Abstract
An enzyme which liberates Pi from myo-inositol hexaphosphate (phytic acid) was shown to be present in culture filtrates of Bacillus subtilis. It was purified until it was homogeneous by ultracentrifugation, but it still showed two isozymes on polyacrylamide gel electrophoresis. The enzyme differed from other previously known phytases in its metal requirement and in its specificity for phytate. It had a specific requirement for Ca2+ for its activity. The enzyme hydrolyzed only phytate and had no action on other phosphate esters tested. This B. subtilis phytase is the only known phytate-specific phosphatase. The products of hydrolysis of phytate by this enzyme were Pi and myo-inositol monophosphate. The enzyme showed optimum activity at pH 7.5. It was inhibited by Ba2+, Sr2+, Hg2+, Cd2+, and borate. Its activity was unaffected by urea, diisopropylfluorophosphate, arsenate, fluoride, mercaptoethanol, trypsin, papain, and elastase.
Full text
PDF

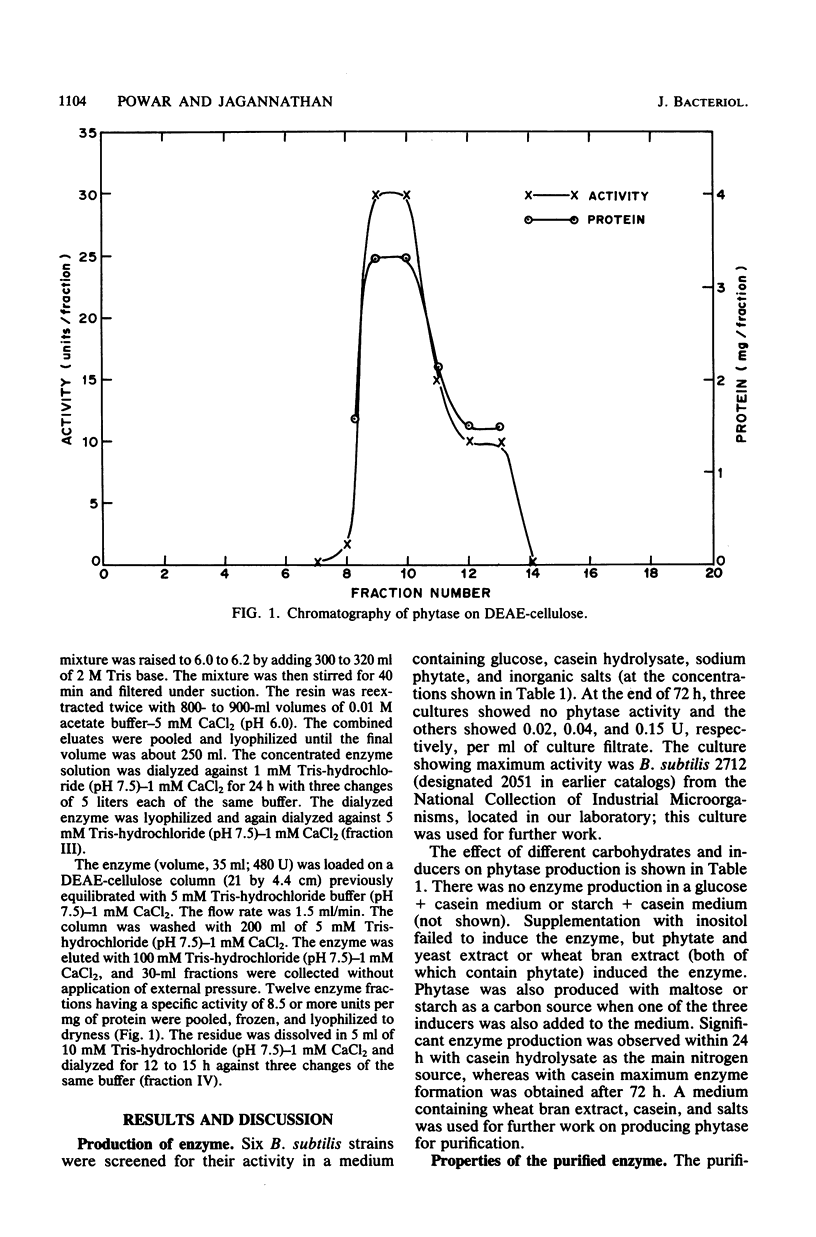
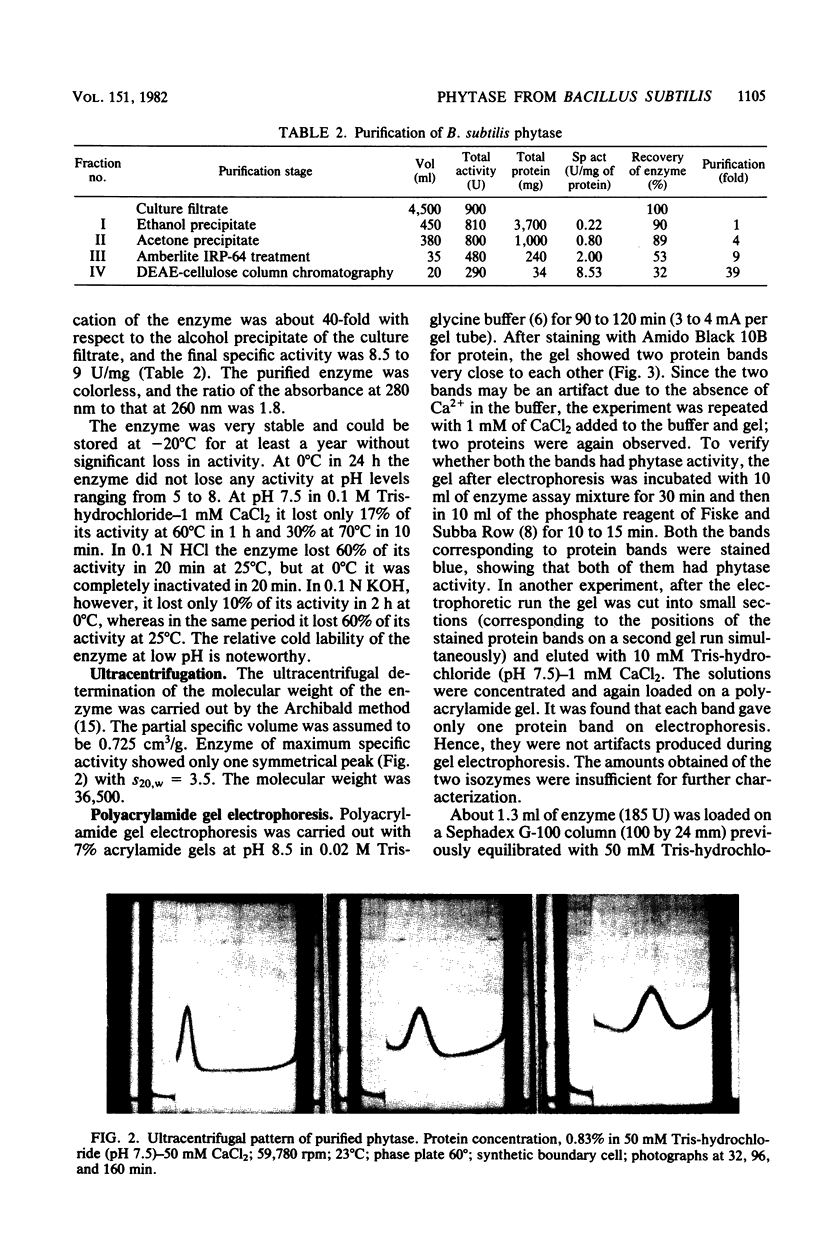



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAGNOSTOPOULOS C., LINO A. Activité de phosphotransférase non spécifique et de phytase dans les graines de bié et l'E. coli. Bull Soc Chim Biol (Paris) 1958;40(7-8):1045–1057. [PubMed] [Google Scholar]
