Abstract
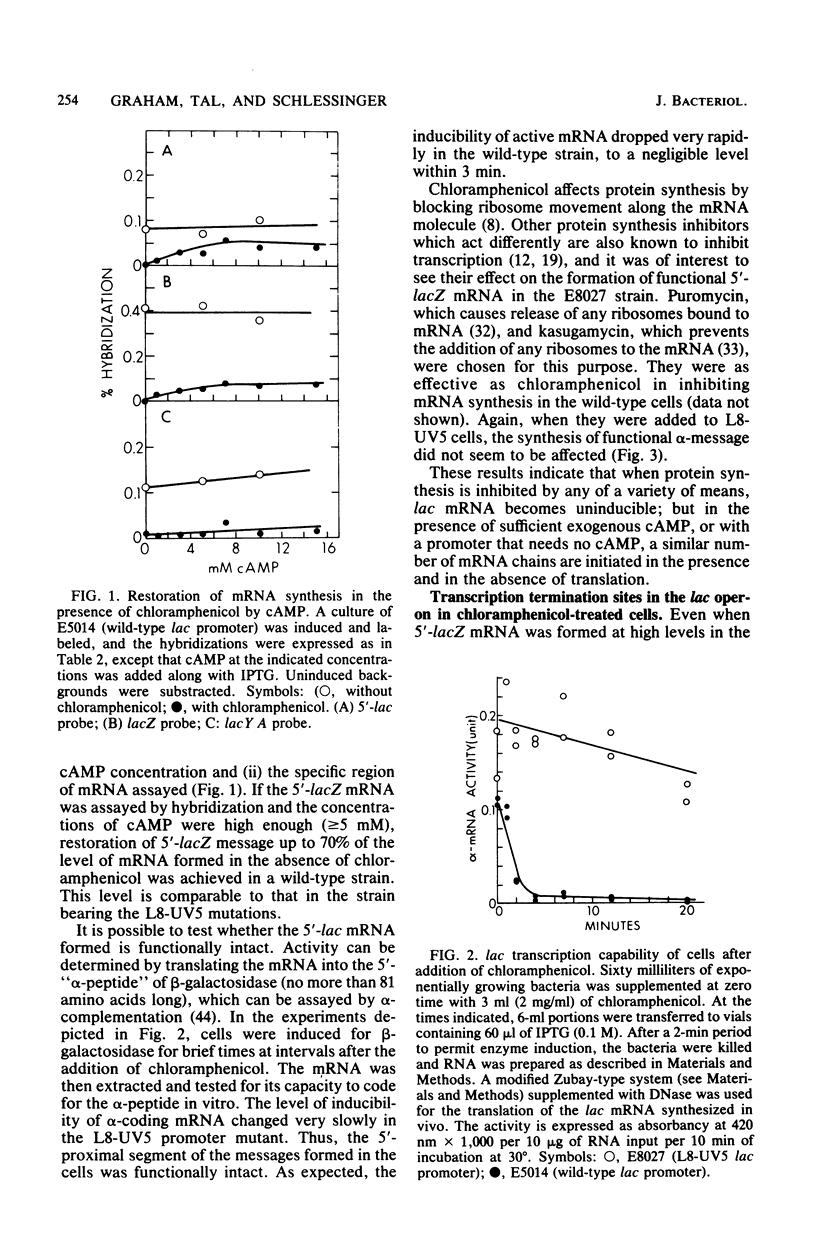
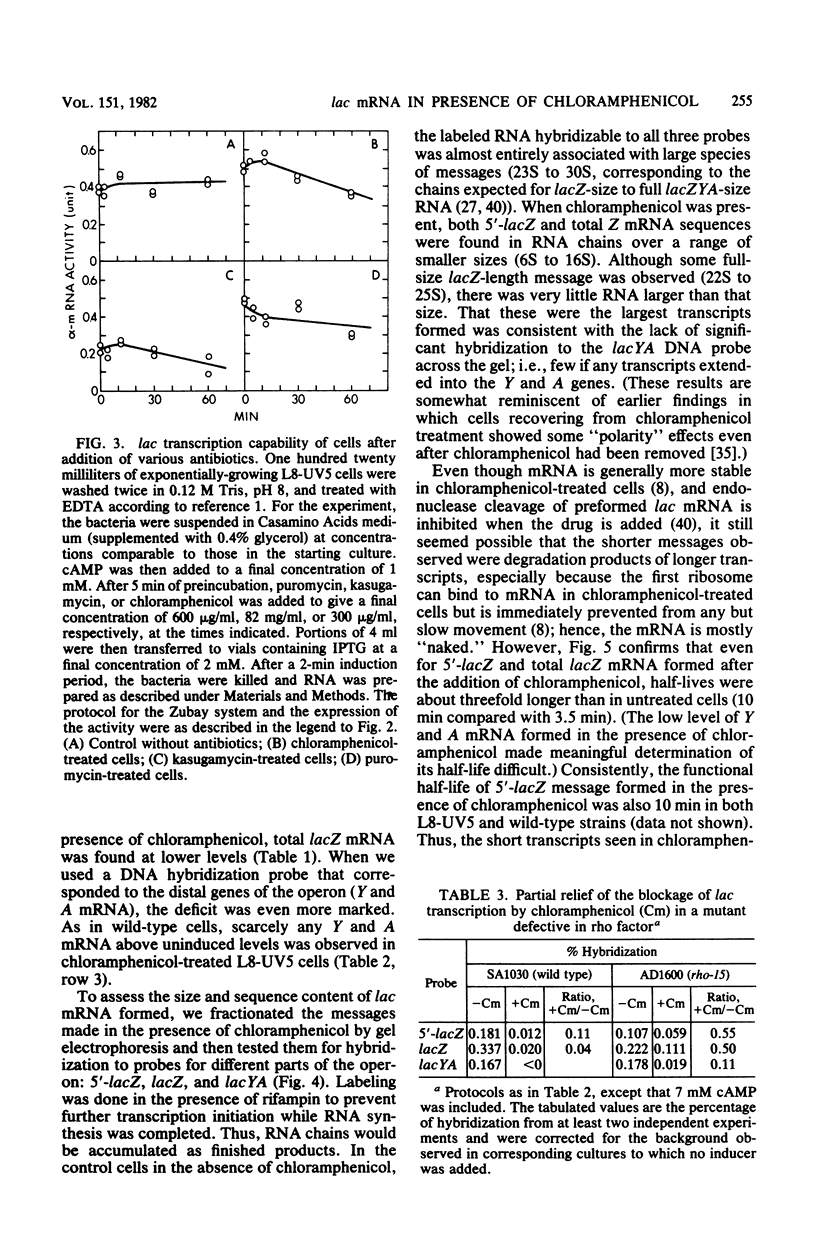
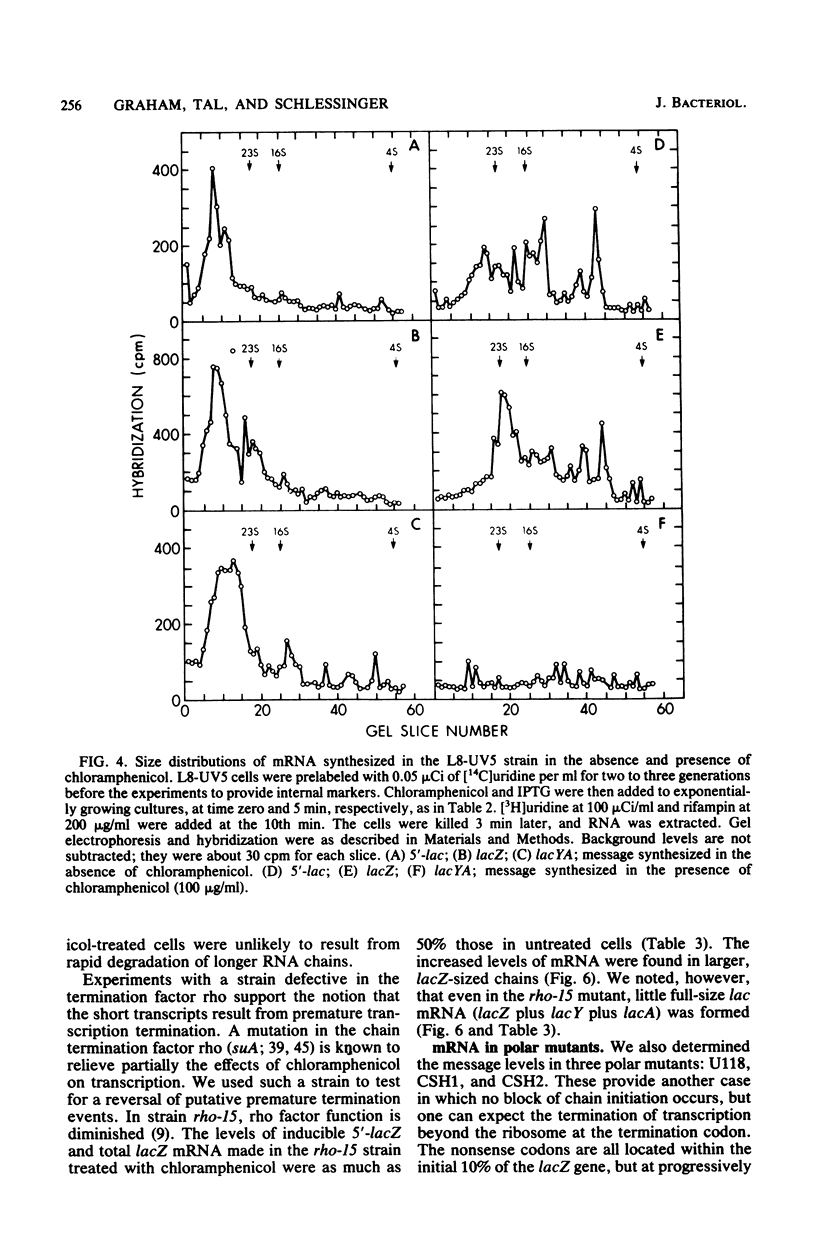
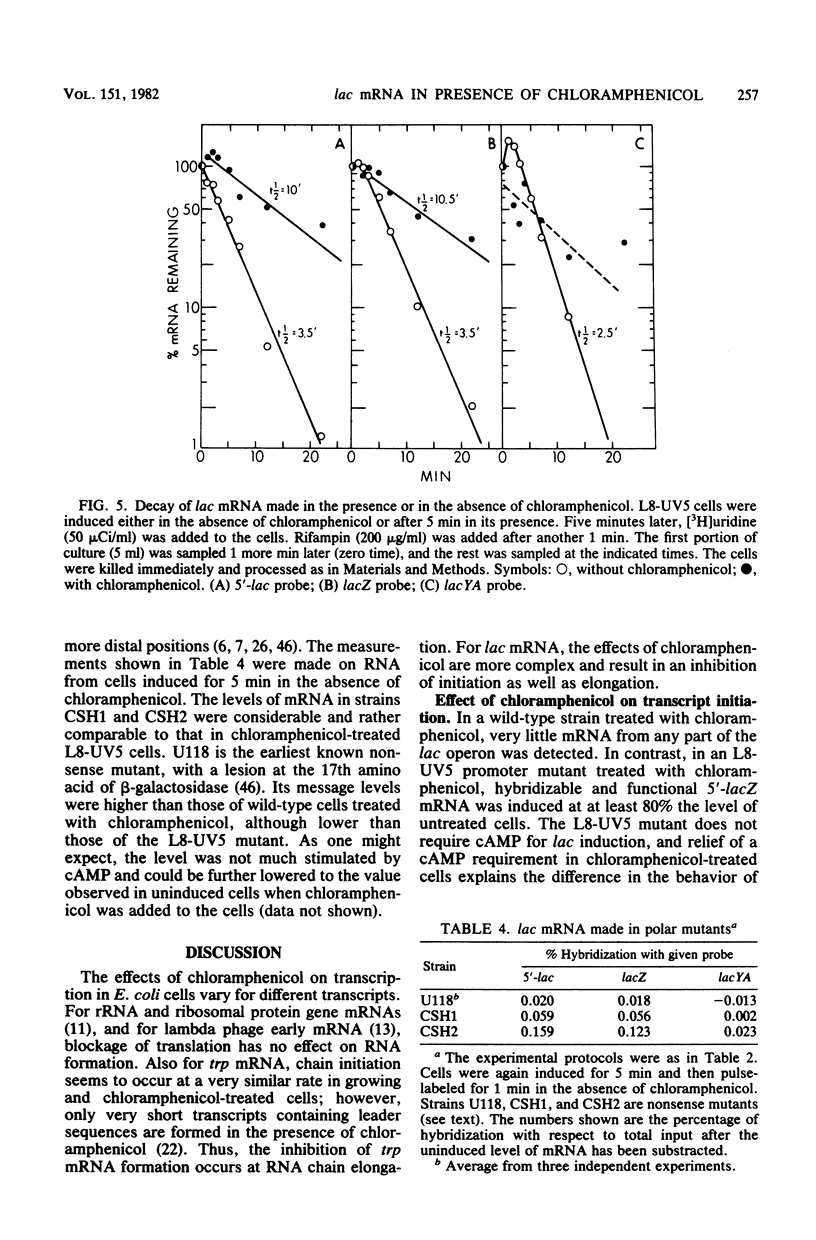
When protein synthesis was blocked by chloramphenicol in vivo, transcription initiation of lac mRNA was severely inhibited. In a promoter mutant (L8-UV5) or in wild-type cells supplemented with adenosine 3',5'-phosphate (greater than or equal to 5 mM), nearly normal initiation could be achieved, and when the mRNA chains formed were extracted, they coded for the 5'-terminal alpha-peptide of the lacZ gene in vitro. However, even under such conditions, only a fraction of RNA polymerases proceeded to the end of the Z gene in the presence of chloramphenicol; as a consequence, a wide range of sizes of mRNA was produced, and very few transcripts were formed all the way to the natural termination site of the operon. In other words, premature transcription termination occurred in chloramphenicol-treated cells, as current models predict, but terminations occurred to variable extents at several intragenic sites and apparently at least one intergenic site. Termination at intragenic sites occurred far less in cells bearing a mutation in the transcription termination factor rho.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D., Kennell D. Metabolism of messenger RNA from the gal operon of Escherichia coli. J Mol Biol. 1974 Dec 15;90(3):581–599. doi: 10.1016/0022-2836(74)90236-8. [DOI] [PubMed] [Google Scholar]
- Adhya S., Gottesman M. Control of transcription termination. Annu Rev Biochem. 1978;47:967–996. doi: 10.1146/annurev.bi.47.070178.004535. [DOI] [PubMed] [Google Scholar]
