Abstract
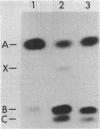
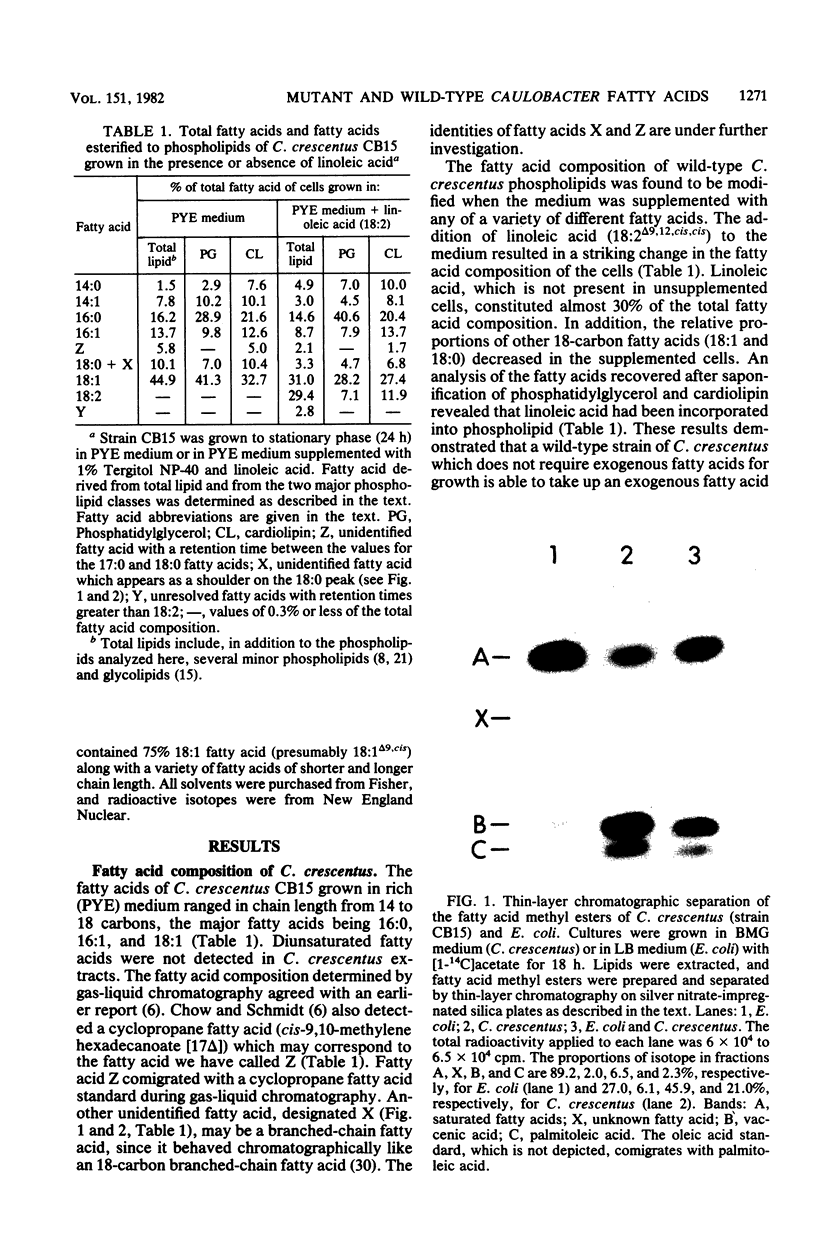
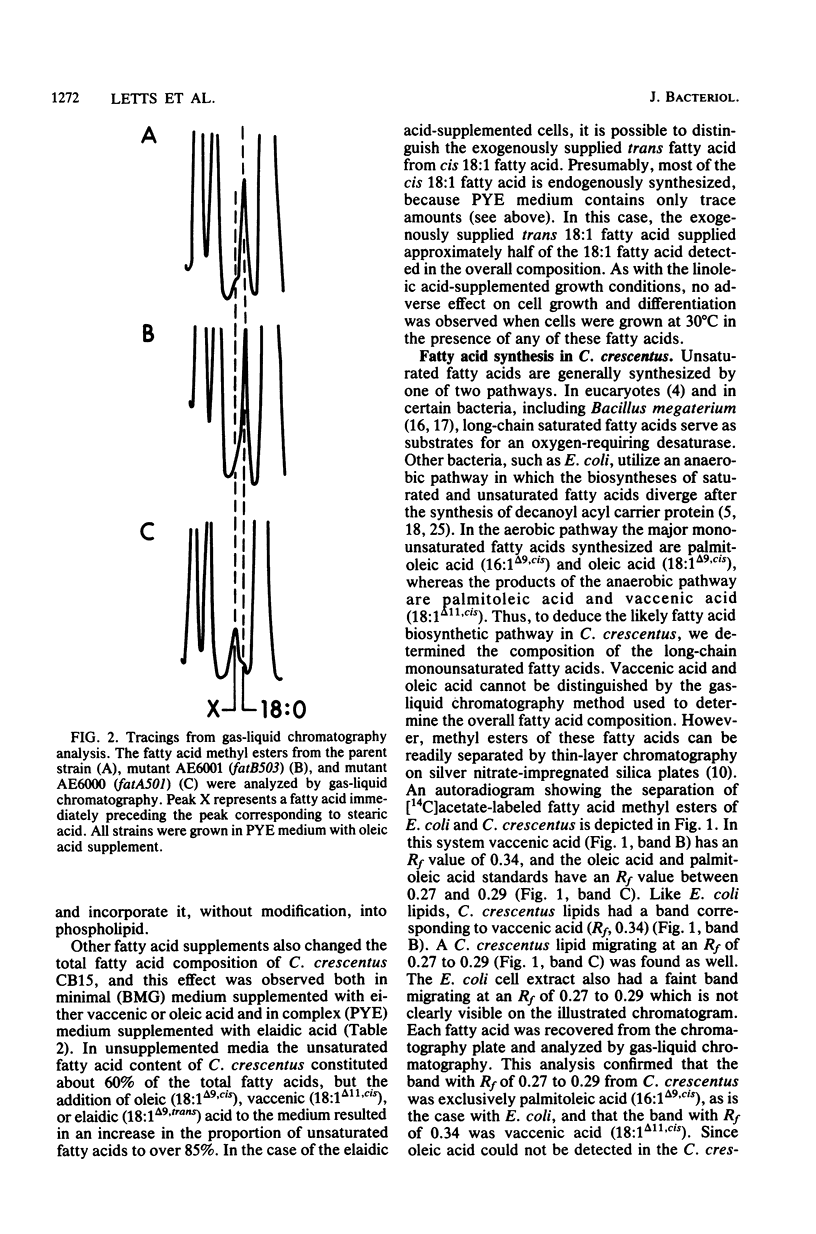
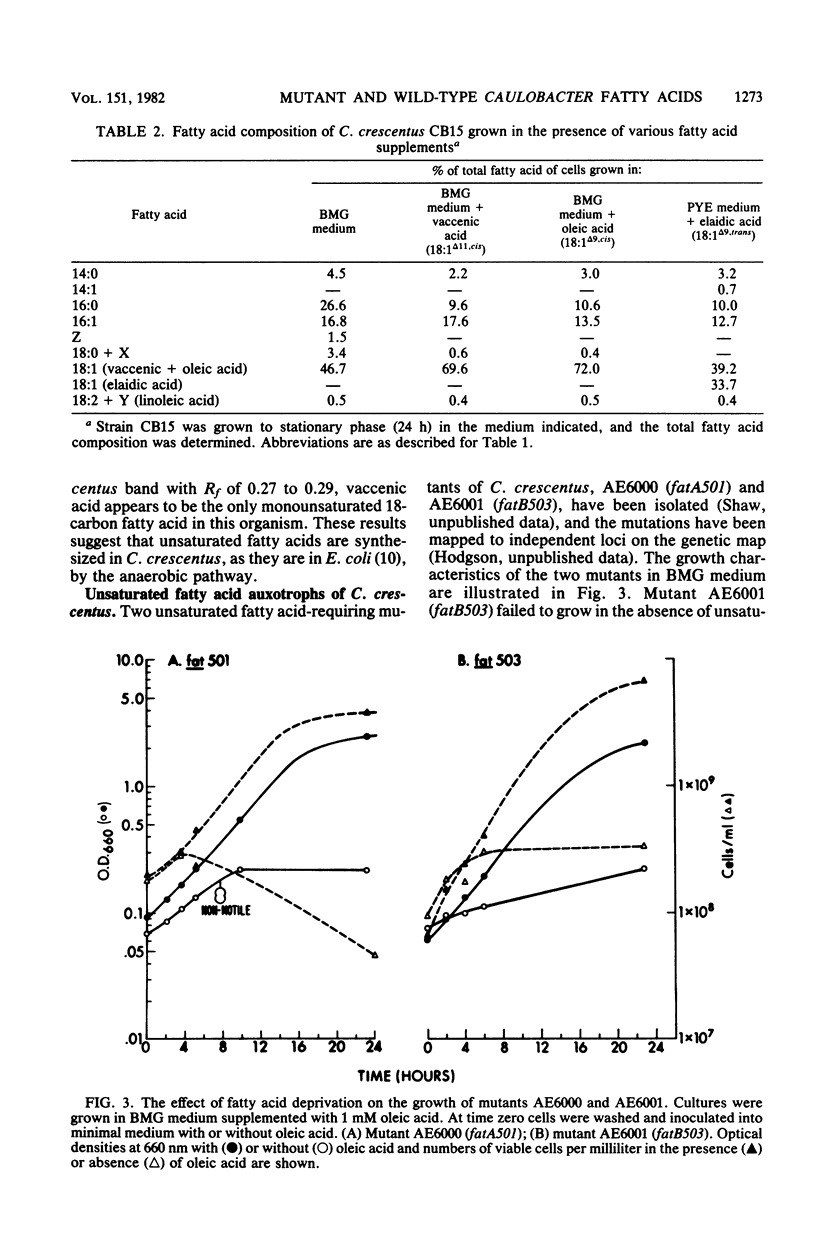
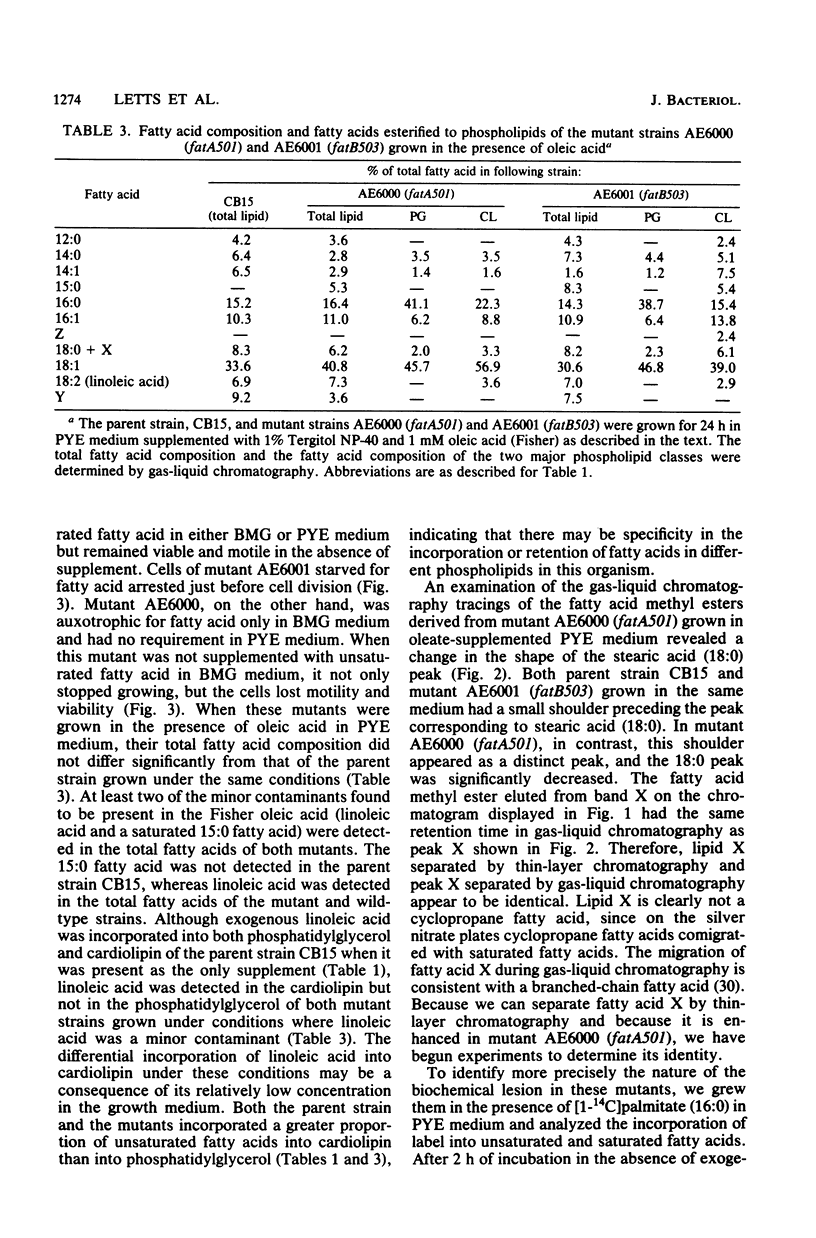
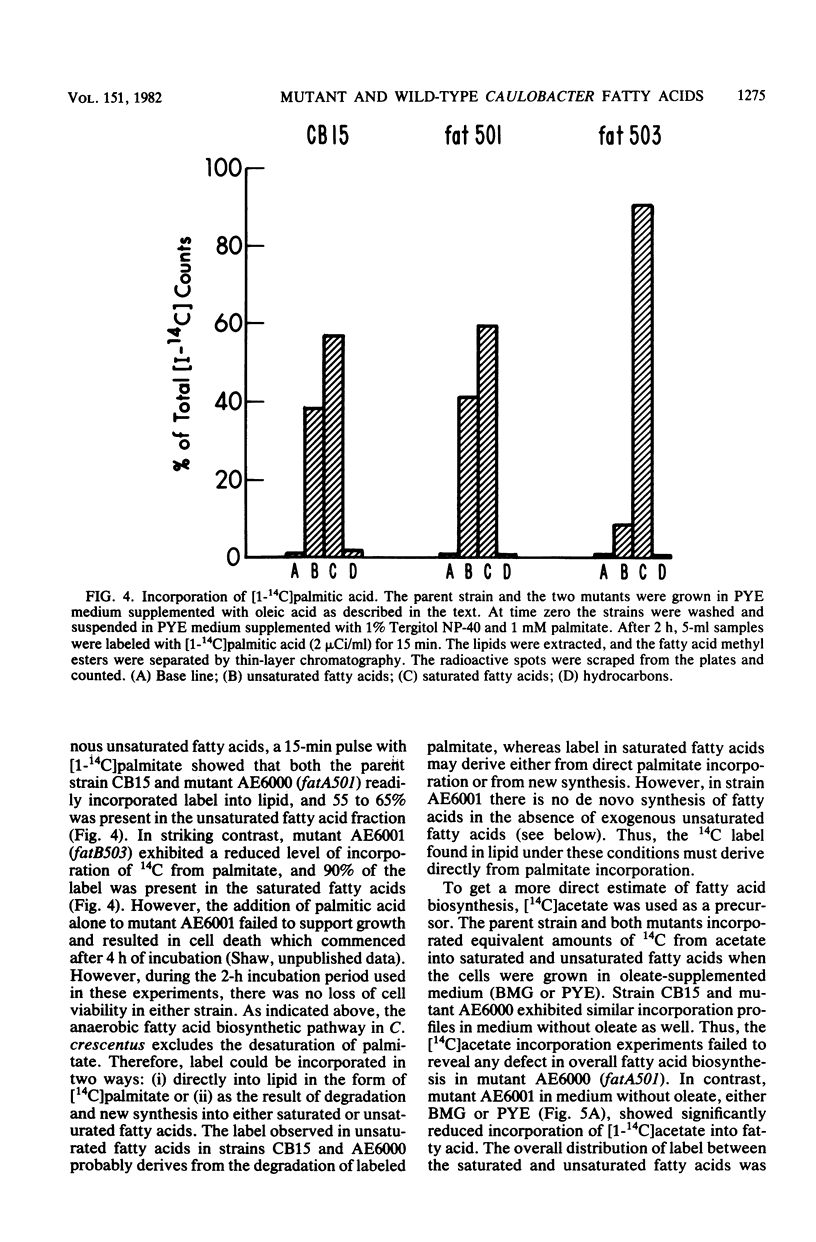
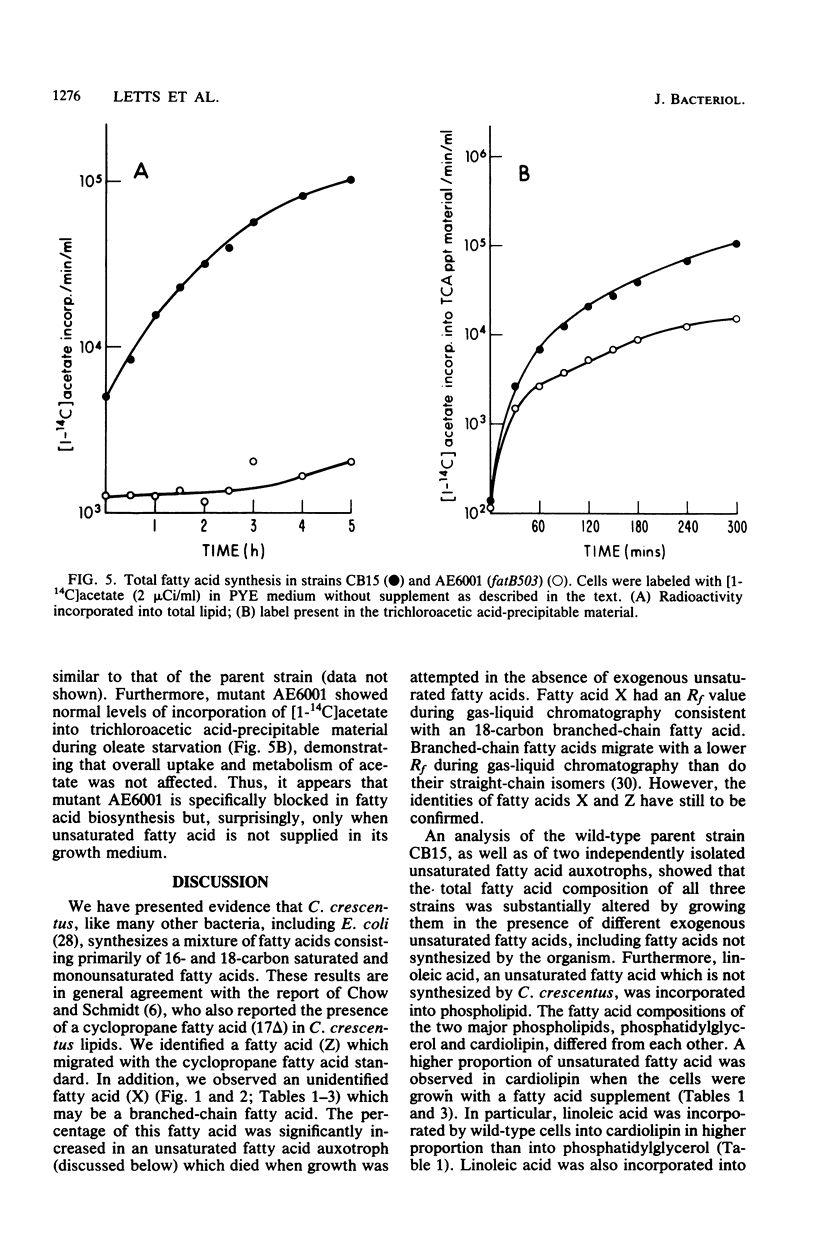
The fatty acid composition of the dimorphic bacterium Caulobacter crescentus was found to consist primarily of 16- and 18-carbon fatty acids, both saturated and monounsaturated, in agreement with the findings of Chow and Schmidt (J. Gen. Microbiol. 83:359-373, 1974). In addition, two minor but as yet unidentified fatty acids were detected. Chromatographic mobilities suggested that these fatty acids may be a cyclopropane and a branched-chain fatty acid. In addition, we demonstrated that the fatty acid composition of wild-type C. crescentus can be altered by growing the cells in medium supplemented with any one of a variety of unsaturated fatty acids. Linoleic acid, a diunsaturated fatty acid which is not synthesized by