Abstract
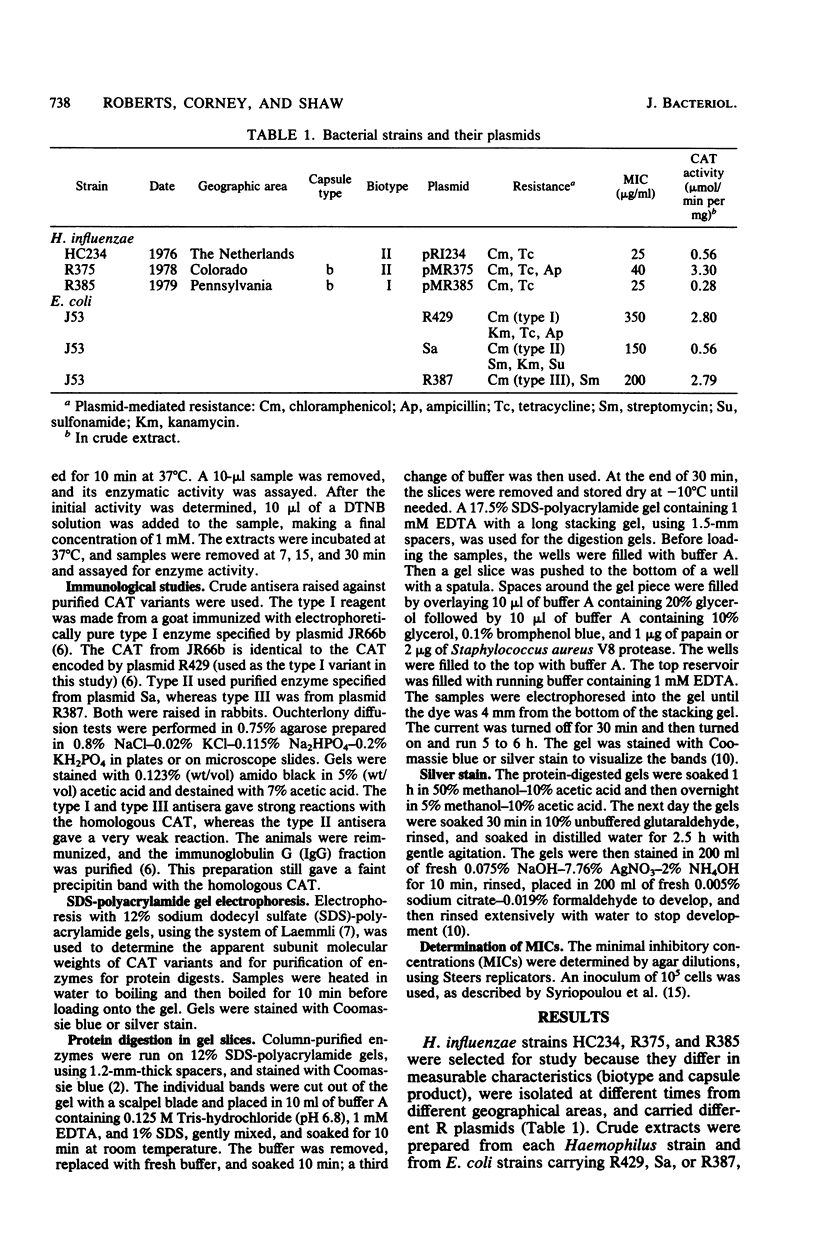
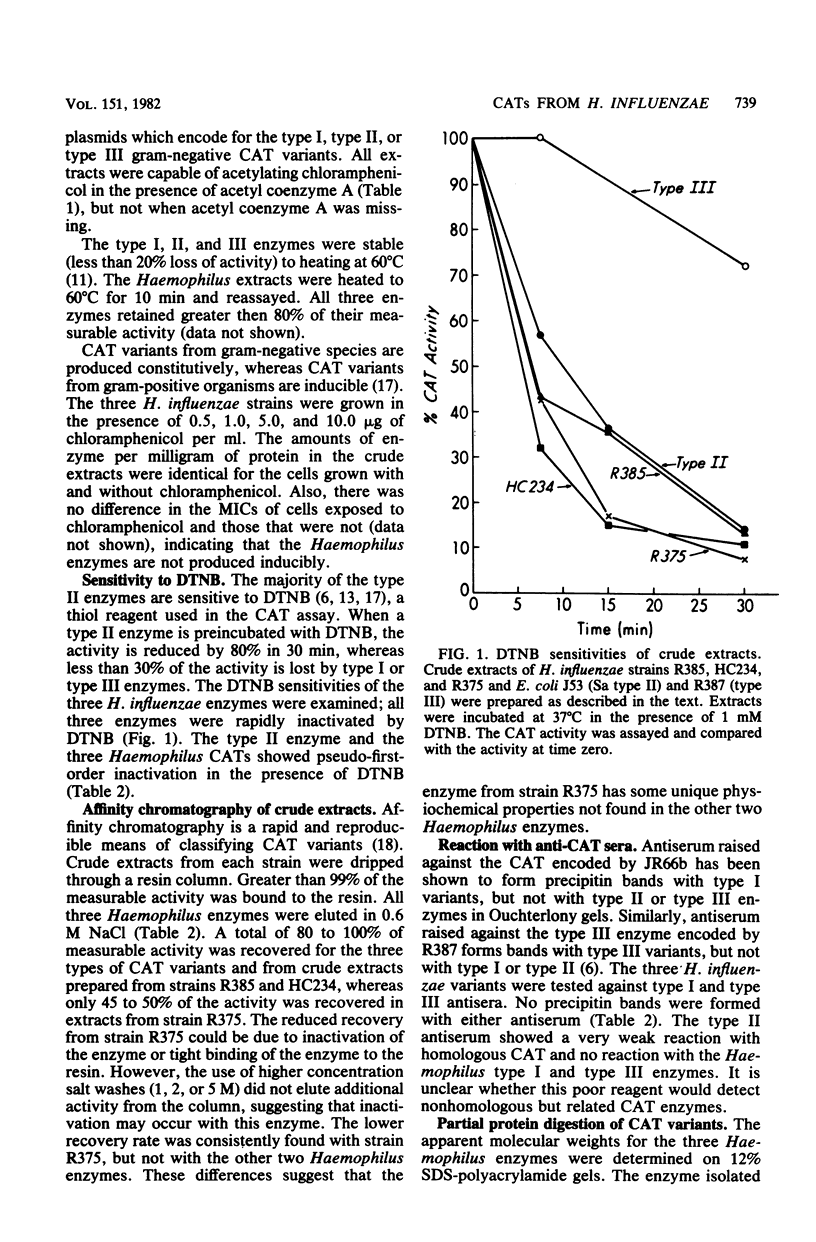
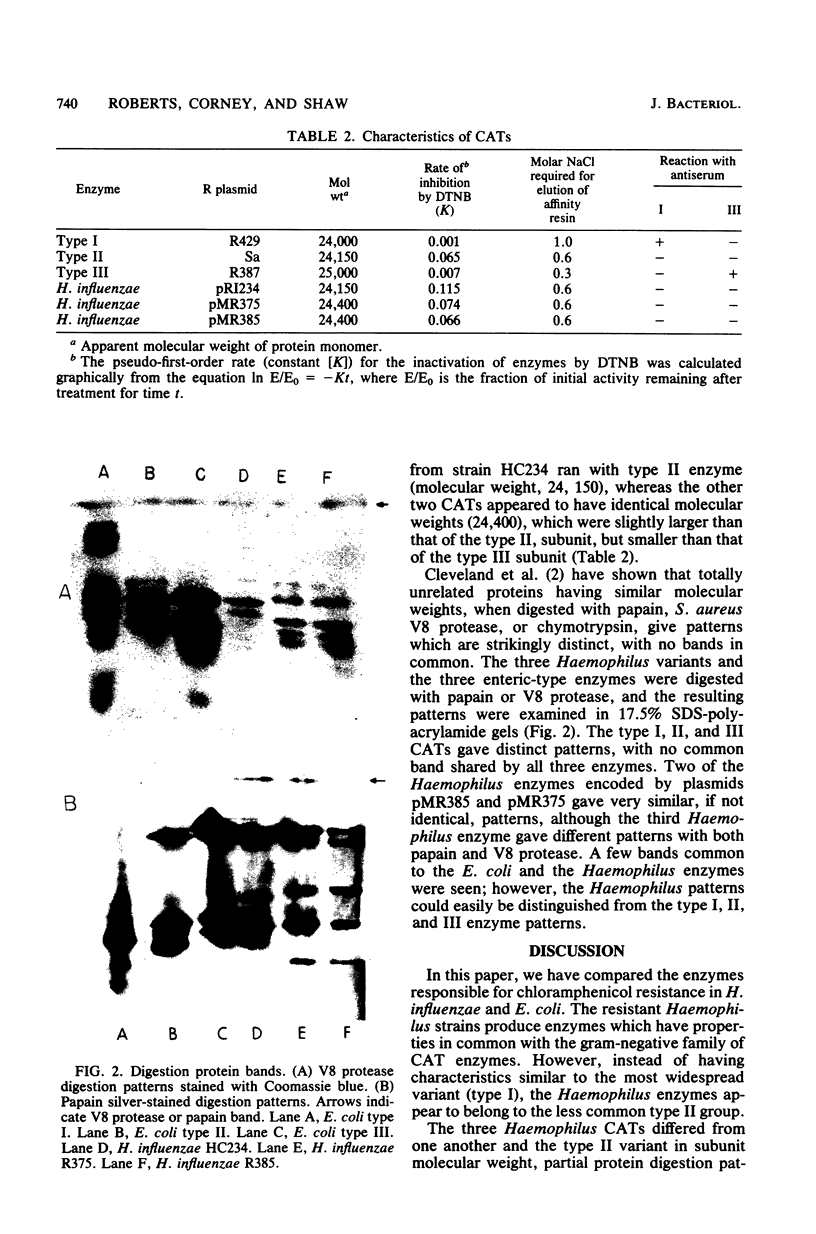
Three plasmid-mediated chloramphenicol acetyltransferases isolated from different Haemophilus influenzae strains were purified and characterized. All three enzymes had properties in common with the gram-negative family of chloramphenicol acetyltransferase. The Haemophilus enzymes and the enteric type II enzyme were sensitive to 5,5'-dithiobis(2-nitrobenzoic acid), gave the same elution patterns from a highly substituted resin containing a bound chloramphenicol base, and had similar reactions to antisera. All four differed from each other in subunit molecular weight, enzyme activity, and partial protein digestion patterns. The data suggest that the three Haemophilus enzymes belong to the less common type II group and are related, but is not identical, to each other and to the enteric type II enzyme.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azemun P., Stull T., Roberts M., Smith A. L. Rapid detection of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1981 Aug;20(2):168–170. doi: 10.1128/aac.20.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- De Graaff J., Elwell L. P., Falkow S. Molecular nature of two beta-lactamase-specifying plasmids isolated from Haemophilus influenzae type b. J Bacteriol. 