- Arditti R., Grodzicker T., Beckwith J. Cyclic adenosine monophosphate-independent mutants of the lactose operon of Escherichia coli. J Bacteriol. 1973 May;114(2):652–655. doi: 10.1128/jb.114.2.652-655.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Cohen T., Silberstein A., Kuhn J., Tal M. Relief of polarity in E. coli depleted of 30S ribosomal subunits. Mol Gen Genet. 1979 Jun 7;173(2):127–134. doi: 10.1007/BF00330302. [DOI] [PubMed] [Google Scholar]
- Cremer K., Silengo L., Schlessinger D. Polypeptide formation and polyribosomes in Escherichia coli treated with chloramphenicol. J Bacteriol. 1974 May;118(2):582–589. doi: 10.1128/jb.118.2.582-589.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crombrugghe B., Adhya S., Gottesman M., Pastan I. Effect of Rho on transcription of bacterial operons. Nat New Biol. 1973 Feb 28;241(113):260–264. doi: 10.1038/newbio241260a0. [DOI] [PubMed] [Google Scholar]
- Dennis P. P. Effects of chloramphenicol on the transcriptional activities of ribosomal RNA and ribosomal protein genes in Escherichia coli. J Mol Biol. 1976 Dec 15;108(3):535–546. doi: 10.1016/s0022-2836(76)80135-0. [DOI] [PubMed] [Google Scholar]
- Dütting D., Hübner L. The effect of antibiotics on the in vivo synthesis of messenger ribonucleic acid from the lactose operon of Escherichia coli. Mol Gen Genet. 1972;116(3):277–290. doi: 10.1007/BF00269771. [DOI] [PubMed] [Google Scholar]
- Friedman D. I., Schauer A. T., Baumann M. R., Baron L. S., Adhya S. L. Evidence that ribosomal protein S10 participates in control of transcription termination. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1115–1118. doi: 10.1073/pnas.78.2.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J., Margason G., Finch B. On the turnover of ppGpp in Escherichia coli. J Biol Chem. 1972 Oct 10;247(19):6055–6058. [PubMed] [Google Scholar]