C. crescentus, was incorporated into phospholipids without apparent modification. In addition, we found that C. crescentus, like Escherichia coli, synthesizes vaccenic acid (18:1 delta 11,cis) rather than oleic acid (18:1 delta 9,cis). This result allowed us to deduce that the mechanism of fatty acid desaturation in C. crescentus is anaerobic, as it is in E. coli. Finally, we examined the fatty acid biosynthesis and composition of two unsaturated fatty acid auxotrophs of C. crescentus. Neither of these mutants resembled the E. coli unsaturated fatty acid auxotrophs, which have defined enzymatic lesions in fatty acid biosynthesis. Rather, the mutants appeared to have defects relating to the complex coordination of membrane biogenesis and cell cycle events in C. crescentus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agabian N., Evinger M., Parker G. Generation of asymmetry during development. Segregation of type-specific proteins in Caulobacter. J Cell Biol. 1979 Apr;81(1):123–136. doi: 10.1083/jcb.81.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- BLOOMFIELD D. K., BLOCH K. The formation of delta 9-unsaturated fatty acids. J Biol Chem. 1960 Feb;235:337–345. [PubMed] [Google Scholar]
- Brock D. J., Kass L. R., Bloch K. Beta-hydroxydecanoyl thioester dehydrase. II. Mode of action. J Biol Chem. 1967 Oct 10;242(19):4432–4440. [PubMed] [Google Scholar]
- Contreras I., Bender R. A., Mansour J., Henry S., Shapiro L. Caulobacter cresentus mutant defective in membrane phospholipid synthesis. J Bacteriol. 1979 Nov;140(2):612–619. doi: 10.1128/jb.140.2.612-619.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras I., Shapiro L., Henry S. Membrane phospholipid composition of Caulobacter crescentus. J Bacteriol. 1978 Sep;135(3):1130–1136. doi: 10.1128/jb.135.3.1130-1136.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras I., Weissborn A., Amemiya K., Mansour J., Henry S., Shapiro L., Bender R. The effect of termination of membrane phospholipid synthesis on cell-dependent events in Caulobacter. J Mol Biol. 1980 Apr;138(2):401–409. doi: 10.1016/0022-2836(80)90295-8. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Gelmann E. P. An estimate of the minimum amount of unsaturated fatty acid required for growth of Escherichia coli. J Biol Chem. 1973 Feb 25;248(4):1188–1195. [PubMed] [Google Scholar]
- Cronan J. E., Jr Molecular biology of bacterial membrane lipids. Annu Rev Biochem. 1978;47:163–189. doi: 10.1146/annurev.bi.47.070178.001115. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr The unsaturated fatty acids of Escherichia coli. Biochim Biophys Acta. 1967 Dec 5;144(3):695–697. doi: 10.1016/0005-2760(67)90063-x. [DOI] [PubMed] [Google Scholar]
- De Siervo A. J., Homola A. D. Analysis of caulobacter crescentus lipids. J Bacteriol. 1980 Sep;143(3):1215–1222. doi: 10.1128/jb.143.3.1215-1222.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnen S. T., Newton A. Chromosome replication during development in Caulobacter crescentus. J Mol Biol. 1972 Mar 14;64(3):671–680. doi: 10.1016/0022-2836(72)90090-3. [DOI] [PubMed] [Google Scholar]
- Degnen S. T., Newton A. Dependence of cell division on the completion of chromosome replication in Caulobacter. J Bacteriol. 1972 Jun;110(3):852–856. doi: 10.1128/jb.110.3.852-856.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FULCO A. J., LEVY R., BLOCH K. THE BIOSYNTHESIS OF DELTA-9 AND DELTA-5-MONOSATURATED FATTY ACIDS BY BACTERIA. J Biol Chem. 1964 Apr;239:998–1003. [PubMed] [Google Scholar]
- Fulco A. J. The biosynthesis of unsaturated fatty acids by bacilli. 3. Uptake and utilization of exogenous palmitate. J Biol Chem. 1972 Jun 10;247(11):3503–3510. doi: 10.2172/4729734. [DOI] [PubMed] [Google Scholar]
- Helmkamp G. M., Jr, Bloch K. Beta-hydroxydecanoyl thioester dehydrase. Studies on molecular structure and active site. J Biol Chem. 1969 Nov 10;244(21):6014–6022. [PubMed] [Google Scholar]
- Johnson R. C., Ely B. Isolation of spontaneously derived mutants of Caulobacter crescentus. Genetics. 1977 May;86(1):25–32. doi: 10.1093/genetics/86.