Abstract
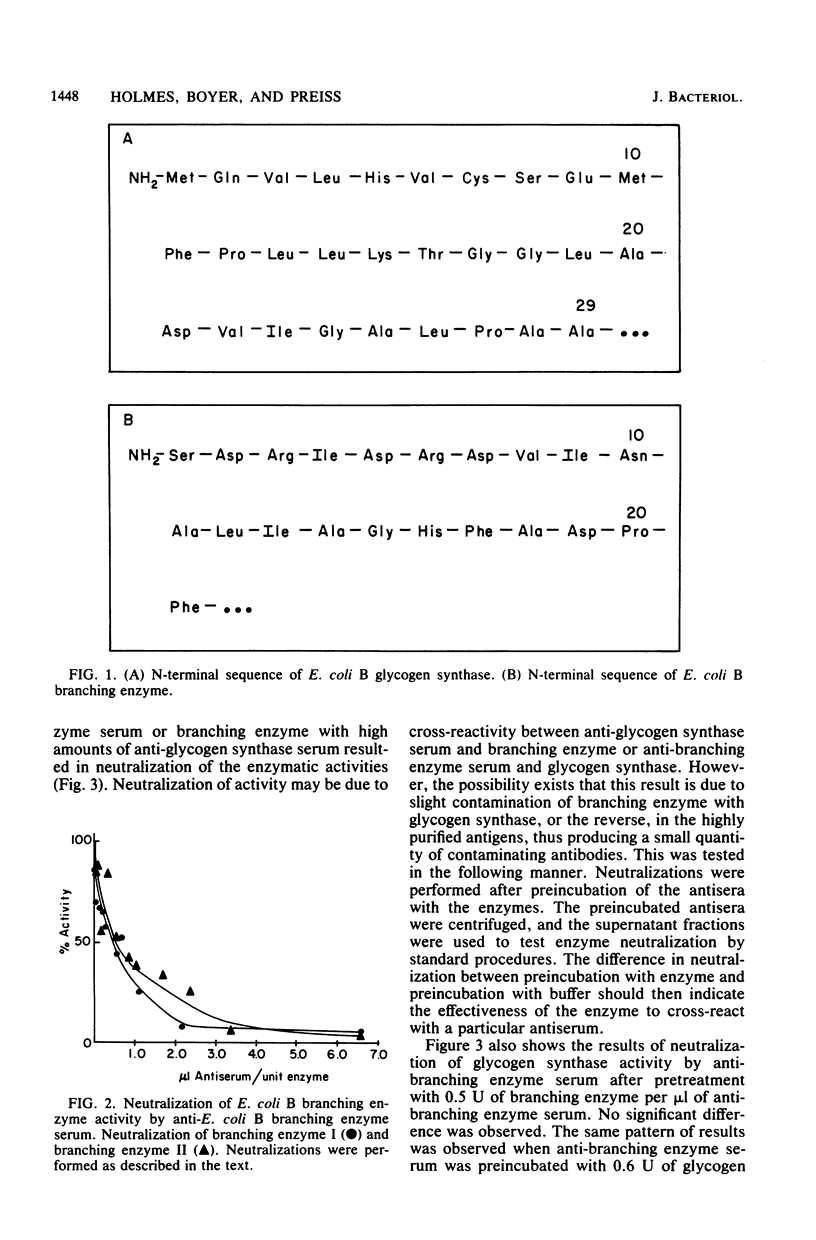
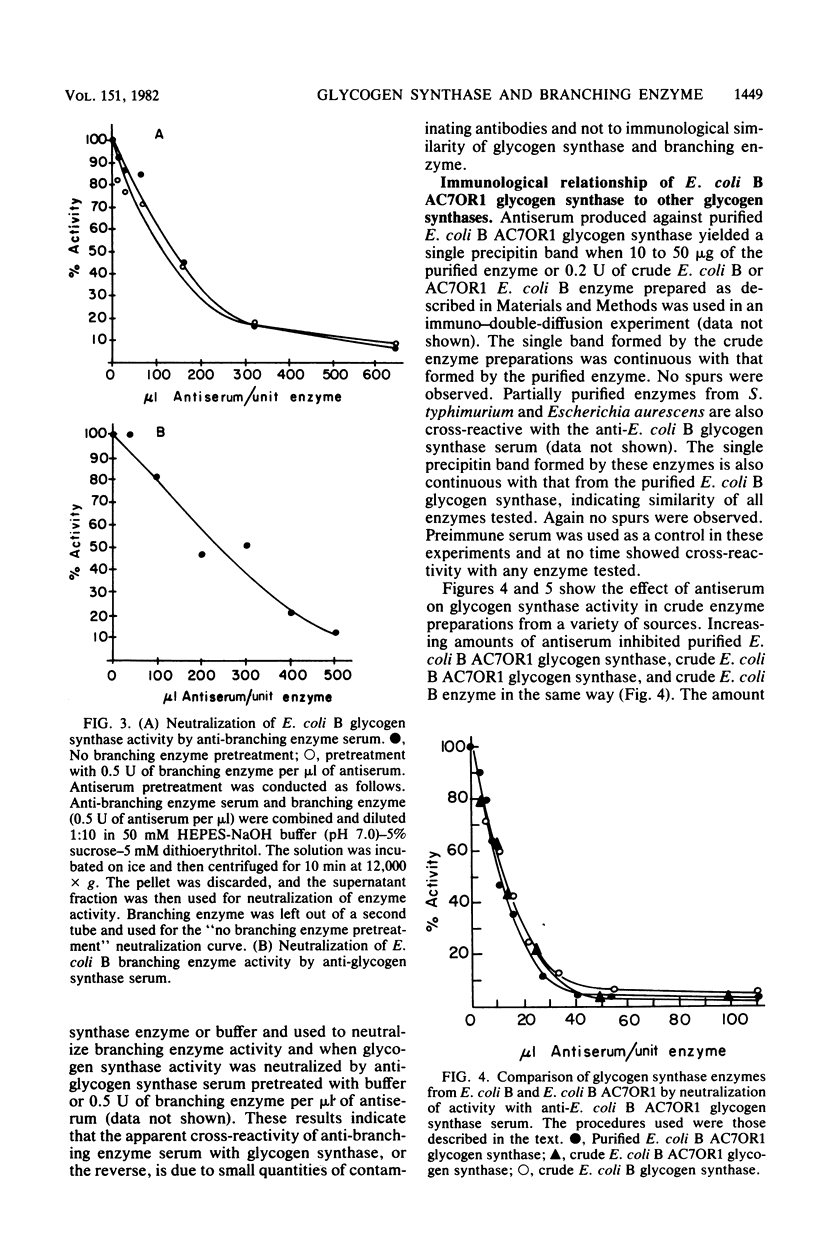
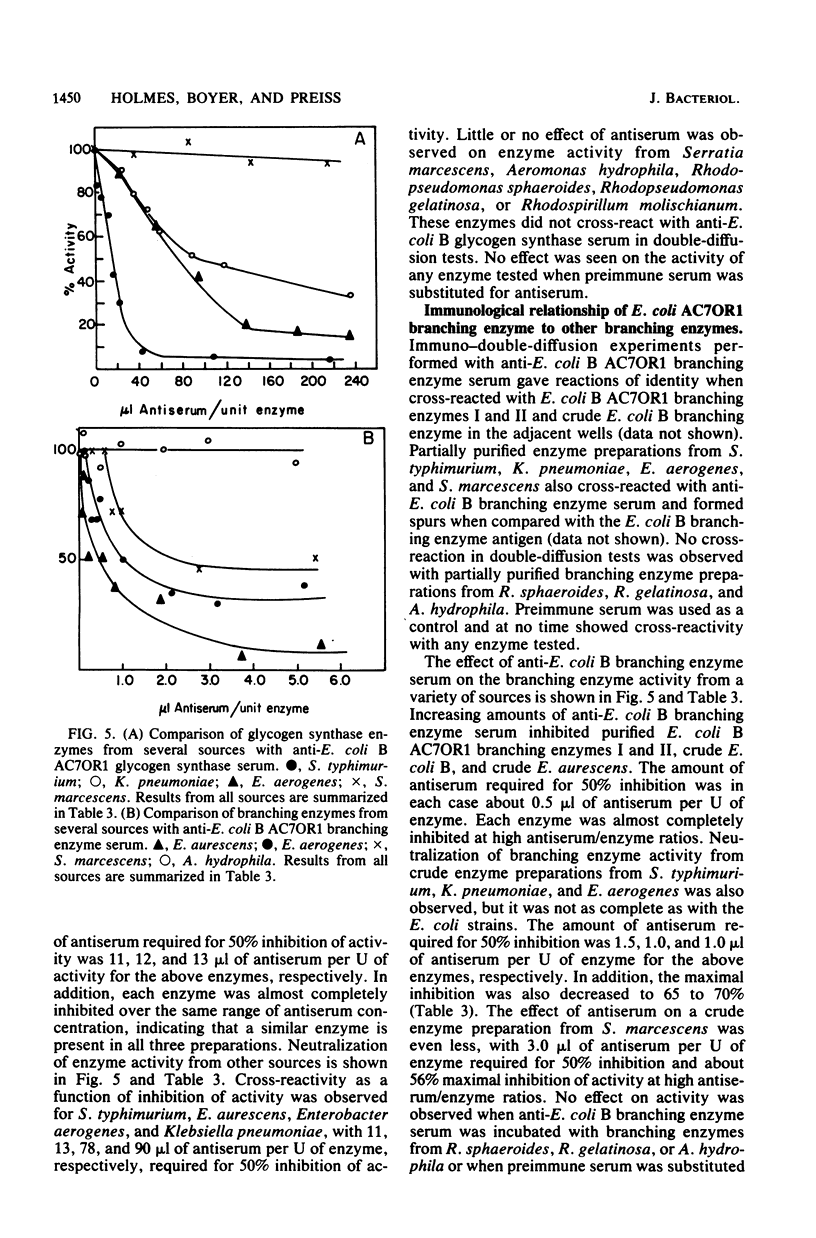
Escherichia coli B glycogen synthase and branching enzyme, although similar in amino acid composition, had no significant immunological cross-reactivity. The N-terminal sequences of the glycogen synthase were rich in hydrophobic residues, whereas branching enzyme had a higher content of acidic and basic residues. However, residues 21 to 28 of glycogen synthase and 7 to 14 of branching enzyme shared six of eight residues in common. Two fractions of branching enzyme, branching enzymes I and II, which can be isolated from E. coli B cell extracts, have been shown to be immunologically identical, suggesting that only one type of branching enzyme activity is present in E. coli B. Evidence has been obtained which indicates that E. coli B glycogen synthase and branching enzyme are antigenically very similar to glycogen synthases and branching enzymes from other enteric bacteria. No cross-reactivity with either enzyme was observed in cell extracts from photosynthetic bacteria.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann L., Baumann P. Studies of relationship among terrestrial Pseudomonas, Alcaligenes, and enterobacteria by an immunological comparison of glutamine synthetase. Arch Microbiol. 1978 Oct 4;119(1):25–30. doi: 10.1007/BF00407923. [DOI] [PubMed] [Google Scholar]
- Boyer C., Preiss J. Biosynthesis of bacterial glycogen. Purification and properties of the Escherichia coli b alpha-1,4,-glucan: alpha-1,4-glucan 6-glycosyltansferase. Biochemistry. 1977 Aug 9;16(16):3693–3699. doi: 10.1021/bi00635a029. [DOI] [PubMed] [Google Scholar]