- Greenblatt J., Li J., Adhya S., Friedman D. I., Baron L. S., Redfield B., Kung H. F., Weissbach H. L factor that is required for beta-galactosidase synthesis is the nusA gene product involved in transcription termination. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1991–1994. doi: 10.1073/pnas.77.4.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Yanofsky C. Hyper-labile messenger RNA in polar mutants of the tryptophan operon of Escherichia coli. J Mol Biol. 1972 Dec 14;72(1):103–110. doi: 10.1016/0022-2836(72)90072-1. [DOI] [PubMed] [Google Scholar]
- Hirschel B. J., Shen V., Schlessinger D. Lactose operon transcription from wild-type and L8-UV5 lac promoters in Escherichia coli treated with chloramphenicol. J Bacteriol. 1980 Sep;143(3):1534–1537. doi: 10.1128/jb.143.3.1534-1537.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto F. Diversity of regulation of genetic transcription. I. Effect of antibiotics which inhibit the process of translation on RNA metabolism in Escherichia coli. J Mol Biol. 1973 Feb 25;74(2):113–136. doi: 10.1016/0022-2836(73)90102-2. [DOI] [PubMed] [Google Scholar]
- Jacobs K. A., Schlessinger D. Escherichia coli DNA-directed beta-galactosidase synthesis in presence and absence of Ca2+. Biochemistry. 1977 Mar 8;16(5):914–920. doi: 10.1021/bi00624a016. [DOI] [PubMed] [Google Scholar]
- Jacobs K. A., Shen V., Schlessinger D. Coupling of lac mRNA transcription to translation in Escherichia coli cell extracts. Proc Natl Acad Sci U S A. 1978 Jan;75(1):158–161. doi: 10.1073/pnas.75.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano Y., Kuwano M., Imamoto F. Initial trp operon sequence in Escherichia coli is transcribed without coupling to translation. Mol Gen Genet. 