Abstract
The human 25-hydroxyvitamin D3-1α-hydroxylase (1α-OHase) gene has been cloned. It contained nine exons and eight introns spanning ≈6.5 kb and a 1.4-kb 5′-flanking region. The 5′-flanking region contains consensus or highly conserved sequences for TATA, Pu, and CCAAT boxes, four cAMP response elements, two activator protein-1 (AP-1) response elements, two AP-2 response elements, three specific protein-1 (Sp1) response elements, and four NF-κB binding sites, but no vitamin D response element. By using luciferase reporter gene constructs of truncated forms of the 1α-OHase promoter transfected into a modified pig kidney cell line, AOK-B50, we identified regulatory regions of the 1.4-kb 1α-OHase promoter for parathyroid hormone 1–34 [PTH(1–34)], forskolin, and 1,25-hydroxyvitamin D3 [1,25(OH)2D3]. The 1.4-kb 1α-OHase promoter (AN1) modestly (1.7-fold) induced luciferase activity, whereas 1,100- (AN2), 827- (AN3), 672- (AN4), 463-(AN5), and 363-bp (AN6)-truncated promoters greatly stimulated luciferase activity by 494-fold, 18.4-fold, 55.3-fold, 643-fold, and 56.4-fold, respectively. PTH(1–34) and forskolin stimulated the activity of all constructs to varying degrees with significantly greater responsiveness for both compounds on AN2 and AN5. 1,25(OH)2D3 suppressed PTH(1–34)-induced activity on AN2 and AN5 constructs by 58% and 52%, respectively, but had no effect on the other constructs. These studies characterize the regulatory regions of the human 1α-OHase gene and provide insight into the physiologic basis for regulation of the expression of this gene by PTH and 1,25(OH)2D3.
Vitamin D is metabolized by sequential hydroxylations in the liver to 25-hydroxyvitamin D3 [25(OH)D3] and in the kidney to form 1,25-hydroxyvitamin D3 [1, 25(OH)2D3] (1). 1,25(OH)2D3 is the biologically active form and plays an essential role in the maintenance of calcium homeostasis (1–4). 1,25(OH)2D3 is synthesized in the proximal renal tubule by the 25-hydroxyvitamin D-1α-hydroxylase (1α-OHase) (5). This enzyme has also been found in nonrenal tissues, such as macrophages and keratinocytes (6–8). The renal synthesis of 1,25(OH)2D3 is tightly regulated by circulating parathyroid hormone (PTH), insulin-like growth factor I, calcium, phosphorus, and 1,25(OH)2D3 (1, 2, 9–13). The nonrenal synthesis of 1,25(OH)2D3 is not tightly regulated (6, 8). The expression of the renal 1α-OHase has been shown to be inhibited by its end product, 1,25(OH)2D3 (1, 2). Mice lacking the 1,25-hydroxyvitamin D3 receptor (VDR) developed an abnormally high serum concentration of 1,25(OH)2D3 (14), suggesting that the expression of 1α-OHase was regulated by the 1,25(OH)2D3 through liganded VDR. The cDNA encoding the mouse 1α-OHase was cloned by Takeyama et al. (14) in 1997, followed by the cloning of rat and human cDNAs (15–18). The human renal and nonrenal 1α-OHase cDNA sequences have been cloned and are 100% identical (18). The complete mouse gene and a portion of the 5′-flanking region of the human 1α-OHase gene have been cloned (19–22).
PTH appears to be a critical up-regulator of 1α-OHase activity in vivo. Serum calcium levels are sensed in the parathyroid glands through the calcium receptor (23, 24). When calcium levels drop, PTH is secreted, which targets the PTH/PTH-related peptide (PTHrP) receptor in bone to stimulate resorption and in the proximal tubule to stimulate 1α-OHase activity (19, 25, 26). PTH/PTHrP receptor agonists stimulate intracellular cAMP levels activating the cAMP-dependent protein kinase A, which phosphorylates cAMP-responsive element (CRE)-binding protein that subsequently modulates transcription through CRE in the promoters of target genes (19, 27, 28). Additional pathways of cAMP-dependent modulation of transcription may include activator protein (AP)-1, AP-2, and specific protein-1 (Sp1) sites in target genes (29). The capacity of a PTH/PTHrP receptor agonist to induce the expression of a reporter construct driven by a portion of the mouse 1α-hydroxylase promoter has been demonstrated in the modified porcine kidney cell line, AOK-B50 (19).
To better understand the role of PTH and other factors in the regulation of the metabolism of 25(OH)D3 to 1,25(OH)2D3, we cloned the human 1α-OHase gene, including the promoter region. A series of truncated luciferase reporter gene constructs was transfected into AOK-B50 cells and evaluated for their transcription-promoting activity in the presence of PTH and other factors known to modulate serum 1,25(OH)2D3 levels in vivo.
