Abstract
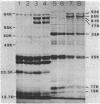
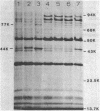
Azotobacter vinelandii produced three major proteins of 93,000, 85,000, and 81,000 daltons and a minor 77,000-dalton protein in the outer membrane of Fe-limited cells, and these cells were competent for transformation by DNA. The synthesis of these proteins was repressed in Fe-sufficient medium. Mo limitation of nitrogen-fixing cells resulted in the hyperproduction of a 44,000-dalton protein and the production of a minor 77,000-dalton protein in the outer membrane. Mo limitation enhanced competence in Fe-limited medium and induced competence in Fe-sufficient medium. The 44,000-dalton protein was replaced by a 45,000-dalton protein when Fe-sufficient medium also contained NH4+, but the cells were noncompetent. The synthesis of these proteins was repressed in Mo-sufficient medium and by NH4+ in Fe-limited medium. All of the culture supernatants contained a blue-white fluorescent material (absorbance maximum, 214 nm) which appeared to coordinate Fe3+, Fe2+, MoO4(2-), WO3(2-), and VO3(-).
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BULEN W. A., LECOMTE J. R. Isolation and properties of a yellow-green fluorescent peptide from azotobacter medium. Biochem Biophys Res Commun. 1962 Dec 19;9:523–528. doi: 10.1016/0006-291x(62)90119-5. [DOI] [PubMed] [Google Scholar]
- Benemann J. R., McKenna C. E., Lie R. F., Traylor T. G., Kamen M. D. The vanadium effect in nitrogen fixation by azotobacter. Biochim Biophys Acta. 1972 Mar 30;264(1):25–38. doi: 10.1016/0304-4165(72)90113-4. [DOI] [PubMed] [Google Scholar]
- Bishop P. E., Jarlenski D. M., Hetherington D. R. Evidence for an alternative nitrogen fixation system in Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7342–7346. doi: 10.1073/pnas.77.12.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Hancock R. E., Hantke K., Hartmann A. Functional organization of the outer membrane of escherichia coli: phage and colicin receptors as components of iron uptake systems. J Supramol Struct. 1976;5(1):37–58. doi: 10.1002/jss.400050105. [DOI] [PubMed] [Google Scholar]
- Brill W. J. Biochemical genetics of nitrogen fixation. Microbiol Rev. 1980 Sep;44(3):449–467. doi: 10.1128/mr.44.3.449-467.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin J. L., Bulen W. A. The isolation and identification of 2,3-dihydroxybenzoic acid and 2-N,6-N-di-92,3-dihydroxybenzoyl)-L-lysine formed by iron-deficient Azotobacter vinelandii. Biochemistry. 1969 Mar;8(3):757–762. doi: 10.1021/bi00831a002. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Frost G. E., Rosenberg H. The inducible citrate-dependent iron transport system in Escherichia coli K12. Biochim Biophys Acta. 1973 Nov 30;330(1):90–101. doi: 10.1016/0005-2736(73)90287-3. [DOI] [PubMed] [Google Scholar]
- KEELER R. F., VARNER J. E. Tungstate as an antagonist of molybdate in Azotobacter vinelandii. Arch Biochem Biophys. 1957 Aug;70(2):585–590. doi: 10.1016/0003-9861(57)90146-7. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Heller K., Coulton J. W., Braun V. Genetic control of hydroxamate-mediated iron uptake in Escherichia coli. J Bacteriol. 1980 Jul;143(1):256–264. doi: 10.1128/jb.143.1.256-264.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketchum P. A., Owens M. S. Production of molybdenum-coordinating compound by Bacillus thuringiensis. J Bacteriol. 1975 May;122(2):412–417. doi: 10.1128/jb.122.2.412-417.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagatani H. H., Brill W. J. Nitrogenase V. The effect of Mo, W and V on the synthesis of nitrogenase components in Azotobacter vinelandii. Biochim Biophys Acta. 1974 Aug 7;362(1):160–166. doi: 10.1016/0304-4165(74)90037-3. [DOI] [PubMed] [Google Scholar]
- Ohkawa I., Shiga S., Kageyama M. Effect of iron concentration in the growth medium on the sensitivity of Pseudomonas aeruginosa to pyocin S2. J Biochem. 1980 Jan;87(1):323–331. doi: 10.1093/oxfordjournals.jbchem.a132740. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Page W. J., Sadoff H. L. Physiological factors affecting transformation of Azotobacter vinelandii. J Bacteriol. 1976 Mar;125(3):1080–1087. doi: 10.1128/jb.125.3.1080-1087.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Induction of transformation competence in Azotobacter vinelandii iron-limited cultures. Can J Microbiol. 1978 Dec;24(12):1590–1594. doi: 10.1139/m78-254. [DOI] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Optimal conditions for transformation of Azotobacter vinelandii. J Bacteriol. 1979 Sep;139(3):1058–1061. doi: 10.1128/jb.139.3.1058-1061.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienkos P. T., Brill W. J. Molybdenum accumulation and storage in Klebsiella pneumoniae and Azotobacter vinelandii. J Bacteriol. 1981 Feb;145(2):743–751. doi: 10.1128/jb.145.2.743-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Stiefel E. I., Watt G. D. Azotobacter cytochrome b557.5 is a bacterioferritin. Nature. 1979 May 3;279(5708):81–83. doi: 10.1038/279081a0. [DOI] [PubMed] [Google Scholar]