Abstract
In a recent study, we found that newly isolated clones of NIH 3T3 mouse cells undergo neoplastic transformation more readily than uncloned cultures from which they were derived. After eleven low-density passages (LDPs), most of the 29 clones produced lightly stained early-stage transformed foci when grown to confluence in a primary assay for transformation, and one of them consistently produced a few tiny dense foci. In the present work, six of the clones were kept in LDPs for 56 passages and assayed for focus formation at confluence at six passage levels. The clone that produced tiny dense foci switched to light foci during the LDPs, four others produced light foci at different passage levels, and one progressed from light to dense foci after the last passage. By contrast, all the clones progressed to dense focus formation in five or fewer serial repetitions of the assay at confluence. Because all but one of the clones underwent about half as many total divisions at each LDP as they did when grown to the stationary state at confluence, the latter is more efficient in eliciting progression than the exponential growth of the LDPs. Extension of the period at confluence of uncloned cultures results in the appearance of dense foci within light foci. Because the latter are localized clonal populations, the intrafocal progression reinforces the conclusion that clonal expansion favors transformation. We discuss the significance of these results for the clonal origin of human cancer and the increased incidence of cancer with age.
Keywords: clonal selection, carcinogenesis, aging and cancer
It is well established that most mammalian cancers develop progressively through stages of increasing malignancy before the host reaches a terminal state of illness (1). It is also generally accepted that progression involves a series of genetic changes in preneoplastic and neoplastic cells accompanied by selective growth of the cells best suited to multiply in the ambient microenvironment of the developing tumor (2). The concept of progression developed from studies of tumor development in experimental animals (1) where there was little opportunity for analysis of the process at the cellular level. However, one of the most thorough analyses of progression comes from a large-scale study of human melanoma, beginning with the easily detected common mole or melanocytic nevus (3) and proceeding by stages to the relatively rare and fatal metastatic melanoma (4). The sequence of radial and vertical expansion that characterizes melanoma progression resembles the progression of light monolayered foci to dense multilayered foci in cultures of NIH 3T3 mouse cells (5), which follows Foulds’ rules of progressive tumor development (1). The cell culture studies were made with heterogeneous uncloned cultures, which generated multiple transformations (6) and therefore differed from naturally occurring tumors that are clonal in origin (7, 8). Further work on “spontaneous” transformation with large numbers of clones indicated that selection of spontaneously occurring variants under the growth constraint of contact inhibition at confluence plays a significant role in progression to more advanced neoplastic states (6). It also showed that cellular interactions among polyclonal subpopulations of uncloned cultures tend to suppress progression of the neoplastic phenotype. The large-scale clonal studies were pursued for a limited period of time, and the dynamics of transformation were not described for individual clones, so a definitive picture of long-term progression in identified clonal populations was not obtained. In the present work, 6 of the 29 clones from the transformation-sensitive original stock of NIH 3T3 cells (6, 9) were passaged at low density in high-serum concentration that minimizes transformation in uncloned cultures (10, 11). After varying numbers of these low-density passages (LDPs) up to a maximum of 56, aliquots of the cells from each clone were assayed at confluence for transformed focus formation and saturation density. Growth curves of low-density subcultures from the confluent cultures were used to determine the relation of the transformed properties to growth rates. The large number of LDPs followed at different passage levels by serial assays at confluence showed great diversity among the clones in their transformation behavior as well as changes in that behavior at different passage levels. Although it was established that the genetic events underlying the early stages of transformation that yield light foci at confluence do occur during exponential growth of the LDPs, progression to dense focus formation occurs with regularity only during serial rounds of growth constraint at confluence. When combined with our previous finding of a much greater rate of transformation in clones than in their polyclonal parental populations, the results indicate that progression occurs at confluence mainly in early-stage, light-staining foci, which are localized, genetically altered subclonal populations within each clone. The confluent state not only allows for the selective localized growth of the transformed cells, but adds heritable damage to the cells, which must also contribute to progressive neoplastic development. We discuss the significance of clonal expansion and destabilization for tumor progression in humans.
MATERIALS AND METHODS
Cells and Culture Methods.