MATERIALS AND METHODS
Genomic Library Screening and Sequence Analysis.
A human genomic DNA library constructed by introduction of Sau3AI partially digested genomic DNA from normal placenta lymphocytes into the site of the phage vector EMBL3 was purchased from CLONTECH. The library was screened by plaque hybridization (1 × 106 plaques) using the 5′-most fragment (700 bp) of the human 1α-OHase cDNA as a probe, which was labeled with [α-32P]dCTP (NEN) by random priming (Prime-A-Gene, Promega). Nylon membrane replicas of ≈1 × 106 plaques were prehybridized at 42°C for 1 hr with 50% formamide/5× Denhardt’s solution/0.75 M NaCl/0.05 M NaH2PO4, pH 7.4/3 mM EDTA/0.1% SDS/0.1 mg/ml salmon sperm. Hybridization was carried out at 42°C overnight under the same conditions. Membranes were washed for 30 min with 1× SSC/0.1% SDS at 42°C for 30 min at room temperature and exposed to x-ray film at −70°C overnight. Positive plaques were isolated and rescreened. Purified phage DNA digested with SacI generated four fragments of 5.5 kb, 3.5 kb, 3.0 kb, and 1.0 kb. These four fragments were separately subcloned into the pGEM 7 (Promega) vector to perform the sequence analysis in a Prism 373 DNA sequencer (Applied Biosystems) with AmpliTaq DNA polymerase FS (Perkin–Elmer). Sequence analysis using T7 and SP6 promoter primers mapped the fragment order and showed that the 5′ end of the sequence is 1,369 kb from ATG.
Computer-Based Sequence Analysis.
The promoter sequence was analyzed by the Transcription Element Search System (tess of the Computational Biology and Informatics Laboratory, School of Medicine, University of Pennsylvania, http://agave.humgen.upenn.edu/tess/index.html) by using a 5-bp minimum element size limit, a 10% mismatch allowance, a minimum log-likelihood of homology of 10, and a secondary log-likelihood density threshold of 1.6.
Construction of Reporter Plasmids.
PCR was used to generate several truncated forms of the 1α-OHase promoter: the primers used and product sizes are summarized in Table 1. By using genomic clone DNA as a template, PCR was performed at 94°C for 3 min of denaturing, annealing for 1 min at 60°C, and extension for 10 min at 72°C by using Taq polymerase (GIBCO/BRL). PCR products of 5′ flanking fragments of 1α-OHase gene were inserted into the KpnI site of the luciferase basic plasmid vector, pGL2 (Promega). The subcloned PCR products were sequenced by using T7 and SP6 promoter primers to confirm that the products were the authentic promoter fragments.
Table 1.
Primers used for human 1α-OHase
| Human 1α-OHase promoter form | Primers (5′ → 3′) | Direction | Fragment size, bp |
|---|---|---|---|
| AN1 | CACGGTACCTTGGGAAGGTGAGGGGGT | F | 1,469 |
| CACGGTACCTCGGGCGCCCAGCGGACG | R | ||
| AN2 | AN1 cut with KpnI and SacI | 1,100 | |
| AN3 | CACGGTACCAATTAGAACATCCACATC | F | 827 |
| CACGCTAGCTCGGGCGCCCAGCGGACG | R | ||
| AN4 | CACGGTACCAAGAAAGAAAGATGGAGG | F | 672 |
| CACGCTAGCTCGGGCGCCCAGCGGACG | R | ||
| AN5 | CACGGTACCTGAAAGATGATGGGGAATTCC | F | 463 |
| CACGCTAGCTCGGGCGCCCAGCGGACG | R | ||
| AN6 | CACGGTACCGCCTTATAGCCTTTCCTA | F | 363 |
| CACGCTAGCTCGGGCGCCCAGCGGACG | R |
F, forward; R, reverse.
Cell Culture and Treatment.
AOK-B50 cells were maintained in DMEM with 7% FCS. Cells in 35 mm2 dishes at 50–60% confluence were transfected with 2 μg of constructed reporter gene plasmid DNA or with pGL2 plasmid DNA as a negative control for 3 hr in serum-free DMEM by using 4 μl of Lipofectamine (Life Technologies). Media was replaced with DMEM with 7% FCS, and cells were grown for 24 hr. Cells were then treated with PTH agonists, forskolin, 1,25(OH)2D3, or appropriate vehicle controls as indicated for an additional 24 hr in serum-free DMEM.
Luciferase Assays.
After treatment, cells were harvested in 150 μl of lysis buffer (Promega). The protein content of the extracts was determined by the Bradford procedure (Bio-Rad). Extracted samples (50 μg of protein) were subjected to the luciferase assay in duplicate by using a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego).