- Brown D. H., Illingworth B., Cori C. F. THE MECHANISM OF THE DE NOVO SYNTHESIS OF POLYSACCHARIDE BY PHOSPHORYLASE. Proc Natl Acad Sci U S A. 1961 Apr;47(4):479–485. doi: 10.1073/pnas.47.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Cocks G. T., Wilson A. C. Enzyme evolution in the Enterobacteriaceae. J Bacteriol. 1972 Jun;110(3):793–802. doi: 10.1128/jb.110.3.793-802.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P. Gene rearrangements in the evolution of the tryptophan synthetic pathway. Bacteriol Rev. 1975 Jun;39(2):87–120. doi: 10.1128/br.39.2.87-120.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Kawaguchi K., Greenberg E., Preiss J. Biosynthesis of bacterial glycogen. Purification and properties of the Escherichia coli B ADPglucose:1,4-alpha-D-glucan 4-alpha-glucosyltransferase. Biochemistry. 1976 Feb 24;15(4):849–857. doi: 10.1021/bi00649a019. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen T. H., Ishaque A., Preiss J. Biosynthesis of bacterial glycogen. Characterization of the subunit structure of Escherichia coli B glucose-1-phosphate adenylyltransferase (EC 2.7.7.27). J Biol Chem. 1976 Dec 25;251(24):7880–7885. [PubMed] [Google Scholar]
- Holmes E., Preiss J. Characterization of Escherichia coli B glycogen synthase enzymatic reactions and products. Arch Biochem Biophys. 1979 Sep;196(2):436–448. doi: 10.1016/0003-9861(79)90295-9. [DOI] [PubMed] [Google Scholar]