1976 Jul 23;146(2):179–188. doi: 10.1007/BF00268086. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Yanofsky C. Polarity suppressors increase expression of the wild-type tryptophan operon of Escherichia coli. J Mol Biol. 1976 May 15;103(2):395–409. doi: 10.1016/0022-2836(76)90319-3. [DOI] [PubMed] [Google Scholar]
- Kung H. F., Weissbach H. Further characterization of L factor, a protein required for beta-galactosidase synthesis. Arch Biochem Biophys. 1980 May;201(2):544–550. doi: 10.1016/0003-9861(80)90543-3. [DOI] [PubMed] [Google Scholar]
- Kung H., Tainsky M., Weissbach H. Regulation of the in vitro synthesis of the alpha-peptide of beta-galactosidase directed by a restriction fragment of the lactose operon. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1000–1010. doi: 10.1016/0006-291x(78)91450-x. [DOI] [PubMed] [Google Scholar]
- Langley K. E., Villarejo M. R., Fowler A. V., Zamenhof P. J., Zabin I. Molecular basis of beta-galactosidase alpha-complementation. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1254–1257. doi: 10.1073/pnas.72.4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L. W., Kennell D. Models for decay of Escherichia coli lac messenger RNA and evidence for inactivating cleavages between its messages. J Mol Biol. 1979 Dec 5;135(2):369–390. doi: 10.1016/0022-2836(79)90442-x. [DOI] [PubMed] [Google Scholar]
- Mackie G., Wilson D. B. Polarity and transcription in the galactose operon of E. coli. Biochem Biophys Res Commun. 1972 Jul 11;48(1):226–234. doi: 10.1016/0006-291x(72)90367-1. [DOI] [PubMed] [Google Scholar]
- Morse D. E. Polarity induced by chloramphenicol and relief by suA. J Mol Biol. 1971 Jan 14;55(1):113–118. doi: 10.1016/0022-2836(71)90285-3. [DOI] [PubMed] [Google Scholar]
