Abstract
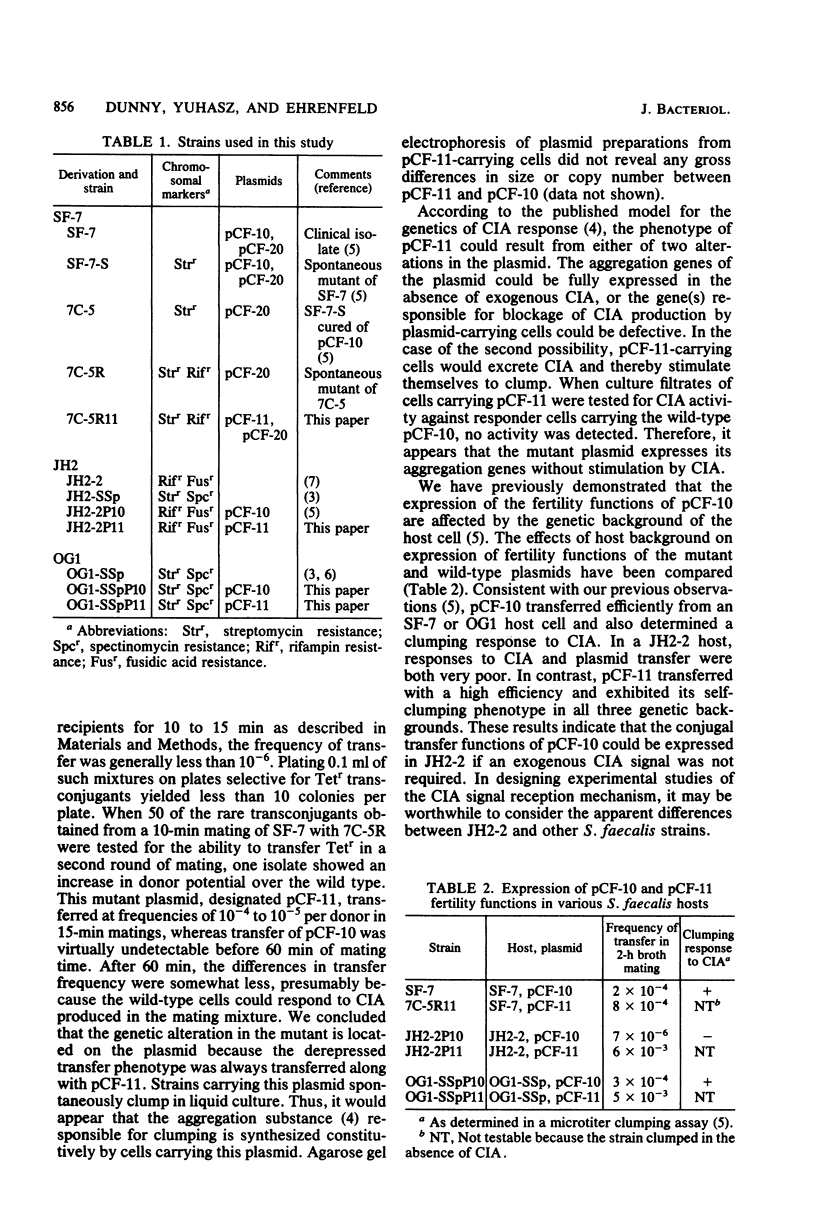
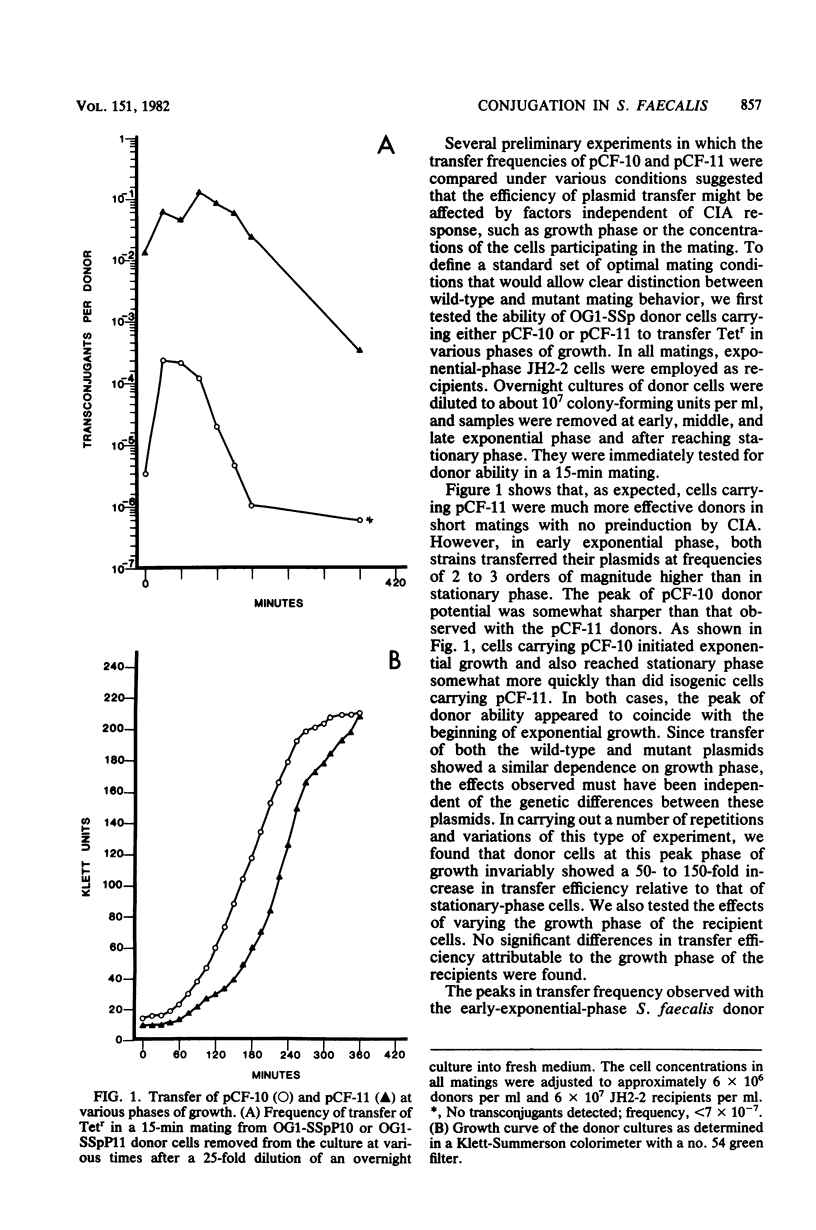
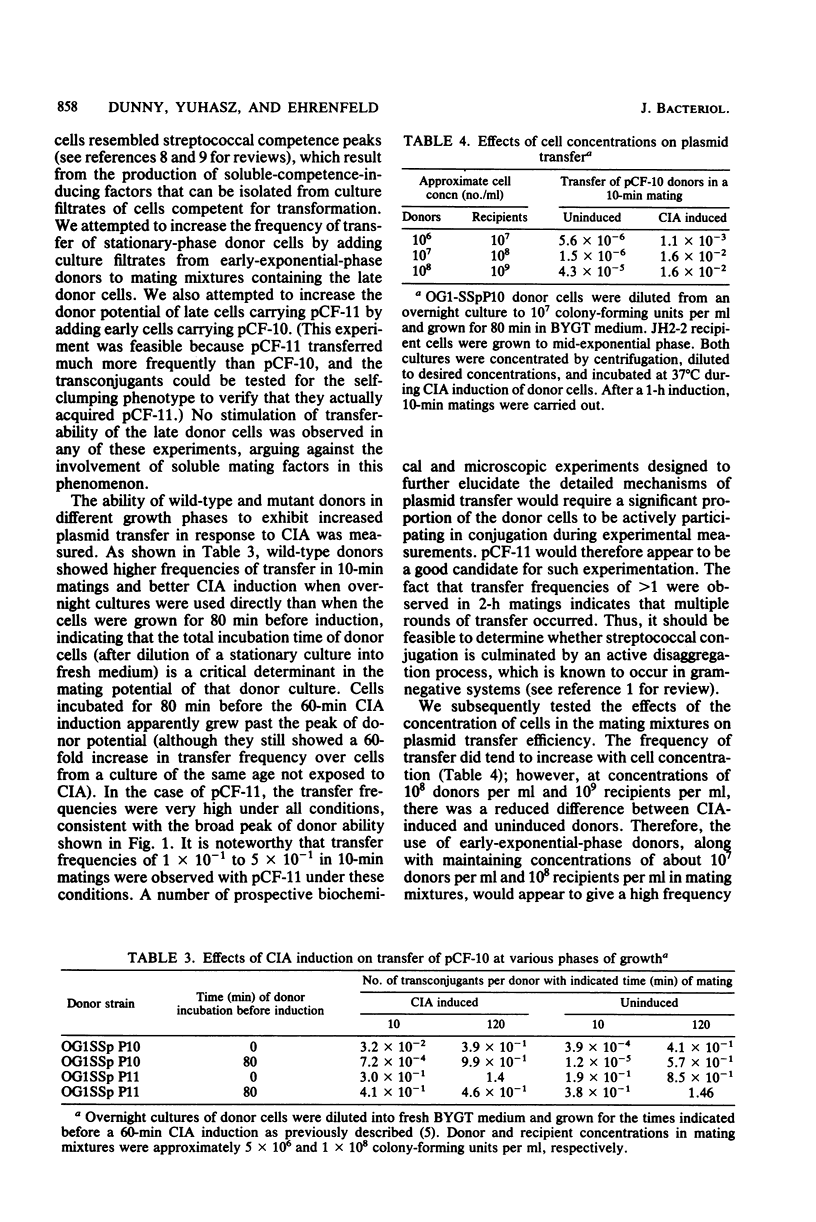
In an effort to elucidate the mechanisms of conjugal plasmid transfer in Streptococcus faecalis, a genetic analysis of the sex pheromone-dependent tetracycline resistance plasmid pCF-10 was initiated. Rare transconjugants obtained from short matings with wild-type donors not exposed to sex pheromones were screened for increased donor potential in a subsequent mating. From this screening, a mutant plasmid, designated pCF-11, whose transfer functions are expressed in the absence of pheromone induction was isolated. Cells carrying pCF-11 spontaneously clump when grown in broth culture but do not excrete sex pheromones active against wild-type donors. In the course of initial experiments, it