1976 Apr;126(1):439–446. doi: 10.1128/jb.126.1.439-446.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Saunders J. R., Richmond M. H., Falkow S. Relationships among some R plasmids found in Haemophilus influenzae. J Bacteriol. 1977 Jul;131(1):356–362. doi: 10.1128/jb.131.1.356-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mendez B., Tachibana C., Levy S. B. Heterogeneity of tetracycline resistance determinants. Plasmid. 1980 Mar;3(2):99–108. doi: 10.1016/0147-619x(80)90101-8. [DOI] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Packman L. C., Shaw W. V. The use of naturally occurring hybrid variants of chloramphenicol acetyltransferase to investigate subunit contacts. Biochem J. 1981 Feb 1;193(2):541–552. doi: 10.1042/bj1930541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Swenson C. D., Owens L. M., Smith A. L. Characterization of chloramphenicol-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 1980 Oct;18(4):610–615. doi: 10.1128/aac.18.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Bouanchaud D. H., Goldstein F. W. Mechanism of transferable resistance to chloramphenicol in Haemophilus parainfluenzae. Antimicrob Agents Chemother. 1978 Feb;13(2):326–330. doi: 10.1128/aac.13.2.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V., Brodsky R. F. Characterization of chloramphenicol acetyltransferase from chloramphenicol-resistant Staphylococcus aureus. J Bacteriol. 