The original line of NIH 3T3 cells (9) used as the source of transformable clones was received from the National Cancer Institute, Bethesda, MD, in 1988, passaged once, and frozen in liquid nitrogen. The cells, designated SA′, were thawed in April 1998, cultured for 2 days, and distributed as single cells in 96-well microtiter plates for cloning as previously described (6). Although 29 clones were used for relatively short-term studies of their transformability, six of them were chosen to represent a range of growth rates and were used for the more detailed long-term studies of individual clones described here. A subline of the original SA′ cells was passaged at low density 146 times and became relatively refractory to “spontaneous” transformation by our standard technique of serial 2-wk assays at high population density in a low concentration (2%) of calf serum (CS) (12). These cells were designated the A′ subline and were used in the experiments to provide a confluent monolayered background for the expression of transformed foci by small numbers of the SA′ cells when the number of transformed cells was too high to produce nonoverlapping foci in direct assay of the SA′ cells by themselves. The procedures used here were described in a survey of transformation among the 29 clones (6), but there are some aspects of the methodology that merit emphasis here. Reference to assay at confluence means the seeding of 105 cells in 21-cm2 culture dishes in growth medium with 2% CS in which they grew to confluence in 4 to 5 days and were kept with medium changes every 3 or 4 days for a total of 14 days. Some of the cultures were then fixed in Bouin’s reagent and stained with Giemsa, this constituting a primary (1°) assay. At the same time, sister cultures were used for a 2° serial assay that replicates the 1° assay, and this procedure was repeated up to five serial assays (3°, 4°, 5°). The purpose of the serial assays is to provide a dynamic picture of the progressive transformation. Because the foci are initiated by proliferation of single cells, any increase in the cell population density or size of foci from one serial assay to the next is an indication of their increased capacity to multiply when surrounded by nontransformed cells and therefore of neoplastic progression. In a 1° assay of the uncloned parental culture, the assay period was extended to observe the origin of dense foci within light foci.
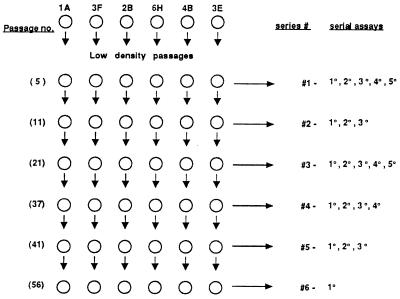
The transformation assays at confluence in 2% CS described above are to be distinguished from serial passages at low population density in the high concentration (10%) of CS used in routine maintenance of the cells that were designed to minimize transformation. The standard LDPs were usually made every 4 days by seeding 5 × 103 cells in 21-cm2 dishes. These cells never reach confluence and are kept in a continuous state of exponential multiplication in 10% CS, reaching 3.0–4.5 × 105 cells per dish in 4 days, except for clone 3E with an average of 1.8 × 105 cells in 4 days (13). The 1° assays at confluence were initiated with aliquots of the cells from the LDP after 5, 11, 21, 37, 41, and 56 of the passages; the various series of focus assays at confluence after increasing numbers of passages are referred to as the first, second, … , sixth series of assays (see Fig. 1). The numbers of serial focus assays at confluence initiated immediately after each of these six passage points were, respectively, 5, 3, 5, 4, 3, and 1. The series and assay number are represented as 1–1°, 1–2°… 2–1°, 2–2°, … , etc. The defined medium used throughout in combination with CS was MCDB 402 (molecular, cellular, and developmental biology medium 402) (14).
Figure 1.
Flow chart of low-density passages and serial assays at confluence. The procedure is described in Materials and Methods.
RESULTS
Clonal Transformation During Exponential Proliferation of Cells in LDP.
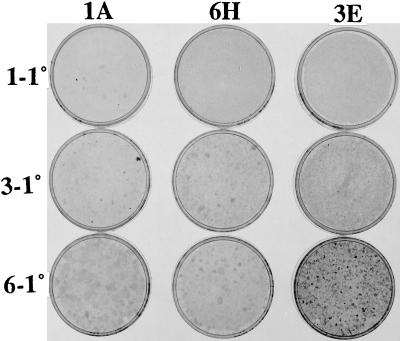
Common experience with uncloned cultures of NIH 3T3 cells is that the capacity to produce transformed foci at confluence decreases over an extended period of frequent passages at low population density (10, 11). Several serial assays at confluence are then required to restore the capacity for focus formation, and sometimes even that does not succeed, as in the case of the A′ subline (6). There is evidence, however, that clones do undergo early stages of transformation during LDP of clones of the SA′ subline, as indicated by the formation of light broad foci in many clones at confluence after they have been passaged at low density more than 10 times (6). To investigate this point further, the six SA′ clones of the present study were assayed for focus formation after various numbers of LDPs up to the 56th passage. The 1A clone that was already producing a few very small dense foci in 1° assays at confluence after five LDPs continued to do so after 11 and 21 LDPs but switched to light broad foci in 1° assays after 37, 41, and 56 passages (Table 1, Fig. 2). Four of the other clones produced no foci in 1° assay after five LDPs but did so after the 11th and all subsequent LDPs tested, as typified in the 1° assays of clone 6H (Fig. 2). The 3E clone actually increased the density of individual transformed foci in 1° assay after the 56th LDP, producing hundreds of small light foci and 20 small moderately dense foci (Fig. 2). By contrast, the 4B clone produced no foci in 1° assays up to and including the 41st LDP but produced a dozen tiny light foci and one broad moderate focus after the 56th passage (Table 1). These results show that early stages of transformation are initiated during LDP. They also indicate that there is variation from clone to clone in the degree and direction of transformation at low density with some clones remaining light focus formers, others regressing from dense to light focus formation, and a minority progressing, but only rarely, to small moderately dense focus formation.
