Abstract
The probability with which different regions of a genome come in contact with one another is a question of general interest. The current study addresses this subject for vegetatively growing diploid cells of fission yeast Schizosaccharomyces pombe by application of the Cre/loxP site-specific recombination assay. High levels of allelic interactions imply a tendency for chromosomes to be colocalized along their lengths. Significant homology-dependent pairing at telomere proximal loci and robust nonspecific clustering of centromeres appear to be the primary determinants of this feature. Preference for direct homolog-directed interactions at interstitial chromosomal regions was ambiguous, perhaps as a consequence of chromosome flexibility and the constraints and dynamic nature of the nucleus. Additional features of the data provide evidence for chromosome territories and reveal an intriguing phenomenon in which interaction frequencies are favored for nonhomologous loci that are located at corresponding relative (rather than absolute) positions within their respective chromosome arms. The latter feature, and others, can be understood as manifestations of transient, variable, and/or occasional nonspecific telomeric associations. We discuss the factors whose interplay sets the probabilities of chromosomal interactions in this organism and implications of the inferred organization for ectopic recombination.
CHROMOSOMAL interactions within the nucleus, and specifically the relationships between homologous chromosomes, is a general question of interest for eukaryotic cells. Homologous chromosome pairing is a prominent feature of meiotic prophase. Although it has been demonstrated and investigated in several model organisms, the exact mechanisms by which homologous chromosomes recognize each other and come into contact are still largely unknown (for a recent review see Zickler 2006). Outside of the meiotic program, the association of homologous chromosomes has been investigated in premeiotic and somatic (mitotically dividing) cells. Pairing in premeiotic cells has been documented in plants (e.g., Prieto et al. 2004) and both in budding and fission yeast (Scherthan et al. 1994; Weiner and Kleckner 1994). Premeiotic pairing has long been proposed to facilitate the process of homolog juxtaposition during meiosis (Stack and Brown 1969).
Observations on the behavior of chromosomes in vegetative cells show a diverse picture. In Dipteran insects the association of homologous chromosomes in vegetative cells is a normal part of nuclear organization. Homologous chromosomes are restricted to subdomains in Drosophila melanogaster interphase nuclei (Hochstrasser et al. 1986; Marshall et al. 1996) and vegetative pairing has been suggested to occur during embryonic development (Hiraoka et al. 1993; Fung et al. 1998; Gemkow et al. 1998) and in plants (Fransz et al. 2002). In other higher eukaryotes, somatic pairing is restricted to specific loci/sequences or cell types or suspected from the existence of transsensing effects. In plants, somatic pairing has been detected in the floral tissue of wheat (Aragon-Alcaide et al. 1997). In mammals' chromosomal regions, somatic pairing is generally absent, but examples of time- and stage-specific pairing of particular loci have been described (e.g., at centromeres in the cerebellum (Arnoldus et al. 1989) and, most recently, as part of X chromosome inactivation in the female (Xu et al. 2006). A tendency for homologous association of oppositely imprinted loci has also been demonstrated, suggesting that pairing may play a role in imprinting (Lasalle and Lalande 1996).
In budding yeast, somatic pairing was detected by fluorescence in situ hybridization (FISH) on spread nuclei (Burgess et al. 1999) and by application of the Cre/loxP system (Burgess and Kleckner 1999) (but for a contrasting view, see Lorenz et al. 2003). In the latter system, the rate of recombination between a particular pair of sites is a function of their relative local concentrations; relative rates of recombination for different pairs of sites can thus be used to determine relative spatial proximities and, thus, overall chromosome disposition (Burgess and Kleckner 1999).
The fission yeast Schizosaccharomyces pombe is a haploid eukaryote in which the diploid state is normally confined to the zygote that immediately undergoes meiosis. Stable diploid strains can be obtained, however, by interrupted mating or by protoplast fusion. The former method results in sporulation-capable diploid strains (heterozygous for the mating type), which can then be used as starting points to induce a relatively synchronous meiosis in the cell population (Egel 1973; Egel and Egel-Mitani 1974). FISH analysis on spread nuclei of such sporulation-capable diploid strains prior to meiotic induction can be interpreted as an evaluation of chromosome pairing in the premeiotic stage (Scherthan et al. 1994).
In this study we set up a system to further investigate overall chromosomal interactions. This system allows evaluation of the existence of somatic pairing in stable fission yeast diploids not on the cusp of entering meiosis. For this purpose, diploid strains were obtained by uniting haploid strains of identical mating types by protoplast fusion and interchromosomal interactions were then examined by application of the Cre/loxP system previously used for budding yeast. These experiments assess overall frequencies of recombination in vegetatively growing populations, thus providing an assessment of overall chromosome disposition as averaged throughout the growth cycle.
MATERIALS AND METHODS
Strains, media, and standard genetic methods:
S. pombe strains used in this study are listed in Table 1. The standard media yeast extract agar (YEA), yeast extract liquid (YEL), minimal medium (MMA), liquid minimal medium (MML), synthetic sporulation medium (SPA), and classical genetic methods (crosses and dissection of asci) were as described by Gutz et al. (1974). MMA and MML were supplemented with nutrients at 100 mg/liter and SPA at 20 mg/liter according to the experimental requirements. For protoplast fusion MMA was supplemented with 1.2 m sorbitol. YEA + 6 contained adenine, uracil, leucine, lysine, histidine, and arginine at 100 mg/liter and was used to grow haploid strains. Diploid strains were maintained on YEA + 5 and were cultivated for transformation in YEL + 5, which contained the above nutrients except for adenine. YEA contains a limiting amount of adenine; as a result, adenine-dependent haploid strains with ade6-M210 or ade6-M216 mutation form red colonies on this medium. Sporulation-incapable (h−/h−) diploid strains were constructed by protoplast fusion (Sipiczki and Ferenczy 1977). Their selection and maintenance were facilitated by the interallelic complementation of the ade6-M210 and ade6-M216 alleles which rendered them adenine independent. Diploid strains formed white colonies on YEA + 5. All incubations were carried out at 30°.