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagenaur C., Farmer S., Agabian N. Adsorption properties of stage-specific Caulobacter phage phiCbK. Virology. 1977 Mar;77(1):401–407. doi: 10.1016/0042-6822(77)90436-6. [DOI] [PubMed] [Google Scholar]
- Mansour J. D., Henry S., Shapiro L. Differential membrane phospholipid synthesis during the cell cycle of Caulobacter crescentus. J Bacteriol. 1980 Jan;141(1):262–269. doi: 10.1128/jb.141.1.262-269.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osley M. A., Sheffery M., Newton A. Regulation of flagellin synthesis in the cell cycle of caulobacter: dependence on DNA replication. Cell. 1977 Oct;12(2):393–400. doi: 10.1016/0092-8674(77)90115-5. [DOI] [PubMed] [Google Scholar]
- POINDEXTER J. S. BIOLOGICAL PROPERTIES AND CLASSIFICATION OF THE CAULOBACTER GROUP. Bacteriol Rev. 1964 Sep;28:231–295. doi: 10.1128/br.28.3.231-295.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott D. J., Vagelos P. R. Acyl carrier protein. Adv Enzymol Relat Areas Mol Biol. 1972;36:269–311. doi: 10.1002/9780470122815.ch8. [DOI] [PubMed] [Google Scholar]
- Shapiro L. Differentiation in the Caulobacter cell cycle. Annu Rev Microbiol. 1976;30:377–407. doi: 10.1146/annurev.mi.30.100176.002113. [DOI] [PubMed] [Google Scholar]
- Shapiro L., Maizel J. V., Jr Synthesis and structure of Caulobacter crescentus flagella. J Bacteriol. 1973 Jan;113(1):478–485. doi: 10.1128/jb.113.1.478-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert D. F. Genetic modification of membrane lipid. Annu Rev Biochem. 1975;44:315–339. doi: 10.1146/annurev.bi.44.070175.001531. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Vagelos P. R. Fatty acid mutant of E. coli lacking a beta-hydroxydecanoyl thioester dehydrase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1579–1586. doi: 10.1073/pnas.58.4.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner S., Lester R. L. Metabolism of diphosphoinositide and triphosphoinositide in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Jan 27;260(1):82–87. doi: 10.1016/0005-2760(72)90076-8. [DOI] [PubMed] [Google Scholar]