1968 Jan;95(1):28–36. doi: 10.1128/jb.95.1.28-36.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syriopoulou V. P., Scheifele D. W., Sack C. M., Smith A. L. Effect of inoculum size on the susceptibility of Haemophilus influenzae b to beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Oct;16(4):510–513. doi: 10.1128/aac.16.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van A. D., Goldstein F., Acar J. F., Bouanchaud D. H. A transferable kanamycin resistance plasmid isolated from Haemophilus influenzae. Ann Microbiol (Paris) 1975 Apr;126(3):397–399. [PubMed] [Google Scholar]
- Zaidenzaig Y., Fitton J. E., Packman L. C., Shaw W. V. Characterization and comparison of chloramphenicol acetyltransferase variants. Eur J Biochem. 1979 Oct 15;100(2):609–618. doi: 10.1111/j.1432-1033.1979.tb04208.x. [DOI] [PubMed] [Google Scholar]
- Zaidenzaig Y., Shaw W. V. Affinity and hydrophobic chromatography of three variants of chloramphenicol acetyltransferases specified by R factors in Escherichia coli. FEBS Lett. 1976 Mar 1;62(3):266–271. doi: 10.1016/0014-5793(76)80072-5. [DOI] [PubMed] [Google Scholar]
- van Klingeren B., van Embden J. D., Dessens-Kroon M. Plasmid-mediated chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1977 Mar;11(3):383–387. doi: 10.1128/aac.11.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]