Table 1.
Focus formation by clones in 1° assays of cells from LDPs
| Clone | Series no. (no. passages)
|
|||||
|---|---|---|---|---|---|---|
| 1 (5) | 2 (11) | 3 (21) | 4 (37) | 5 (41) | 6 (56) | |
| 1A | 4*–7 | 10*–0 | 11*–1 | 0–6 M | 0–41 M | 0–130 |
| 3F | 0–0 | 0–3 | 0–5 | 0–17 M | 1*–15 M | 0–130 |
| 2B | 0–0 | 0–8 | 0–2 | 0–4 M | 0–59 M | 0–3 |
| 6H | 0–0 | 0–17 | 0–10 M | 0–3 M | 0–46 | 0–44 |
| 4B | 0–0 | 0–0 | 0–0 | 0–0 | 0–0 | 0–14B |
| 3E | 0–0 | 0–0 M | 3*–2 | 0–17 M | 0–2 G | 20**–TNTC |
| A′† | ND | ND | ND | 0–0 | 0–0 | ND |
The first number of each pair denotes dense foci (≥1 mm unless noted with ∗), the second number denotes light foci. 1° assays on the uncloned A′ cultures were done only in parallel with the fourth and fifth series and were negative for dense and light foci. See photograph in Fig. 2. ∗, ≪1 mm; ∗∗, <1 mm; M mottled; G, grainy; B, one broad moderate focus; TNTC, too numerous to count; ND, not done.
, Uncloned parental culture.
Figure 2.
Focus formation by clones in 1° assays of cells from low-density passages. The six clones were passaged 56 times at low density and were used for a 1° assay of 105 cells at various passage levels. Selected assays from three of the clones are shown here to illustrate the diverse clone-specific dynamics of transformation during the low-density passages. The clones are listed at the top of each column. The series numbers precede the 1° assay designation on the left. The number of LDPs of each series were first series, 5; third series, 21; sixth series, 56. Note the tiny dense foci of clone 1A assays 1–1° and 3–1°, plus a larger dense focus in 3–1°. There are light foci in clone 6H assays 3–1° and 6–1°, and in 3E assay 3–1°. There are many light foci in clone 3E assay 6–1°, plus small dense foci. See Table 1.
Progression of Transformation in Serial Assays at Confluence.
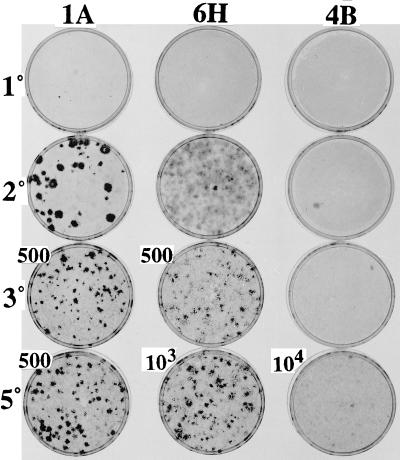
The capacity for progressively increasing degrees of transformation was studied in each of the clones by serial assays at confluence after varying numbers of LDPs. The pattern of progressive transformation differed from clone to clone as illustrated in Table 2 and Fig. 3. Clone 1A, which produced a few small dense foci in 1° assay, produced a number of very large dense foci in a 2° assay. The latter presumably arose from the cells constituting the small dense foci of the 1° assay that had apparently undergone transformation during the later stages of that assay. From the 3° assay onward of clone 1A, there were so many transformed cells that they had to be diluted and seeded with a large excess (105) of nontransformed A′ cells to obtain discrete foci. The A′ cells then multiplied to form a confluent monolayered background that markedly reduced the foci to a much smaller size than those seen in the 2° assay of 105 cells of the 1A clone, with no added A′ cells. The 6H clone, which produced no foci in the 1° assay, displayed a large number of broad light foci in the 2° assay, along with a few dense foci of varying size (Fig. 3). Subsequent assays of the 6H clone with an excess of A′ cells revealed increasing numbers of dense foci and increasing density of the cell populations within the individual foci. The 4B clone, which was the last to produce foci in the 1° assays after varying numbers of LDPs, was the last to produce foci in the serial assays (Table 2). Only single light foci appeared in the 2° and 3° assays of clone 4B, but there was a large number of the light foci in the later assays (Table 2 and Fig. 3). Clone 3E produced a small number of small dense foci in the 2° assay at confluence, which increased progressively in number through the 5° assay, but did not increase in size until the 5° assay (Table 2). Each of the two remaining clones, 3F and 2B, exhibited individualized patterns of increase in number (Table 2), size, and density (not shown) of foci in serial assays. The results show not only that focus formation differs from clone to clone, but that it is subject to change during LDPs within individual clones as exhibited in the serial assays after different numbers of LDP.
