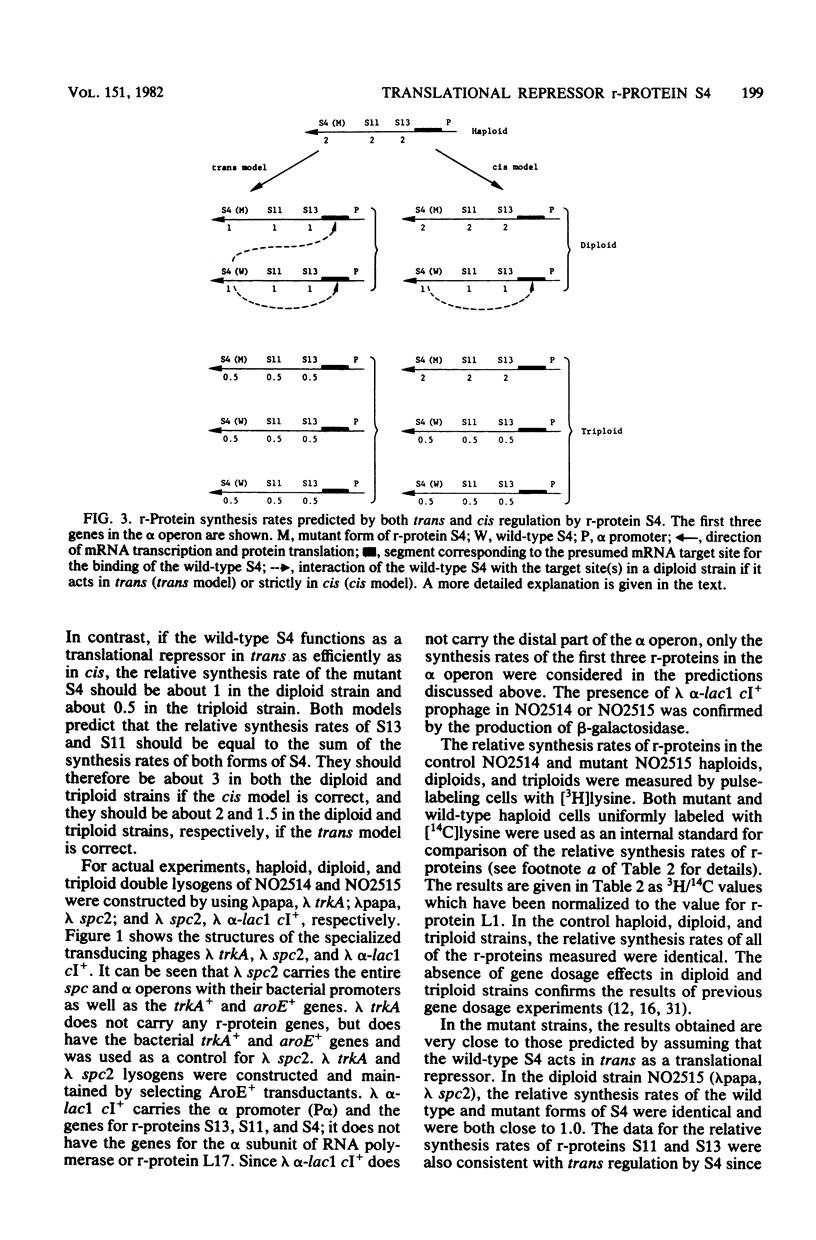
Ribosomal protein (r-protein) S4 is the translational repressor which regulates the synthesis rates of r-proteins whose genes are in the alpha operon: r-proteins S13, S11, S4, and L17. In a strain having a mutation in the gene for r-protein S4 (rpsD), the mutant S4 fails to regulate expression of the alpha operon, resulting in specific and significant overproduction of r-proteins S13, S11, and S4. This confirms and extends similar observations made with rpsD mutants (M. O. Olsson and L. A. Isaksson, Mol. Gen. Genet. 169:271-278, 1979) before post-transcriptional regulation of r-protein synthesis was proposed and is consistent with the established regulatory role of r-protein S4. The rpsD mutant has been used to study the question of whether regulatory r-proteins function in trans or strictly in cis as translational repressors. The mutant strain was lysogenized with one or two specialized transducing phages carrying a wild-type S4 gene to obtain strains which were diploid or triploid with respect to the alpha operon. The wild-type and mutant forms of S4 were separated by two-dimensional polyacrylamide gel electrophoresis, which allowed accurate measurement of the relative contributions of r-proteins from different alpha operons within a single cell. We found that expression of r-proteins from the chromosomal alpha operon containing the rpsD allele was reduced when the wild-type S4 was present, with the effect being greater in the triploid strain than in the diploid strain. We conclude that the wild-type S4 acts in trans as a translational repressor to regulate expression from the chromosomal alpha operon.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barritault D., Expert-Bezancon A., Guérin M. F., Hayes D. The use of acetone precipitation in the isolation of ribosomal proteins. Eur J Biochem. 1976 Mar 16;63(1):131–135. doi: 10.1111/j.1432-1033.1976.tb10215.x. [DOI] [PubMed] [Google Scholar]
- Birge E. A., Kurland C. G. Altered ribosomal protein in streptomycin-dependent Escherichia coli. Science. 1969 Dec 5;166(3910):1282–1284. doi: 10.1126/science.166.3910.1282. [DOI] [PubMed] [Google Scholar]
- Brot N., Caldwell P., Weissbach H. Autogenous control of Escherichia coli ribosomal protein L10 synthesis in vitro. Proc Natl Acad Sci U S A. 1980 May;77(5):2592–2595. doi: 10.1073/pnas.77.5.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler M. G., Pritchard R. H. The effect of gene concentration and relative gene dosage on gene output in Escherichia coli. Mol Gen Genet. 1975;138(2):127–141. doi: 10.1007/BF02428117. [DOI] [PubMed] [Google Scholar]