Chemicals.
PTH(1–34) and PTHrP(1–34) were from Bachem; forskolin, insulin-like growth factor I, and cell culture reagents were from Sigma, restriction enzymes were from Life Technologies, and 1,25(OH)2D3 was a kind gift from Milan Uskokovic (Hoffman–LaRoche).
Statistical Analyses.
Significant differences were identified by using Student’s t test.
RESULTS
Sequence Analysis of Human 1α-OHase 5′-Flanking Sequence.
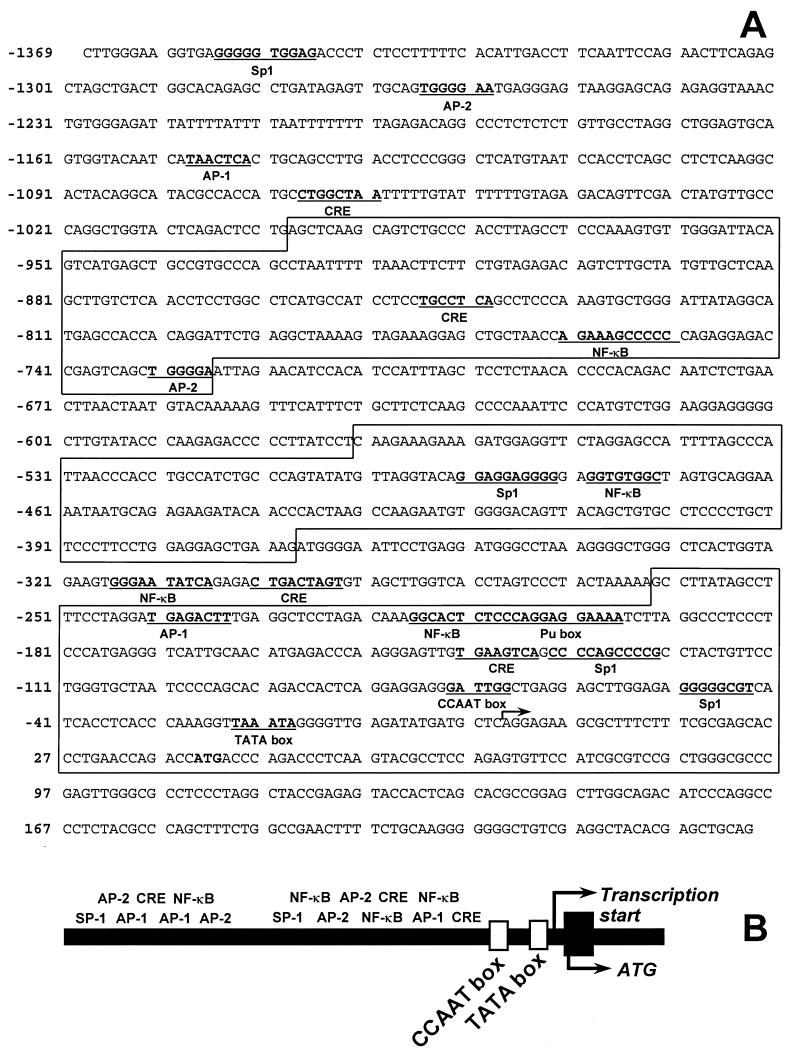
A human genomic library was screened with the most 5′ fragment (700 bp) of the 1α-OHase cDNA as a probe. A positive genomic clone of 13 kb was digested with SacI, which generated 3.5-kb, 3.0-kb, 1.0-kb, and 5.5-kb fragments. These fragments were subcloned into pGEM 7 vector (Promega). Sequence analysis confirmed that the 5.5-kb fragment encoded intron 8 to exon 9, the 1.0-kb fragment encoded exon 6 to intron 8, the 3.5-kb fragment encoded the 5′-flanking region to exon 6, and the 3.0-kb fragment encoded a portion of the 5′-flanking region. The complete sequence analysis showed that there are 1,369 bp of 5′-flanking region upstream from ATG. The TATA box was located 64 bp upstream from ATG, and a CCAAT box was identified 116 bp upstream from ATG. Additional putative regulatory elements including CRE-like sequences, consensus or highly conserved AP-1, AP-2, Sp1, and NF-kB sites were identified by computer search (30). The complete sequence and a map of the landmarks of the human 1α-OHase promoter are shown in Fig. 1.
Figure 1.