Table 2.
Focus formation by clones in serial assays at confluence
| Clone | Focus formers/105 cells
|
||||
|---|---|---|---|---|---|
| 1° | 2° | 3° | 4° | 5° | |
| 1A | 4*–7 | 43–0 | 18,100–0 | 4,800–0 | 17,600–1.200 |
| 3F | 0–0 | 0–116 | 60–56 | 22–300 | 6,000–6,000 |
| 2B | 0–0 | 1–125 | 0–80 | 6–250 M | 200–4,000 |
| 6H | 0–0 | 0–TNTC | 15,000–0 | 4,000–0 | 10,000–0 |
| 4B | 0–0 | 0–1 | 0–1 | 0–200 | 1–TNTC |
| 3E | 0–0 | 11*–4 | 1,450*–0 | 8,500*–0 | 11,000–0 |
| A′† | 0–0 | 0–0 | 0–0 | 0–0 | ND |
The cells used in these serial assays of the clones were from the first series of such assays that were initiated after five low-density passages. The numbers of foci are all normalized to 105 cells, but where they exceed 250, they are extrapolated from dilutions of the cells mixed with a standard passage of nontransformed A′ to form a confluent background. The first number of each pair denotes dense foci (≥1 mm unless noted with ∗); the second number denotes light foci. See photograph in Fig. 3. ∗, ≪1 mm; M, mottled; TNTC, too numerous to count.
The serial assays for the uncloned A′ cells are taken from the fourth series.
Figure 3.
Progressive transformation of clones in serial assays at confluence after five LDPs (first series). The three clones shown here illustrate the variable extent of transformation and progression in serial assays at confluence initiated after five LDPs (first series). The designations on the left side of each row (1°, 2°, 3°, 5°) represent the serial assay. The numbers (500, 103, 104) next to the particular dishes refer to the number of clonal cells seeded together with 105 A′ cells. Where no numbers are shown, 105 cells from the clone were assayed with no A′ cells. See Table 2.
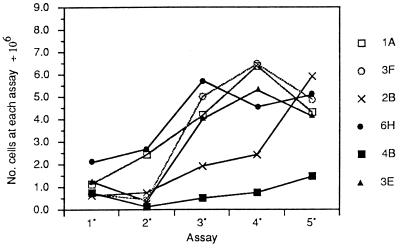
The dynamics of transformation in serial assays of the clones at confluence were also illustrated by the increases in saturation density of assays of 105 cells. These maxima of population density are shown for the first series in Fig. 4. In the first series, clone 1A exhibited a continuous increase in saturation density from the 1° assay and peaked at a high level in the 4° assay. Clone 6H started at a relatively high level, rose slightly in the 2° assay, and precipitously to a maximum in the 3° assay. Two other clones, 3F and 3E, reduced their saturation density in the 2° assay but rose rapidly thereafter, whereas clone 2B rose gradually to the 4° assay, then steeply to the 5° assay. The saturation density of clone 4B rose slowly from the 2° assay onward but reached a maximum that was less than one-fourth that of the other clones. Although the saturation densities corresponded in a general way to the number, size, and density of the foci seen in the photograph of Fig. 3, the foci were seen in large numbers before there were significant increases in saturation density. The results in the third series of serial assays were basically the same as those of the first series, except that clone 4B showed a sharp rise in the 5° assay, which was associated with the appearance of many dense focus formers (not shown).