- Chang F. N., Wang Y. J., Fetterolf C. J., Flaks J. G. Letter: Unequal contribution to ribosomal assembly of both str alleles in Escherichia coli merodiploids and its relationship to the dominance phenomenon. J Mol Biol. 1974 Jan 15;82(2):273–277. doi: 10.1016/0022-2836(74)90345-3. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Dean D., Nomura M. Feedback regulation of ribosomal protein gene expression in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3590–3594. doi: 10.1073/pnas.77.6.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean D., Yates J. L., Nomura M. Escherichia coli ribosomal protein S8 feedback regulates part of spc operon. Nature. 1981 Jan 1;289(5793):89–91. doi: 10.1038/289089a0. [DOI] [PubMed] [Google Scholar]
- Dean D., Yates J. L., Nomura M. Identification of ribosomal protein S7 as a repressor of translation within the str operon of E. coli. Cell. 1981 May;24(2):413–419. doi: 10.1016/0092-8674(81)90331-7. [DOI] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon A. M., Jinks C. S., Strycharz G. D., Nomura M. Regulation of ribosomal protein synthesis in Escherichia coli by selective mRNA inactivation. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3411–3415. doi: 10.1073/pnas.76.7.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda R. Autogenous regulation of the synthesis of ribosomal proteins, L10 and L7/12, in Escherichia coli. Mol Gen Genet. 1980;178(2):483–486. doi: 10.1007/BF00270505. [DOI] [PubMed] [Google Scholar]
- Funatsu G., Puls W., Schiltz E., Reinbolt J., Wittmann H. G. Ribosomal proteins. XXXI. Comparative studies on altered proteins S4 of six Escherichia coli revertants from streptomycin dependence. Mol Gen Genet. 1972;115(2):131–139. doi: 10.1007/BF00277293. [DOI] [PubMed] [Google Scholar]
- Geyl D., Böck A. Synthesis of ribosomal proteins in merodiploid strains and in minicells of Escherichia coli. Mol Gen Genet. 1977 Sep 9;154(3):327–334. doi: 10.1007/BF00571290. [DOI] [PubMed] [Google Scholar]
- Green M., Kurland C. G. Mutant ribosomal protein with defective RNA binding site. Nat New Biol. 1971 Dec 29;234(52):273–275. doi: 10.1038/newbio234273a0. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Hasenbank R., Guthrie C., Stöffler G., Wittmann H. G., Rosen L., Apirion D. Electrophoretic and immunological studies on ribosomal proteins of 100 Escherichia coli revertants from streptomycin dependence. Mol Gen Genet. 1973 Dec 14;127(1):1–18. doi: 10.1007/BF00267778. [DOI] [PubMed] [Google Scholar]