Structure of the human 1α-OHase promoter. A 13-kb genomic clone of the human 1α-OHase gene was sequenced, mapped, and analyzed for consensus sequences to known mammalian transcription factors. (A) The nucleotide sequence for the promoter region is given. Underlined sequences correspond to conservative homologous matches to the noted transcription factor or promoter element. The alternating blocked and unblocked sections indicate the truncated promoters AN1–6. (B) A map of the 1α-OHase promoter showing the relative locations of the putative cAMP-inducible transcription factor response elements.
Promoter Activity of Human 1α-Hydroxylase Gene.
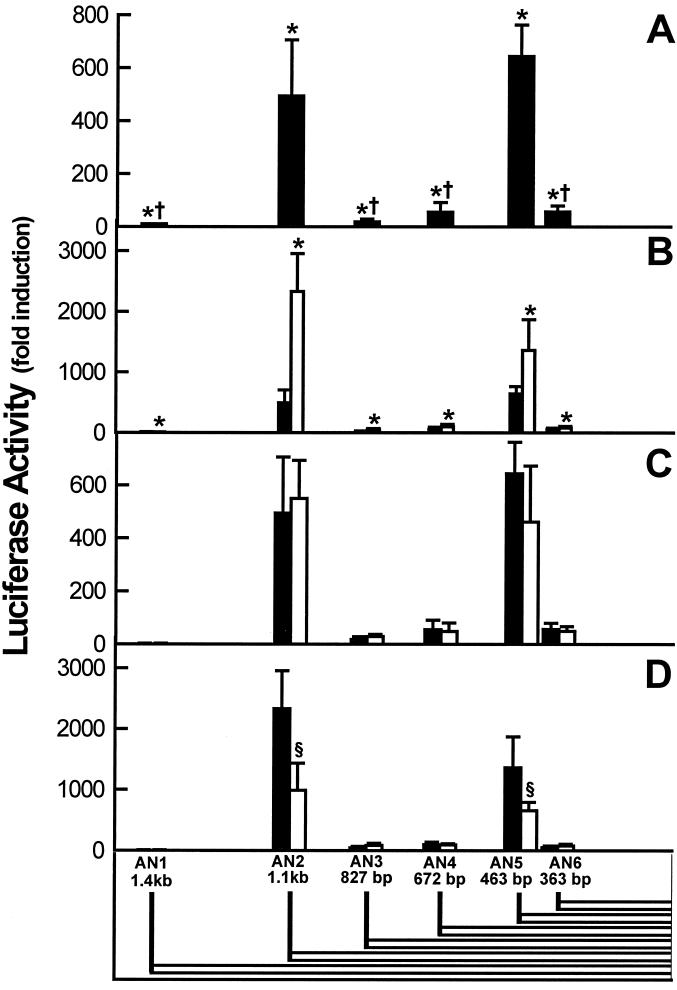
The transcriptional activity profile of 1α-OHase 5′-flanking sequences was studied. A series of truncated portions of the promoter were created extending up to 1.4 kb from the transcription start site of the human 1α-OHase gene. These constructs were inserted upstream of a luciferase reporter gene in the pGL2 basic vector and designated AN1, AN2, AN3, AN4, AN5, and AN6, the lengths of which are enumerated in Table 1 and illustrated by alternating outlined blocks in Fig. 1. Each construct was transiently transfected into AOK-B50 cells (a pig kidney cell line) and subsequently subjected to a luciferase activity assay. The basal luciferase activity of cells transfected with the 1.4-kb construct (AN1) was 1.7-fold greater than the promoter-free construct. The cells transfected with the shorter (AN2–6) constructs exhibited markedly increased promoter activity with the AN2 (490-fold) and AN5 (650-fold) constructs relative to cells transfected with the promoterless vector (Table 2, Fig. 2A).
Table 2.
Luciferase activity in AOK-B50 cells
| Human 1α-OHase promoter form | Size, bp | Basal promoter activity | Forskolin-induced promoter activity |
|---|---|---|---|
| AN1 | 1,469 | 1.7 ± 0.6 | 3.5 ± 1.8 |
| AN2 | 1,100 | 494.0 ± 211.6 | 1,414 ± 369 |
| AN3 | 827 | 18.4 ± 10.4 | 41 ± 20 |
| AN4 | 672 | 55.3 ± 36.1 | 57 ± 26 |
| AN5 | 463 | 643.4 ± 118.8 | 806 ± 252 |
| AN6 | 363 | 56.4 ± 23.1 | 129 ± 40 |
Activity data are given as mean ± SEM of fold induction vs. promoterless (basal) or untreated (forskolin-induced) vector.
Figure 2.