was observed that physiological conditions could affect plasmid transfer frequency. Therefore, a set of standardized optimal mating conditions was defined. The experiments carried out to determine these conditions revealed that a transient increase in transfer frequency of about 2 order of magnitude occurred in early-exponential-phase donor cells. This peak of activity is independent of sex pheromone response, since it was observed with induced or uninduced donor cells carrying either pCF-11 or pCF-10.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clewell D. B., Brown B. L. Sex pheromone cAD1 in Streptococcus faecalis: induction of a function related to plasmid transfer. J Bacteriol. 1980 Aug;143(2):1063–1065. doi: 10.1128/jb.143.2.1063-1065.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Brown B. L., Clewell D. B. Induced cell aggregation and mating in Streptococcus faecalis: evidence for a bacterial sex pheromone. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Craig R. A., Carron R. L., Clewell D. B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979 Jul;2(3):454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- Dunny G., Funk C., Adsit J. Direct stimulation of the transfer of antibiotic resistance by sex pheromones in Streptococcus faecalis. Plasmid. 1981 Nov;6(3):270–278. doi: 10.1016/0147-619x(81)90035-4. [DOI] [PubMed] [Google Scholar]
- Gold O. G., Jordan H. V., van Houte J. The prevalence of enterococci in the human mouth and their pathogenicity in animal models. Arch Oral Biol. 1975 Jul;20(7):473–477. doi: 10.1016/0003-9969(75)90236-8. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