- Howard G. A., Traut R. R. Separation and radioautography of microgram quantities of ribosomal proteins by two-dimensional polyacrylamide gel electrophoresis. FEBS Lett. 1973 Jan 15;29(2):177–180. doi: 10.1016/0014-5793(73)80555-1. [DOI] [PubMed] [Google Scholar]
- Jinks-Robertson S., Nomura M. Regulation of ribosomal protein synthesis in an Escherichia coli mutant missing ribosomal protein L1. J Bacteriol. 1981 Mar;145(3):1445–1447. doi: 10.1128/jb.145.3.1445-1447.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L., Post L., Zengel J., Gilbert S. F., Strycharz W. A., Nomura M. Mapping of ribosomal protein genes by in vitro protein synthesis using DNA fragments of lambdafus3 transducing phage DNA as templates. J Biol Chem. 1977 Oct 25;252(20):7365–7383. [PubMed] [Google Scholar]
- Mitchell D. H., Reznikoff W. S., Beckwith J. Genetic fusions that help define a transcription termination region in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):441–457. doi: 10.1016/0022-2836(76)90239-4. [DOI] [PubMed] [Google Scholar]
- Miura A., Krueger J. H., Itoh S., de Boer H. A., Nomura M. Growth-rate-dependent regulation of ribosome synthesis in E. coli: expression of the lacZ and galK genes fused to ribosomal promoters. Cell. 1981 Sep;25(3):773–782. doi: 10.1016/0092-8674(81)90185-9. [DOI] [PubMed] [Google Scholar]
- Mizushima S., Nomura M. Assembly mapping of 30S ribosomal proteins from E. coli. Nature. 1970 Jun 27;226(5252):1214–1214. doi: 10.1038/2261214a0. [DOI] [PubMed] [Google Scholar]
- Murray N. E., Murray K. Manipulation of restriction targets in phage lambda to form receptor chromosomes for DNA fragments. Nature. 1974 Oct 11;251(5475):476–481. doi: 10.1038/251476a0. [DOI] [PubMed] [Google Scholar]
- Nomura M., Morgan E. A. Genetics of bacterial ribosomes. Annu Rev Genet. 1977;11:297–347. doi: 10.1146/annurev.ge.11.120177.001501. [DOI] [PubMed] [Google Scholar]
- Nomura M., Yates J. L., Dean D., Post L. E. Feedback regulation of ribosomal protein gene expression in Escherichia coli: structural homology of ribosomal RNA and ribosomal protein MRNA. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7084–7088. doi: 10.1073/pnas.77.12.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M. O. Analysis of rpsD mutations in Escherichia coli. II. Physiology of some representative mutants. Mol Gen Genet. 1979 Feb 1;169(3):259–269. doi: 10.1007/BF00382272. [DOI] [PubMed] [Google Scholar]