Activity of the human 1α-OHase promoter. A 1.4-kb clone and several truncated forms of the human 1α-OHase promoter were inserted into a luciferase reporter expression vector, transfected into AOK-B50 kidney cells, grown for 24 hr with and without factors, and then assayed for luciferase activity. (Bottom) The length of the various truncated promoter constructs. The graphs represent the luciferase activity of the constructs relative to that of the promoterless vector. Bars represent the mean ± SEM of three independent determinations. (A) Basal activity of the human 1α-OHase promoter. Transfected cells were assayed for luciferase activity. ∗, P < 0.01 vs. promoterless vector; †, P < 0.001 vs. AN2 and AN5. (B) PTH induction of the activity of the human 1α-OHase promoter. Transfected cells were treated with vehicle alone (filled bar) or 100 nM PTH (1–34) (open bar) for 24 hr and assayed for luciferase activity. ∗, P < 0.01 vs. vehicle alone. (C) Effect of 1,25(OH)2D3 on the activity of the human 1α-OHase promoter. Transfected cells were treated with vehicle alone (filled bar) or with 10 nM 1,25(OH)2D3 (open bar). (D) Effect of 1,25(OH)2D3 on the PTH (1–34)-inducible activity of the human 1α-OHase promoter. Transfected cells were treated with 100 nM PTH (1–34) with vehicle alone (filled bar) or with 10 nM 1,25(OH)2D3 (open bar). §, P < 0.01 vs. the same construct in the absence of 1,25(OH)2D3.
Regulation of the Promoter Activity of the Human 1α-Hydroxylase Gene.
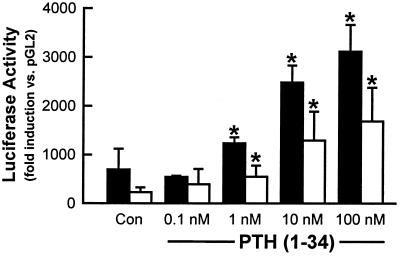
The effects of several factors suspected to be involved in the regulation of the expression of the 1α-OHase gene were examined. The luciferase reporter gene activity was determined in cells transfected with each construct and treated with 10 μM forskolin, a stimulator of cAMP, or vehicle (sterile water). All six promoter constructs responded to forskolin, with AN2 and AN5 being the most responsive (Table 2). Transfected AOK-B50 kidney cells also were tested for PTH responsiveness by treating with PTH(1–34) or vehicle (sterile water) for 24 hr. Induced luciferase reporter gene activity was observed for each of the constructs tested with 100 nM PTH(1–34), with significantly greater induction noted in the AN2 (≈2,300-fold) and AN5 (≈1,500-fold) constructs (Fig. 2B). The PTH/PTHrP receptor agonist PTHrP(1–34) also induced comparable luciferase activity with each of the constructs (data not shown). The enhancement of luciferase reporter gene activity in the AN2 and AN5 constructs by PTH(1–34) was concentration-dependent (Fig. 3). Neither 1,25(OH)2D3 (Fig. 2C) nor insulin-like growth factor I alone (data not shown) had significant stimulatory or suppressive effects with any of the constructs. However, 1,25(OH)2D3 suppressed PTH-stimulated luciferase reporter gene activity by 58% in the AN2 construct and 52% with the AN5 construct. No significant effect was observed on any of the other promoter constructs (Fig. 2D).
Figure 3.
Dose-dependent induction of the AN2 and AN5 truncated forms of the human 1α-OHase promoter by PTH (1–34). AN2- (1,100 bp, filled bar) and AN5- (463 bp, open bar) truncated forms of the human 1α-OHase promoter were inserted into a luciferase reporter expression vector, transfected into AOK-B50 kidney cells, and treated with increasing concentrations of PTH (1–34) for 24 hr. The AOK-B50 cells were then assayed for luciferase activity. Each bar represents the mean fold induction in luciferase activity relative to that of the promoterless construct in the absence of PTH ± SEM of three independent determinations. ∗, P < 0.01 vs. the same construct in the absence of PTH.
DISCUSSION
The results of structure–function and regulatory analyses of the human 1α-OHase gene promoter are reported here. We have mapped the 1α-OHase gene from −3 kb 5′ to +5 kb 3′, including 5 kb of coding sequence. A 1.4-kb segment of the promoter was sequenced, and several putative cAMP-responsive regulatory elements were found. Functional analyses identified distinct regions of the promoter that regulate expression of this gene. Our studies document strong induction of the expression of the 1α-OHase gene by PTH/PTHrP agonists and show that 1,25(OH)2D3 is antagonistic to PTH-induced transcription. The presence of putative cAMP-responsive regulatory elements and PTH-inducible and 1,25(OH)2D3-suppressible regions suggests a complex regulatory pathway. Because the regulation of 1α-OHase activity in renal and nonrenal tissues appears to be different, this complex promoter structure may also be related to the tissue-specific expression of this gene. A role for additional transcription factors or a secondary pathway of action in the regulation of the 1α-OHase promoter activity is indicated by the observation that 1,25(OH)2D3 suppresses PTH-stimulated induction despite the lack of a classical vitamin D response element in the promoter for this gene.