Figure 4.
Saturation densities of the clones from serial assays at confluence of the first series begun after five LDPs.
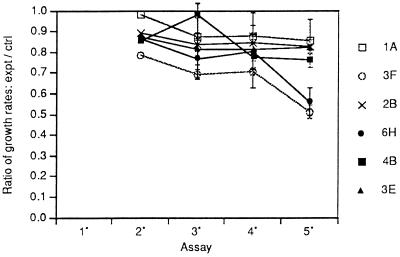
We have found a significant tendency of uncloned cultures to develop a persistent reduction in growth rate at low densities after recovery from the contact inhibition of repeated rounds of confluence (15). This reduction usually accompanies an increase in capacity to overgrow confluent monolayers and thereby to form foci. However, only two of the clones (3F and 6H) from the first series of assays exhibited a continuing decrease in growth rate at low densities with an increase in the number of serial assays at confluence (Fig. 5). The remaining four clones showed some reduction in relative growth rate after the first few assays, but that rate remained relatively constant thereafter. A similar pattern was seen in the third series of assays with the exception of clone 4B, which joined 3F and 6H in a continuous reduction in growth rate (not shown). This may be related to the appearance of over 500 dense foci in the 5° assay of clone 4B in the third series (not shown).
Figure 5.
Reductions in growth rates of the clones after recovery from serial assays at confluence of the first series. Beginning with the 2° assay at confluence, cells from each of the confluent assays were passaged once at low density for 2 days to recover from the inhibitory effects of crowding and passaged once again to determine growth rates. At the same time, the growth rate of the standard passage cells of each clone was determined, and the ratio of growth rate (PD/D) of the postconfluent passage to that of the standard passage of the clone at low density is shown.
The Origin of Dense Foci Within Light Foci in an Extended 1° Assay of the Uncloned Parental Population.
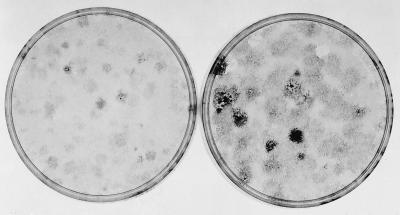
Although dense foci do not appear in the standard 14-day assay of the uncloned parental culture of the clones, they do appear if the assay is extended. When 105 freshly thawed uncloned cells were cultured for 3 days and then assayed for focus formation, a number of light foci appeared at 14 days, and a few densely stained cells were present in the center of some of the light foci (Fig. 6). Therefore, the assay was continued for another 7 days in sister dishes. Dense foci could then be detected forming within the boundaries of the light foci. This was usually followed by retraction in the center of the light foci caused apparently by the decreased adhesiveness of the dense focus-forming cells. The retraction in the midst of progression contrasts with the uniformity of the dense foci initiated by cells that had already progressed before seeding in later serial assays, as in Fig. 3.
Figure 6.
The appearance of dense foci within light foci in an extended 1° assay of the original uncloned SA′ cells. The original uncloned SA′ cells were thawed, and 105 cells were cultured for 3 days in 10% CS before subculture of 105 cells for a 1° assay in 2% CS. A culture was fixed and stained at 14 days, when light foci were apparent, with occasional more densely stained cells in the center of a few foci. Other cultures were fixed and stained at 21 days when there was grossly visible evidence that denser foci were forming within the light ones. (Left) 14 days; (Right) 21 days.
DISCUSSION
Although the NIH 3T3 cells used in the present study had been cloned five times, they had also been passaged 120 times (9) before we received them. Given the high rate of chromosome variation in established cell lines in general (16) and in NIH 3T3 cells in particular (17), it would be surprising if heterogeneous subpopulations did not appear in the cultures. It is known that clonal populations of cells initially generate homogeneous subclones, but variants quickly appear in serial passages that restore heterogeneity to a level comparable to that of the population from which the clones originally arose (18). It is not surprising, therefore, that our parental NIH 3T3 culture would give rise to clones that varied widely in their competence to undergo transformation. Clone 1A produced tiny dense foci in 1° assay at confluence carried out after 5, 11, and 21 LDPs, whereas clone 4B failed to produce any foci in 1° assay until the 56th LDP, and the other clones exhibited intermediate behavior during LDPs. These consistent differences in focus formation in 1° assay were reflected in the serial assays at confluence in which clone 1A produced the largest number of dense foci and clone 4B, the smallest number of dense foci in serial assays (Table 2). The consistency of the rankings in both categories showed that the competence for transformation is a heritable characteristic that is expressed both in serial LDPs and in serial assays at confluence. There was, however, a drift in these properties that was most apparent in clone 1A, which lost the capacity to produce the tiny dense foci when tested in 1° assay after LDPs 37, 41, and 56.