- Huang T. S., Krebs E. G. Effect of proteases on the structure and activity of rabbit skeletal muscle glycogen synthetase. FEBS Lett. 1979 Feb 1;98(1):66–70. doi: 10.1016/0014-5793(79)80153-2. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K., Fox J., Holmes E., Boyer C., Preiss J. De novo synthesis of Escherichia coli glycogen is due to primer associated with glycogen synthase and activation by branching enzyme. Arch Biochem Biophys. 1978 Oct;190(2):385–397. doi: 10.1016/0003-9861(78)90291-6. [DOI] [PubMed] [Google Scholar]
- Kulbe K. D. Micropolyamide thin-layer chromatography of phenylthiohydantoin amino acids (PTH) at subnanomolar level. A rapid microtechnique for simultaneous multisample identification after automated Edman degradations. Anal Biochem. 1974 Jun;59(2):564–573. doi: 10.1016/0003-2697(74)90310-8. [DOI] [PubMed] [Google Scholar]
- Lehmann M., Preiss J. Biosynthesis of bacterial glycogen: purification and properties of Salmonella typhimurium LT-2 adenosine diphosphate glucose pyrophosphorylase. J Bacteriol. 1980 Jul;143(1):120–127. doi: 10.1128/jb.143.1.120-127.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- Murphy T. M., Mills S. E. Immunochemical and enzymatic comparisons of the tryptophan synthase alpha subunits from five species of Enterobacteriaceae. J Bacteriol. 1969 Mar;97(3):1310–1320. doi: 10.1128/jb.97.3.1310-1320.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Preiss J., Greenberg E. Biosynthesis of bacterial glycogen. 3. The adenosine diphosphate-glucose: alpha-4-glucosyl transferase of Escherichia coli B. Biochemistry. 1965 Nov;4(11):2328–2334. doi: 10.1021/bi00887a010. [DOI] [PubMed] [Google Scholar]