Sequence analysis revealed that the 1α-OHase promoter contains putative CRE as well as AP-1, AP-2, Sp1, and NF-κb response elements. Each of these transcription factors has been independently demonstrated to be induced by cAMP (33–34). The presence of conserved cis elements for these factors suggests additional pathways of cAMP regulation of 1α-OHase expression through AP-1, AP-2, Sp1, and NF-κB sites in the 1α-OHase promoter (33–35). It is significant to note that our computer-based sequence analysis of the promoter revealed several sequences with high homology to cAMP-responsive transcription factors. In contrast, cloning and analysis of the 1α-OHase promoter by Murayama et al. (22) showed fewer of these response elements, only two of which correspond to ones we identified.
The 1.4-kb 1α-OHase promoter (AN1) had modest endogenous transcriptional activity. However, because a 5′ portion of the promoter was truncated, activity was dramatically increased. Truncation constructs induced significant transcriptional activity, with AN2 and AN5 inducing the highest activity. AN1 induced modest transcriptional activity over baseline, AN2 and AN5 showed dramatic basal activity, and AN3, AN4, and AN6 exhibited more basal activity compared with AN1 but significantly less basal activity compared with AN2 and AN5. Therefore, we conclude that AN1 and AN4 possess suppressive cis regulatory elements and that AN6 contains few positive regulatory elements.
The low basal activity of AN1 sharply contrasts the relatively high endogenous activity of the mouse 1α-OHase promoter in the same system (19). Only ≈44% sequence identity exists between the human and mouse promoters, which is probably the basis for these observations. It is possible, therefore, that the mouse promoter clone does not include a suppressor element(s) homologous to those in the 5′ region of AN1.
PTH is one of two major regulators of 1α-OHase activity. In our system, PTH/PTHrP receptor agonists and forskolin modulated 1α-OHase promoter activity. These results, in combination with the identification of four putative CRE and several cis elements for cAMP-inducible factors in the promoter, suggest a direct role for PTH in the regulation of 1α-OHase expression. PTH/PTHrP receptor agonists significantly stimulated 1α-OHase promoter activity from basal activity in all truncation constructs; however, constructs AN2 and AN5 were significantly more sensitive than AN1, AN3, AN4, and AN6. CRE and several other putative cAMP-sensitive regulatory elements appear to be present in AN2 and AN5, whereas none were found in AN3 and few are present in AN1, AN4, and AN6. These observations suggest a degree of bifunctional regulation of the 1α-OHase promoter. The comparatively low promoter activity of AN1, which contains the most 5′ portions of the 1α-OHase promoter, supports our hypothesis that the 5′ region of AN1 contains suppressor sequences.
The other major regulator of 1α-OHase activity is 1,25(OH)2D3. Similar to the mouse promoter, 1,25(OH)2D3 did not suppress basal 1α-OHase promoter activity. However, 1,25(OH)2D3 did suppress luciferase reporter gene expression stimulated by PTH(1–34), and this suppression was exclusive to the two high-activity regions (AN2 and AN5) of the 1α-OHase promoter. Sequence analysis of the 1α-OHase promoter indicates that there are no canonical vitamin D response elements in either region. Together, these observations suggest that 1,25(OH)2D3 suppresses 1α-OHase expression by an indirect cascade that interferes with the PTH-signaling pathway. However, it is also possible that an atypical suppressive vitamin D response element(s) is/are located in the 1α-OHase promoter. These results indicate a sophisticated degree of regulation that may not be entirely dependent on PTH or 1,25(OH)2D3 levels.
Our results differ significantly from those presented for another genomic clone of the human 1α-OHase promoter (22). Those investigators found induction by PTH in a region 4 kb 5′ to the transcription start site and suppression by 1,25(OH)2D3 in 0.9 kb of the 3′-most region of the promoter. No mention is made as to whether any PTH sensitivity was found in the 3′-most region or whether any suppression by 1,25(OH)2D3 was found in the −4-kb region. More significantly, suppression by 1,25(OH)2D3 was shown in the absence of PTH. Major differences between the results may be because of experimental differences. Our constructs contained up to −1.4 kb of the 1α-OHase gene promoter, including the native transcription and translation start sites and the first 19 residues of the 1α-OHase, and our transfections were performed in a porcine kidney cell line. In contrast, Murayama et al. (22) substituted the 3′-most region of the promoter with the thymidine kinase promoter, presented analyses of fragments −0.9 kb and ≈4 kb upstream of the promoter, used a mouse kidney cell line, and cotransfected with VDR. The use of the thymidine kinase promoter could be responsible for the observation that 1,25(OH)2D3 independently suppressed 1α-OHase promoter activity. Although this is consistent with our results, it has been shown that thymidine kinase promoter-driven expression may give rise to aberrant results (36).