Until recently, we held that the genetic changes underlying transformation occurred almost exclusively when the cells were kept under contact inhibition at confluence (12). Then we discovered that freshly cloned populations were more susceptible to transformation than their uncloned parents and observed that the clones gained the capacity to produce light foci in 1° assays at confluence after they had undergone a minimum of five LDPs (ref. 6 and Table 1). There was no progression to dense focus formation by five of the six clones in any 1° assay done at any LDP level; in fact, the 1A clone regressed from tiny dense to light focus formation, presumably by selection against the more transformation-prone cells in LDPs. The sole exception to the failure to progress during LDPs was clone 3E, which produced small moderately dense foci in 1° assay after the 56th LDP.
The infrequency of progression during LDP stands in sharp contrast to progression in serial assays at confluence. All of the clones, except 4B, progressed to the production of many dense foci in the serial assays, and even 4B produced over 500 dense foci in the 5° assay of the third series (not shown). Yet all the clones except 3E grew to about half as high a density at each LDP, in which they were kept in continuous exponential growth in the relatively high (10%) serum, as they did in the assays where they reached saturation density in 2% serum by 5–6 days and showed no net growth for the remaining 8–9 days of the assay. So, in spite of their going through about half as many cell divisions per culture at each LDP as in each preprogression assay at confluence, only one clone progressed to dense focus formation by the 56th LDP, whereas five of six of them did so by the fourth serial assay at confluence. The remaining clone (4B) progressed in the 5° assay. The capacity to produce dense foci does not commence until a few days after a naive culture of NIH 3T3 cells has reached maximum density (12, 19). In a critical test of the role of stasis at confluence in progression to dense focus formation, it was shown that cells seeded in dishes of small area and quickly grown to confluence transform sooner than cells seeded in dishes of much greater area that reached confluence later with three times as many cells (20). Unlike conventional point mutations that are maximized during exponential growth and DNA replication (21), progression is maximized when cells are subjected to the growth constraint of contact inhibition. The type of genetic change that occurs at confluence is suggested by the observation that inhibition of DNA synthesis by methotrexate or hydroxyurea results in chromosome aberrations (22, 23). Although a variety of genetic changes are induced in cells by mutagens, there is evidence that chromosome rearrangement is the rate-limiting step in carcinogenesis (24). A similar view was expressed about the origin of human cancer (25). In addition to driving neoplastic progression, long-term constraint of growth at confluence also causes heritable reduction in growth rate of cells (15). This slow-growth phenotype is associated with chromosome rearrangements and large-scale deletions (26, 27). Further indication that cells are genetically damaged in prolonged confluence is the significant decrease in total cell number that is frequently seen after the cells have reached a maximum number (12, 20). It may therefore be no coincidence that progression to dense focus formation begins after cells have reached their maximum density, often when the cell number is declining (12, 20). It is interesting to note that the only clone that ultimately progressed in the LDPs to dense focus formation was clone 3E, which consistently had the lowest growth rate.
A potential source of genetic change in the long-term stationary confluent cultures is the accretion of secondary lysosomes and residual bodies that fill the cytoplasm at 2 weeks under standard assay conditions (36, 37). These contain nucleases and proteases that are released to the cytosol under stressful conditions (38). Fibroblasts subjected to anoxia increase the expression of an endonuclease associated with DNA breakage followed by genomic instability (39). Such induced genomic instability would explain the progressive neoplastic transformation initiated at confluence as well as the persistently reduced growth rate seen in subsequent low-density subcultures (15).
Our finding of the increase in transformation in freshly isolated clonal populations (6) introduced another element into the cause of progression at confluence. Each light focus that occurred in 1° assays after LDPs is a clone of cells that has some growth advantage over surrounding cells at confluence. As it expands laterally, it is forming a local colony increasingly isolated from moderating effects of surrounding cells and therefore more likely to undergo transformation, as seen in Fig. 6. It had earlier been proposed that transformation in C3H 10T1/2 mouse cells treated with a chemical carcinogen depended on the altered cells reaching a minimal colony size (28). This view was validated by a series of experiments with a population of C3H 10T1/2 cells, in which the proclivity for transformation had been initiated but not expressed (29). A mathematical model for this critical size factor was developed and found to agree with several earlier reports on transformation of the 10T1/2 cells. This model is in basic agreement with one developed for tumor development in vivo based on the requirement for a critical number of cells bearing the same or similar mutations (30). It should be noted, however, that dense focus formers can sometimes arise without going through the stage of light focus formation as they did in the case of the 1° assay of clone 1A in Fig. 2. The occasional appearance of advanced transformation without intermediate stages was described previously (5) and parallels observations made in experimental animals (1).
