Abstract
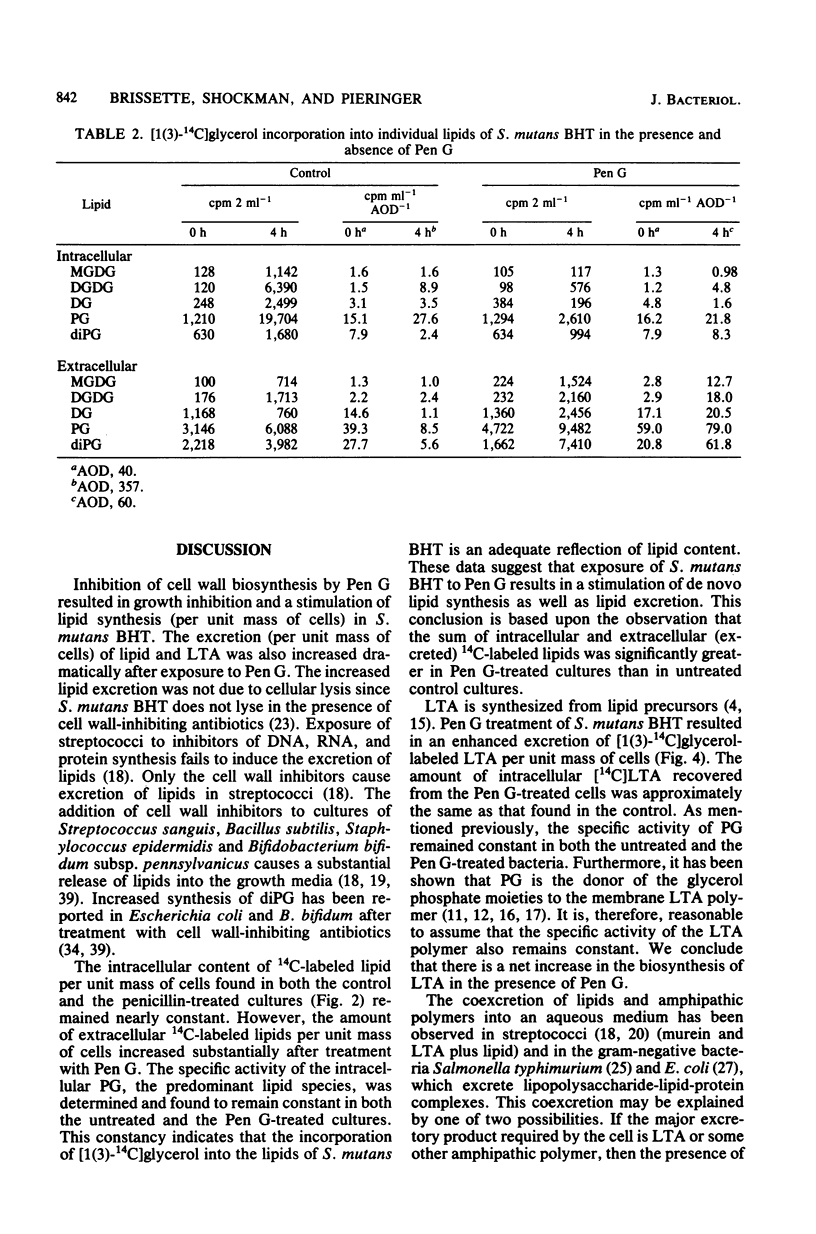
Cultures of Streptococcus mutans BHT grown for at least eight generations in a chemically defined medium containing [1(3)-14C]glycerol, when treated with growth-inhibitory concentrations (0.2 micrograms/ml) of benzylpenicillin (Pen G), produced and excreted increased amounts of lipid and lipoteichoic acid per unit of cells. Cellular lysis was not observed. Compared with untreated controls, lipid excretion increased 15-fold, and lipoteichoic acid excretion increased 6-fold, 4 h after the addition of Pen G. All lipid species showed increased synthesis and excretion after exposure to Pen G. Although the same lipid types were found in both the Pen G-treated and the untreated cultures, the percent composition was altered after treatment with Pen G. The most dramatic example of this was the percentage of intracellular diphosphatidylglycerol found in the Pen G-treated cultures, 22.6%, in contrast to 5.3% found in the untreated cultures.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambron R. T., Pieringer R. A. The metabolism of glyceride glycolipids. V. Identification of the membrane lipid formed from diglucosyl diglyceride in Streptococcus faecalis ATCC 9790 as an acylated derivative of glyceryl phosphoryl diglucosyl glycerol. J Biol Chem. 1971 Jul 10;246(13):4216–4225. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabacungan E., Pieringer R. A. Excretion of extracellular lipids by Streptococcus mutans BHT and FA-1. Infect Immun. 1980 Feb;27(2):556–562. doi: 10.1128/iai.27.2.556-562.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. D., Pieringer R. A., Daneo-Moore L. Effect of cerulenin on cellular autolytic activity and lipid metabolism during inhibition of protein synthesis in Streptococcus faecalis. J Bacteriol. 1981 May;146(2):590–604. doi: 10.1128/jb.146.2.590-604.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Redman B. E., Shockman G. D. Autolytic defective mutant of Streptococcus faecalis. J Bacteriol. 1978 Feb;133(2):631–640. doi: 10.1128/jb.133.2.631-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowfoot P. D., Esfahani M., Wakil S. J. Relation between protein synthesis and phospholipid synthesis and turnover in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1408–1415. doi: 10.1128/jb.112.3.1408-1415.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emdur L. I., Chiu T. H. Turnover of phosphatidylglycerol in Streptococcus sanguis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1137–1144. doi: 10.1016/s0006-291x(74)80097-5. [DOI] [PubMed] [Google Scholar]
- Emdur L., Chiu T. The role of phosphatidylglycerol in the in vitro biosynthesis of teichoic acid and lipoteichoic acid. FEBS Lett. 1975 Jul 15;55(1):216–219. doi: 10.1016/0014-5793(75)80995-1. [DOI] [PubMed] [Google Scholar]
- FRISELL W. R., MEECH L. A., MACKENZIE C. G. A simplified photometric analysis for serine and formaldehyde. J Biol Chem. 1954 Apr;207(2):709–716. [PubMed] [Google Scholar]
- Ferretti J. J., Ward M. Susceptibility of Streptococcus mutans to antimicrobial agents. Antimicrob Agents Chemother. 1976 Aug;10(2):274–276. doi: 10.1128/aac.10.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfield M. C., Pieringer R. A. Phosphatidylkojibiosyl diglyceride. The covalently linked lipid constituent of the membrane lipoteichoic acid from Streptococcus faecalis (faecium) ATCC 9790. J Biol Chem. 1975 Jan 25;250(2):702–709. [PubMed] [Google Scholar]
- Ganfield M. C., Pieringer R. A. The biosynthesis of nascent membrane lipoteichoic acid of Streptococcus faecium (S. faecalis ATCC 9790) from phosphatidylkojibiosyl diacylglycerol and phosphatidylglycerol. J Biol Chem. 1980 Jun 10;255(11):5164–5169. [PubMed] [Google Scholar]