- NATHANS D. PUROMYCIN INHIBITION OF PROTEIN SYNTHESIS: INCORPORATION OF PUROMYCIN INTO PEPTIDE CHAINS. Proc Natl Acad Sci U S A. 1964 Apr;51:585–592. doi: 10.1073/pnas.51.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura H., Kano Y., Schlessinger D., Imamoto F., McPartland A., Somerville R. L. Translation-uncoupled transcription of promoter-proximal DNA sequences in E. coli strains harboring mutationally-generated constitutive promoters within genes of the trp operon. Mol Gen Genet. 1979 May 4;172(2):127–136. doi: 10.1007/BF00268273. [DOI] [PubMed] [Google Scholar]
- Okuyama A., Machiyama N., Kinoshita T., Tanaka N. Inhibition by kasugamycin of initiation complex formation on 30S ribosomes. Biochem Biophys Res Commun. 1971 Apr 2;43(1):196–199. doi: 10.1016/s0006-291x(71)80106-7. [DOI] [PubMed] [Google Scholar]
- Oxender D. L., Zurawski G., Yanofsky C. Attenuation in the Escherichia coli tryptophan operon: role of RNA secondary structure involving the tryptophan codon region. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5524–5528. doi: 10.1073/pnas.76.11.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastushok C., Kennell D. Residual polarity and transcription-translation coupling during recovery from chloramphenicol or fusidic acid. J Bacteriol. 1974 Feb;117(2):631–640. doi: 10.1128/jb.117.2.631-640.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P., Artz S. W. Positive control of lac operon expression in vitro by guanosine 5'-diphosphate 3'-diphosphate. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1726–1730. doi: 10.1073/pnas.76.4.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff P. In vivo role of the relA+ gene in regulation of the lac operon. J Bacteriol. 