Studies on mammary carcinogenesis in rats lend further support to the model. Young Fischer 344 female rats have naturally occurring patches of mammary epithelial cells with a Ha-ras 1 mutation (31). These patches of mutated cells increase selectively in size as the rats mature, but the patches behave normally (32). However, they develop into mammary cancer if their growth is specifically promoted by treatment with the mutagen N-nitroso-N-methylurea (31). During this excess growth, the cells apparently accumulate further genetic change that results in malignancy.
The closest analogy in human cancer to the progression in neoplastic foci seen in the NIH 3T3 cells has been extensively described in the mole-to-melanoma sequence. Moles, also known as melanocytic nevi, occur naturally in most people, and the vast majority of the lesions are harmless. Rarely, hyperplasia with aberrant differentiation and dysplasia occur in a mole (3). Such moles may progress to a radial growth phase that resembles the lateral growth pattern of the thin lightly stained foci seen in 1° assays of the NIH 3T3 cells. The radial growth phase in vivo, which remains less than 1 mm in thickness, marks the onset of malignant melanoma. These thin radially extending lesions progress to a vertical growth phase in which the lesion becomes much thicker and is usually accompanied by metastasis (4). The vertical growth phase resembles the multilayered dense foci of the NIH 3T3 cells, which are highly tumorigenic in nude mice (33). The loose relationship of cells in the dense multilayered foci and the ease of their detachment represent features that are associated with the in vivo capacity to metastasize. It is plausible that the hyperplasia and radial growth of dysplastic nevi provide the critical mass that facilitates further genetic changes, including deletions and chromosome aberrations, which underlie progression to fully autonomous growth and malignancy (25, 34). The parallels of melanoma development with the cell culture model of progression described here recommend the model as an easily manipulated and quantitative tool to deepen our understanding of neoplastic development in humans and its sharply increased clinical manifestation in aging individuals (35).
Acknowledgments
We thank Professor Morgan Harris for his helpful comments and Dorothy M. Rubin for preparing the manuscript. The research was supported by grants from the Council for Tobacco Research and the Elsasser Family Fund.
ABBREVIATIONS
- 1°
2°, etc., primary, secondary, etc
- LDP(s)
low-density passage(s)
- CS
calf serum
References
- 1.Foulds L. Neoplastic Development, Vol. I. New York: Academic; 1969. [Google Scholar]
- 2.Nowell P. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 3.Clark W H, Jr, Elder D E, Guerry D, IV, Epstein M N, Greene M H, Van Horne M. Hum Pathol. 1984;15:1147–1165. doi: 10.1016/s0046-8177(84)80310-x. [DOI] [PubMed] [Google Scholar]
- 4.Clark W H, Elder D E, Van Horn M. Hum Pathol. 1986;17:443–450. doi: 10.1016/s0046-8177(86)80032-6. [DOI] [PubMed] [Google Scholar]
- 5.Rubin H. Proc Natl Acad Sci USA. 1994;91:6619–6623. doi: 10.1073/pnas.91.14.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chow M, Rubin H. Proc Natl Acad Sci USA. 1999;96:2093–2098. doi: 10.1073/pnas.96.5.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fialkow P J. Biochim Biophys Acta. 1976;458:283–321. doi: 10.1016/0304-419x(76)90003-2. [DOI] [PubMed] [Google Scholar]
- 8.Bedi G C, Westra W H, Gabrielson E, Koch W, Sidransky D. Cancer Res. 1996;56:2484–2487. [PubMed] [Google Scholar]