- Cavallini D., Graziani M. T., Dupré S. Determination of disulphide groups in proteins. Nature. 1966 Oct 15;212(5059):294–295. doi: 10.1038/212294a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A., ADA G. L. The separation and quantitative determination of the component sugars of mucoproteins. Biochem J. 1956 Apr;62(4):681–686. doi: 10.1042/bj0620681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. W., MOORE S., STEIN W. H. A chromatographic investigation of pancreatic ribonuclease. J Biol Chem. 1953 Feb;200(2):493–506. [PubMed] [Google Scholar]
- HIRS C. H., STEIN W. H., MOORE S. The amino acid composition of ribonuclease. J Biol Chem. 1954 Dec;211(2):941–950. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Powar V. K., Jagannathan V. Phytase from Bacillus subtilis. Indian J Biochem. 1967 Sep;4(3):184–185. [PubMed] [Google Scholar]
- Skowroński T. Some properties of partially purified phytase from Aspergillus niger. Acta Microbiol Pol. 1978;27(1):41–48. [PubMed] [Google Scholar]
- Valikhanov M. N., Abdullaeva M. M., Rakhimov M. M. Nekotorye svoistva fitazy khlopchatnika. Biokhimiia. 1981 Jan;46(1):100–102. [PubMed] [Google Scholar]