- Preiss J. Regulation of adenosine diphosphate glucose pyrophosphorylase. Adv Enzymol Relat Areas Mol Biol. 1978;46:317–381. doi: 10.1002/9780470122914.ch5. [DOI] [PubMed] [Google Scholar]
- Rocha V., Crawford I. P., Mills S. E. Comparative immunological and enzymatic study of the tryptophan synthetase beta 2 subunit in the Enterobacteriaceae. J Bacteriol. 1972 Jul;111(1):163–168. doi: 10.1128/jb.111.1.163-168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Ciardi J. E., Stadtman E. R. Comparative biochemical and immunological studies of bacterial glutamine synthetases. J Bacteriol. 1973 Sep;115(3):858–868. doi: 10.1128/jb.115.3.858-868.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vretblad P. Immobilization of ligands for biospecific affinity chromatography via their hydroxyl groups. The cyclohexaamylose-beta-amylase system. FEBS Lett. 1974 Oct 1;47(1):86–89. doi: 10.1016/0014-5793(74)80431-x. [DOI] [PubMed] [Google Scholar]
- Yung S. G., Preiss J. Biosynthesis of bacterial glycogen: purification and structural properties of Rhodospirillum tenue adenosine diphosphate glucose synthetase. J Bacteriol. 1981 Jul;147(1):101–109. doi: 10.1128/jb.147.1.101-109.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakin M. M., Garel J. R., Dautry-Varsat A., Cohen G. N., Boulot G. Detection of the homology among proteins by immunochemical cross-reactivity between denatured antigens. Application to the threonine and methionine regulated aspartokinases-homoserine dehydrogenases from Escherichia coli K 12. Biochemistry. 1978 Oct 3;17(20):4318–4323. doi: 10.1021/bi00613a032. [DOI] [PubMed] [Google Scholar]