It has been shown that 1,25(OH)2D3 suppresses PTH/PTHrP receptor number (37). In VDR knockout mice, the 1α-OHase transcript was present at 50 times the levels of wild-type littermates (14). It has also been demonstrated that PTH agonist treatment led a 2- to 3-fold increase in VDR expression in MC3T3-E1 cells (29). Regulation of receptor number influences the biologic activity of that receptor’s ligand. In this context, it is plausible that the combinatorial regulation of 1α-OHase and PTH/PTHrP receptor expression exerted by 1,25(OH)2D3 are the fine controls for PTH responsiveness, which ultimately maintain serum calcium homeostasis. Although the crosstalk between these receptors and their ligands remains unclear, these observations strongly support the hypothesis that PTH and 1,25(OH)2D3 are critical physiologic regulators of 1α-OHase expression in the kidney. The regulation of 1α-OHase expression in nonrenal tissues may use different regions of the promoter dependent on the expression of receptors for PTH and 1,25(OH)2D3. This work provides valuable insights into the regulation of the expression in the kidney of the most critical enzyme in the biosynthetic pathway for 1,25(OH)2D3, which is primarily responsible for systemic calcium homeostasis, and therefore, of great physiologic relevance.
Acknowledgments
We thank Drs. F. Binghurst, J. Potts, and H. Kronenberg of the Massachusetts General Hospital for the AOK-B50 cells and Kelly Persons for assistance with cell culture. This work was made possible in part by National Institutes of Health Grant RO1 AR36963.
ABBREVIATIONS
- 1α-OHase
25-hydroxyvitamin D3–1α-hydroxylase
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- PTH
parathyroid hormone
- CRE
cAMP response element
- AOK-B50
modified pig kidney cell line
- AP
activator protein
- Sp1
specific protein-1
- VDR
vitamin D receptor
- PTHrP
PTH-related peptide
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF072470).
References
- 1.Holick M F. In: Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Favus M J, editor. New York: Raven; 1996. pp. 74–81. [Google Scholar]
- 2.Holick M F. In: Endocrinology. DeGroot L, editor. Philadelphia: Saunders; 1995. pp. 990–1013. [Google Scholar]
- 3.Darwish H, DeLuca H F. Crit Rev Eukaryotic Gene Expression. 1993;3:89–116. [PubMed] [Google Scholar]
- 4.Norman A W. J Clin Nutr. 1998;67:1108–1110. doi: 10.1093/ajcn/67.6.1108. [DOI] [PubMed] [Google Scholar]
- 5.Suda T, Kurukowa K. In: Perinatal Calcium and Phosphorus Metabolism. Holick M F, Gray T K, Anast C S, editors. Amsterdam: Elsevier; 1983. pp. 57–69. [Google Scholar]
- 6.Bikle D D, Nemanic M K, Whitney J O, Elias P W. Biochemistry. 1986;25:1545–1548. doi: 10.1021/bi00355a013. [DOI] [PubMed] [Google Scholar]
- 7.Adams J S, Sharma O P, Gacad M A, Singer F R. J Clin Invest. 1983;72:1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams J S, Singer F R, Gacad M A. J Clin Endocrinol Metab. 1985;60:960–966. doi: 10.1210/jcem-60-5-960. [DOI] [PubMed] [Google Scholar]
- 9.Fraser D R. Physiol Rev. 1980;60:551–613. doi: 10.1152/physrev.1980.60.2.551. [DOI] [PubMed] [Google Scholar]
- 10.Boyle I T, Gray R W, DeLuca H F. Proc Natl Acad Sci USA. 1971;68:2131–2134. doi: 10.1073/pnas.68.9.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garabedian M, Holick M F, DeLuca H F, Boyle I T. Proc Natl Acad Sci USA. 1972;69:1673–1676. doi: 10.1073/pnas.69.7.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portale A A, Halloran B P, Murphy M M, Morris R C. J Clin Invest. 1986;77:7–12. doi: 10.1172/JCI112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nesbitt T, Drezner M K. Endocrinology. 1993;132:133–138. doi: 10.1210/endo.132.1.8419119. [DOI] [PubMed] [Google Scholar]