1981 Jan;145(1):410–416. doi: 10.1128/jb.145.1.410-416.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson J. P., Grimley C., Lowery C. Transcription termination factor rho activity is altered in Escherichia coli with suA gene mutations. Proc Natl Acad Sci U S A. 1975 May;72(5):1725–1728. doi: 10.1073/pnas.72.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Blundell M., Kennell D. Translation and mRNA decay. Mol Gen Genet. 1978 Apr 6;160(2):121–129. doi: 10.1007/BF00267473. [DOI] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann A., Joseph E., Danchin A. Cyclic AMP as a modulator of polarity in polycistronic transcriptional units. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3194–3197. doi: 10.1073/pnas.76.7.3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in antibiotic-treated E. coli. Nat New Biol. 1971 Mar 10;230(10):41–44. doi: 10.1038/newbio230041a0. [DOI] [PubMed] [Google Scholar]
- Welply J. K., Fowler A. V., Beckwith J. R., Zabin I. Positions of early nonsense and deletion mutations in lacZ. J Bacteriol. 1980 May;142(2):732–734. doi: 10.1128/jb.142.2.732-734.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. L., Zubay G., Urm E., Heiness G., Cashel M. Effects of guanosine tetraphosphate, guanosine pentaphosphate, and beta-gamma methylenyl-guanosine pentaphosphate on gene expression of Escherichia coli in vitro. Proc Natl Acad Sci U S A. 1974 Jan;71(1):63–67. doi: 10.1073/pnas.71.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser D., Zabell S., Rothman J., Grodzicker T., Wenk M. Fine structure of the gradient of polarity in the z gene of the lac operon of Escherichia coli. J Mol Biol. 1970 Apr 14;49(1):251–254. doi: 10.1016/0022-2836(70)90392-x. [DOI] [PubMed] [Google Scholar]