- 9.Jainchill J L, Aaronson S A, Todaro G J. J Virol. 1969;4:549–553. doi: 10.1128/jvi.4.5.549-553.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rubin H. Proc Natl Acad Sci USA. 1992;89:977–981. doi: 10.1073/pnas.89.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubin H. Proc Natl Acad Sci USA. 1993;90:10715–10719. doi: 10.1073/pnas.90.22.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubin H, Xu K. Proc Natl Acad Sci USA. 1989;86:1860–1864. doi: 10.1073/pnas.86.6.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow M, Koo J J, Ng P, Rubin H. Mutat Res. 1998;413:251–264. doi: 10.1016/s1383-5718(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 14.Shipley G D, Ham R G. In Vitro. 1981;17:656–670. doi: 10.1007/BF02628401. [DOI] [PubMed] [Google Scholar]
- 15.Rubin H, Yao A, Chow M. Proc Natl Acad Sci USA. 1995;92:4843–4847. doi: 10.1073/pnas.92.11.4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullen V R, Barnett C A. In Vitro. 1976;12:658–664. doi: 10.1007/BF02797467. [DOI] [PubMed] [Google Scholar]
- 17.Rubin H. Differentiation (Berlin) 1993;53:123–137. doi: 10.1111/j.1432-0436.1993.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 18.Poste G, Doll J, Fidler I J. Proc Natl Acad Sci USA. 1981;78:6226–6230. doi: 10.1073/pnas.78.10.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grundel R, Rubin H. Cancer Res. 1991;51:1003–1013. [PubMed] [Google Scholar]
- 20.Yao A, Rubin H. Proc Natl Acad Sci USA. 1994;91:7712–7716. doi: 10.1073/pnas.91.16.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stent G. Molecular Biology of Bacterial Viruses. San Francisco: Freeman; 1963. [Google Scholar]
- 22.Schimke R T. Mutat Res. 1992;276:145–149. doi: 10.1016/0165-1110(92)90004-s. [DOI] [PubMed] [Google Scholar]
- 23.Hahn P, Kapp L N, Morgan W F, Painter R B. Cancer Res. 1986;46:4607–4612. [PubMed] [Google Scholar]
- 24.Kinsella A R, Radman M. Proc Natl Acad Sci USA. 1980;77:3544–3547. doi: 10.1073/pnas.77.6.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cairns J. Nature (London) 1981;289:353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- 26.Hozier J, Sawyer J, Clive D, Moore M. Mutat Res. 1985;147:237–242. doi: 10.1016/0165-1161(85)90064-0. [DOI] [PubMed] [Google Scholar]
- 27.Xia F, Amundson S A, Nickoloff J A, Liber H L. Mol Cell Biol. 1994;14:5850–5857. doi: 10.1128/mcb.14.9.5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haber D A, Fox D A, Dynan W S, Thilly W G. Cancer Res. 1977;37:1644–1648. [PubMed] [Google Scholar]
- 29.Mordan L J, Martner J E, Bertram J S. Cancer Res. 1983;43:4062–4067. [PubMed] [Google Scholar]
- 30.Fisher J C, Hollomon J H. Cancer. 1951;4:916–918. doi: 10.1002/1097-0142(195109)4:5<916::aid-cncr2820040504>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 31.Cha R S, Thilly W G, Zarbl H. Proc Natl Acad Sci USA. 1994;91:3749–3753. doi: 10.1073/pnas.91.9.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cha R S, Guerra L, Thilly W G, Zarbl H. Carcinogenesis. 1996;17:2519–2524. doi: 10.1093/carcin/17.11.2519. [DOI] [PubMed] [Google Scholar]
- 33.Rubin A L, Arnstein P, Rubin H. Proc Natl Acad Sci USA. 1990;87:10005–10009. doi: 10.1073/pnas.87.24.10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez E, Sreekanitaiah C, Chaganti R S K. Cancer Res. 1994;54:3398–3406. [PubMed] [Google Scholar]
- 35.Dix D, Cohen P. J Theor Biol. 1980;83:163–173. doi: 10.1016/0022-5193(80)90377-x. [DOI] [PubMed] [Google Scholar]
- 36.Rubin H, Chow M, Yao A. Proc Natl Acad Sci USA. 1996;93:1825–1830. doi: 10.1073/pnas.93.5.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chow M, Rubin H. Mech Ageing Dev. 1996;89:165–183. doi: 10.1016/0047-6374(96)01744-7. [DOI] [PubMed] [Google Scholar]
- 38.Roberg K, Ollinger K. Am J Pathol. 1998;152:1151–1156. [PMC free article] [PubMed] [Google Scholar]
- 39.Russo C A, Weber T K, Volpe C M, Stoler D L, Petrelli N J, Rodriguez-Bigas M, Burhans W C, Anderson G R. Cancer Res. 1995;55:1122–1128. [PubMed] [Google Scholar]