- 14.Takeyama K-I, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. Science. 1997;277:1827–1829. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 15.Shinki T, Shimada H, Wakino S, Anzawa H, Hayashi M, Saruta T, DeLuca H F, Suda T. Proc Natl Acad Sci USA. 1997;94:12920–12925. doi: 10.1073/pnas.94.24.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Monkawa T, Yoshida T, Wakino S, Shinki T, Anazawa H, DeLuca H F, Suda T, Hayashi M, Saruta T. Biochem Biophys Res Commun. 1997;239:527–533. doi: 10.1006/bbrc.1997.7508. [DOI] [PubMed] [Google Scholar]
- 17.Fu G K, Lin D, Zhang M Y H, Bikle D D, Shackleton C H L, Miller W L, Portale A A. Mol Endocrinol. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- 18.St.-Arnaud R, Messerlian S, Moir J M, Omdahl J L, Glorieux F H. J Bone Miner Res. 1997;12:1552–1559. doi: 10.1359/jbmr.1997.12.10.1552. [DOI] [PubMed] [Google Scholar]
- 19.Brenza H L, Kimmerl-Jehan C, Jehan F, Shinki T, Wakino S, Anazawa H, Suda T, DeLuca H F. Proc Natl Acad Sci USA. 1998;95:1387–1391. doi: 10.1073/pnas.95.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fu G K, Portale A A, Miller W L. DNA Cell Biol. 1997;16:1499–1507. doi: 10.1089/dna.1997.16.1499. [DOI] [PubMed] [Google Scholar]
- 21.Kitanaka S, Takeyama K-I, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T, Kato S. N Engl J Med. 1998;338:653–661. doi: 10.1056/NEJM199803053381004. [DOI] [PubMed] [Google Scholar]
- 22.Murayama A, Takeyama K-I, Kitanaka S, Kodera Y, Hosoya T, Kato S. Biochem Biophys Res Commun. 1998;249:11–16. doi: 10.1006/bbrc.1998.9098. [DOI] [PubMed] [Google Scholar]
- 23.Brown E M, Pollak M, Hebert S C. Annu Rev Med. 1998;49:15–29. doi: 10.1146/annurev.med.49.1.15. [DOI] [PubMed] [Google Scholar]
- 24.Autry C P, Kifor O, Brown E M, Fuller F H, Rogers K V, Halloran B P. J Endocrinol. 1997;153(3):437–444. doi: 10.1677/joe.0.1530437. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka Y, DeLuca H F. Arch Biochem Biophys. 1973;154:566–576. doi: 10.1016/0003-9861(73)90010-6. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka Y, DeLuca H F. Am J Physiol. 1984;246:E168–E173. doi: 10.1152/ajpendo.1984.246.2.E168. [DOI] [PubMed] [Google Scholar]
- 27.Jüppner H, Abou-Samra A B, Freeman M, Kong X F, Schipani E, Ricards J, Kolakowski L, Hock J, Potts J T, Jr, Kronenberg H, Segre G. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 28.Kong X F, Urena P, Schipani E, Jüppner H, Kronenberg H M, Potts J T, Jr, Segre G V, Abou-Samra A B. J Endocr Invest. 1992;15:97–101. [PubMed] [Google Scholar]
- 29.Krishnan A V, Cramer S D, Bringhurst F R, Feldman D. Endocrinology. 1995;136(2):705–712. doi: 10.1210/endo.136.2.7835303. [DOI] [PubMed] [Google Scholar]
- 30.Schug J, Overton G C. tess: Transcription Element Search System on the World Wide Web. Philadelphia: Univ. of Pennsylvania; 1997. , Technical Report CBIL-TR-1997-1001-v0.0. [Google Scholar]
- 31.Karin M. Trends Genet. 1989;5:65–67. doi: 10.1016/0168-9525(89)90027-9. [DOI] [PubMed] [Google Scholar]
- 32.Michael M D, Michael L F, Simpson E R. Mol Cell Endocrinol. 1997;134:147–156. doi: 10.1016/s0303-7207(97)00178-0. [DOI] [PubMed] [Google Scholar]
- 33.Parry G C, Mackman N. J Immunol. 1997;159:5450–5456. [PubMed] [Google Scholar]
- 34.Monnier D, Loeffler J P. DNA Cell Biol. 1998;17:151–159. doi: 10.1089/dna.1998.17.151. [DOI] [PubMed] [Google Scholar]
- 35.Bringhurst F R, Jüppner H, Guo J. Endocrinology. 1993;132:2090–2098. doi: 10.1210/endo.132.5.8386606. [DOI] [PubMed] [Google Scholar]
- 36.Kerry D M, Dwivedi P P, Hahn C N, Morris H A, Omdahl JL, May B K. J Biol Chem. 1996;271:29715–29721. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- 37.Titus L, Jackson E, Nanes M S, Rubin J E, Catherwood B D. J Bone Miner Res. 1991;6:631–637. doi: 10.1002/jbmr.5650060614. [DOI] [PubMed] [Google Scholar]