- Olsson M. O., Gausing K. Post-transciptional control of coordinated ribosomal protein synthesis in Escherichia coli. Nature. 1980 Feb 7;283(5747):599–600. doi: 10.1038/283599a0. [DOI] [PubMed] [Google Scholar]
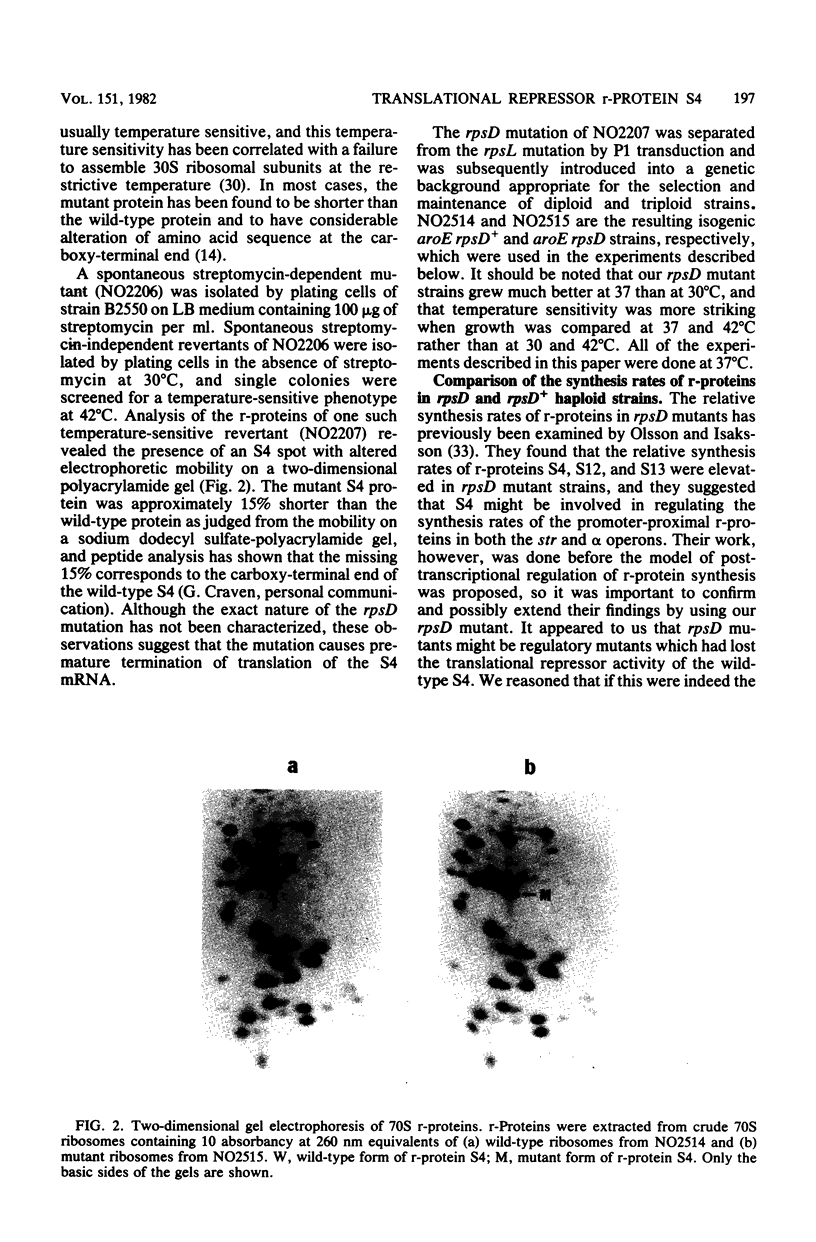
- Olsson M. O., Isaksson L. A. Analysis of rpsD mutations in Escherichia coli. I. Comparison of mutants with various alterations in ribosomal protein S4. Mol Gen Genet. 1979 Feb 1;169(3):251–257. doi: 10.1007/BF00382271. [DOI] [PubMed] [Google Scholar]
- Olsson M. O., Isaksson L. A. Analysis of rpsD mutations in Escherichia coli. III. Effects of rpsD mutations on expression of some ribosomal protein genes. Mol Gen Genet. 1979 Feb 1;169(3):271–278. doi: 10.1007/BF00382273. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling P. F., Modolell J., Takeda Y., Davis B. D. Ribosomes from Escherichia coli merodiplods heterozygous for resistance to streptomycin and to spectinomycin. J Mol Biol. 1968 Nov 14;37(3):407–421. doi: 10.1016/0022-2836(68)90111-3. [DOI] [PubMed] [Google Scholar]
- Stöffler G., Hasenbank R., Dabbs E. R. Expression of the L11-L1 operon in mutants of Escherichia coli lacking the ribosomal proteins L1 or L11. Mol Gen Genet. 1981;181(2):164–168. doi: 10.1007/BF00268422. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Arfsten A. E., Nomura M. In vitro expression of Escherichia coli ribosomal protein genes: autogenous inhibition of translation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1837–1841. doi: 10.1073/pnas.77.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Dean D., Strycharz W. A., Nomura M. E. coli ribosomal protein L10 inhibits translation of L10 and L7/L12 mRNAs by acting at a single site. Nature. 1981 Nov 12;294(5837):190–192. doi: 10.1038/294190a0. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Nomura M. E. coli ribosomal protein L4 is a feedback regulatory protein. Cell. 1980 Sep;21(2):517–522. doi: 10.1016/0092-8674(80)90489-4. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Nomura M. Feedback regulation of ribosomal protein synthesis in E. coli: localization of the mRNA target sites for repressor action of ribosomal protein L1. Cell. 1981 Apr;24(1):243–249. doi: 10.1016/0092-8674(81)90520-1. [DOI] [PubMed] [Google Scholar]
- Zengel J. M., Mueckl D., Lindahl L. Protein L4 of the E. coli ribosome regulates an eleven gene r protein operon. Cell. 1980 Sep;21(2):523–535. doi: 10.1016/0092-8674(80)90490-0. [DOI] [PubMed] [Google Scholar]