Abstract
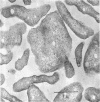
Three morphologically similar strains of halophilic, box-shaped procaryotes have been isolated from brines collected in the Sinai, Baja California (Mexico), and southern California (United States). Although the isolates in their morphology resemble Walsby's square bacteria, which are a dominant morphological type in the Red Sea and Baja California brines, they are probably not identical to them. The cells show the general characteristics of extreme halophiles and archaebacteria. They contain pigments similar to bacteriorhodopsin which apparently mediate light-driven ion translocation and photophosphorylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaurock A. E., Stoeckenius W., Oesterhelt D., Scherfhof G. L. Structure of the cell envelope of Halobacterium halobium. J Cell Biol. 1976 Oct;71(1):1–22. doi: 10.1083/jcb.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogomolni R. A., Baker R. A., Lozier R. H., Stoeckenius W. Action spectrum and quantum efficiency for proton pumping in Halobacterium halobium. Biochemistry. 1980 May 13;19(10):2152–2159. doi: 10.1021/bi00551a024. [DOI] [PubMed] [Google Scholar]
- Bogomolni R. A., Baker R. A., Lozier R. H., Stoeckenius W. Light-driven proton translocations in Halobacterium halobium. Biochim Biophys Acta. 1976 Jul 9;440(1):68–88. doi: 10.1016/0005-2728(76)90114-6. [DOI] [PubMed] [Google Scholar]
- Casadio R., Stoeckenius W. Effect of protein-protein interaction on light adaptation of bacteriorhodopsin. Biochemistry. 1980 Jul 8;19(14):3374–3381. doi: 10.1021/bi00555a043. [DOI] [PubMed] [Google Scholar]
- Danon A., Stoeckenius W. Photophosphorylation in Halobacterium halobium. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1234–1238. doi: 10.1073/pnas.71.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Kessel M., Cohen Y. Ultrastructure of square bacteria from a brine pool in Southern Sinai. J Bacteriol. 1982 May;150(2):851–860. doi: 10.1128/jb.150.2.851-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Light energy conversion in Halobacterium halobium. Microbiol Rev. 1978 Dec;42(4):682–706. doi: 10.1128/mr.42.4.682-706.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley E. V., MacDonald R. E. A second mechanism for sodium extrusion in Halobacterium halobium: a light-driven sodium pump. Biochem Biophys Res Commun. 1979 May 28;88(2):491–499. doi: 10.1016/0006-291x(79)92075-8. [DOI] [PubMed] [Google Scholar]
- MacDonald R. E., Greene R. V., Clark R. D., Lindley E. V. Characterization of the light-driven sodium pump of Halobacterium halobium. Consequences of sodium efflux as the primary light-driven event. J Biol Chem. 1979 Dec 10;254(23):11831–11838. [PubMed] [Google Scholar]
- Mescher M. F., Strominger J. L. Structural (shape-maintaining) role of the cell surface glycoprotein of Halobacterium salinarium. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2687–2691. doi: 10.1073/pnas.73.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullakhanbhai M. F., Larsen H. Halobacterium volcanii spec. nov., a Dead Sea halobacterium with a moderate salt requirement. Arch Microbiol. 1975 Aug 28;104(3):207–214. doi: 10.1007/BF00447326. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Rowen R. A morphological study of Halobacterium halobium and its lysis in media of low salt concentration. J Cell Biol. 1967 Jul;34(1):365–393. doi: 10.1083/jcb.34.1.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckenius W. Walsby's square bacterium: fine structure of an orthogonal procaryote. J Bacteriol. 1981 Oct;148(1):352–360. doi: 10.1128/jb.148.1.352-360.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland F., Dompert W., Bernhardt G., Sumper M. Halobacterial glycoprotein saccharides contain covalently linked sulphate. FEBS Lett. 1980 Oct 20;120(1):110–114. doi: 10.1016/0014-5793(80)81058-1. [DOI] [PubMed] [Google Scholar]