- Glaser L., Lindsay B. The synthesis of lipoteichoic acid carrier. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1131–1136. doi: 10.1016/s0006-291x(74)80096-3. [DOI] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral T. A., Daneo-Moore L. Glycerol incorporation in certain oral streptococci. Infect Immun. 1980 Dec;30(3):759–765. doi: 10.1128/iai.30.3.759-765.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J. L., Knox K. W., Wicken A. J., Hewett M. J. Formation of extracellular lipoteichoic acid by oral streptococci and lactobacilli. Infect Immun. 1975 Aug;12(2):378–386. doi: 10.1128/iai.12.2.378-386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly S. J., Daneo-Moore L., Shockman G. D. Factors regulating cell wall thickening and intracellular iodophilic polysaccharide storage in Streptococcus mutans. Infect Immun. 1977 Jun;16(3):967–973. doi: 10.1128/iai.16.3.967-973.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mychajlonka M., McDowell T. D., Shockman G. D. Inhibition of peptidoglycan, ribonucleic acid, and protein synthesis in tolerant strains of Streptococcus mutans. Antimicrob Agents Chemother. 1980 Apr;17(4):572–582. doi: 10.1128/aac.17.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Nov;100(2):617–624. doi: 10.1128/jb.100.2.617-624.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothfield L., Pearlman-Kothencz M. Synthesis and assembly of bacterial membrane components. A lipopolysaccharide-phospholipid-protein complex excreted by living bacteria. J Mol Biol. 1969 Sep 28;44(3):477–492. doi: 10.1016/0022-2836(69)90374-x. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. Relations between bacterial cell wall synthesis, growth phase, and autolysis. J Biol Chem. 1958 Feb;230(2):961–977. [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Cornett J. B., Mychajlonka M. Does penicillin kill bacteria?. Rev Infect Dis. 1979 Sep-Oct;1(5):787–796. doi: 10.1093/clinids/1.5.787. [DOI] [PubMed] [Google Scholar]
- Shockman G. D. Symposium on the fine structure and replication of bacteria and their parts. IV. Unbalanced cell-wall synthesis: autolysis and cell-wall thickening. Bacteriol Rev. 1965 Sep;29(3):345–358. doi: 10.1128/br.29.3.345-358.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Lipids and lipoteichoic acid of autolysis-defective Streptococcus faecium strains. J Bacteriol. 1980 Jun;142(3):741–746. doi: 10.1128/jb.142.3.741-746.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Morphological and physiological study of autolytic-defective Streptococcus faecium strains. J Bacteriol. 1979 May;138(2):598–608. doi: 10.1128/jb.138.2.598-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stárka J., Moravová J. Phospholipids and cellular division of Escherichia coli. J Gen Microbiol. 1970 Feb;60(2):251–257. doi: 10.1099/00221287-60-2-251. [DOI] [PubMed] [Google Scholar]
- Sund M. L., Linder L. Autolysis in strains of viridans streptococci. J Gen Microbiol. 1976 Sep;96(1):87–94. doi: 10.1099/00221287-96-1-87. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J. Mode of action of beta-lactam antibiotics. Rev Infect Dis. 1979 Jan-Feb;1(1):39–54. doi: 10.1093/clinids/1.1.39. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H. Biochemical changes in Bifidobacterium bifidum var. pennsylvanicus after cell wall inhibition. IX. Metabolism and release of cellular lipids in the presence of antibiotics. Biochim Biophys Acta. 1976 Dec 20;450(3):277–287. doi: 10.1016/0005-2760(76)90001-1. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]