TABLE 1.
Strains
| Strain | Genotype | Origin |
|---|---|---|
| C1 | h− leu1-32 ura4-D18 ade6-M210 | J. Kohli |
| C2 | h− leu1-32 ura4-D18 ade6-M216 | J. Kohli |
| C3 | h+leu1-32 ura4-D18 ade6-M210 | J. Kohli |
| C4 | h+leu1-32 ura4-D18 ade6-M216 | J. Kohli |
| 1A/1 | h− lys3∷padh1-loxP-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 1A/3 | h+lys3∷padh1-loxP-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 2A/1 | h− his1∷padh1-loxP-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 2A/2 | h− his1∷padh1-loxP-kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| 2A/4 | h+his1∷padh1-loxP-kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| 3A/1 | h− arg7∷padh1-loxP-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 4A/1 | h− lys4∷padh1-loxP-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 4A/3 | h+lys4∷padh1-loxP-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 5A/2 | h− arg1∷padh1-loxP-kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| 1U/2 | h− lys3∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| 2U/1 | h− his1∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 2U/2 | h− his1∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| 3U/1 | h− arg7∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 3U/2 | h− arg7∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| 4U/1 | h− lys4∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 4U/2 | h− lys4∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| 4U/4 | h+lys4∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| 5U/1 | h− arg1∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M210 | This study |
| 5U/2 | h− arg1∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M216 | This study |
| 1A2U | h− lys3∷padh1-loxP-kanMX6 his1∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M210 | Cross: 1A/3 × 2U/2 |
| 2A1U | h− his1∷padh1-loxP-kanMX6 lys3∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M210 | Cross: 2A/4 × 1U/1 |
| 3A4U | h− arg7∷padh1-loxP-kanMX6 lys4∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M216 | Cross: 3A/1 × 4U/4 |
| 4A3U | h− lys4∷padh1-loxP-kanMX6 arg7∷loxP-ura4-kanMX6 leu1-32 ura4-D18 ade6-M210 | Cross: 4A/3 × 3U/2 |
| 1UDIP | h−/h− lys3∷loxP-ura4-kanMX6/lys3+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 1U/2 × C1 |
| 2UDIP | h−/h− his1∷loxP-ura4-kanMX6/his1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 2U/2 × C1 |
| 3UDIP | h−/h− arg7∷loxP-ura4-kanMX6/arg7+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 3U/2 × C1 |
| 4UDIP | h−/h− lys4∷loxP-ura4-kanMX6/lys4+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 4U/2 × C1 |
| 5UDIP | h−/h− arg1∷loxP-ura4-kanMX6/arg1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 5U/2 × C1 |
| 1A1U | h−/h− lys3∷padh1-loxP-kanMX6/lys3∷loxP-ura4-kanMX6 leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 1A/1 × 2U/2 |
| 2A2U | h−/h− his1∷padh1-loxP-kanMX6/his1∷loxP-ura4-kanMX6 leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 2A/1 × 2U/2 |
| 3A3U | h−/h− arg7∷padh1-loxP-kanMX6/arg7∷loxP-ura4-kanMX6 leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 3A/1 × 3U/2 |
| 4A4U | h−/h− lys4∷padh1-loxP-kanMX6/lys4∷loxP-ura4-kanMX6 leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 4A/1 × 4U/2 |
| 5A5U | h−/h− arg1∷padh1-loxP-kanMX6/arg1∷loxP-ura4-kanMX6 leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 5A/2 × 5U/1 |
| 1A2U cisa | h−/h− lys3∷padh1-loxP-kanMX6/lys3+ his1∷loxP-ura4-kanMX6/his1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 1A2U × C2 |
| 1A2U transb | h−/h− lys3∷padh1-loxP-kanMX6/lys3+ his1∷loxP-ura4-kanMX6/his1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 1A/1 × 2U/2 |
| 1A3U | h−/h− lys3∷padh1-loxP-kanMX6/lys3+ arg7∷loxP-ura4-kanMX6/arg7+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 1A/1 × 3U/2 |
| 1A4U | h−/h− lys3∷padh1-loxP-kanMX6/lys3+ lys4∷loxP-ura4-kanMX6/lys4+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 1A/1 × 4A/2 |
| 1A5U | h−/h− lys3∷padh1-loxP-kanMX6/lys3+ arg1∷loxP-ura4-kanMX6/arg1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 1A/1 × 5U/2 |
| 2A1U cis | h−/h− his1∷padh1-loxP-kanMX6/his1+ lys3∷loxP-ura4-kanMX6/lys3+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 2A1U × C2 |
| 2A1U trans | h−/h− his1∷padh1-loxP-kanMX6/his1+ lys3∷loxP-ura4-kanMX6/lys3+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 2A/1 × 1U/2 |
| 2A3U | h−/h− his1∷padh1-loxP-kanMX6/his1+ arg7∷loxP-ura4-kanMX6/arg7+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 2A/2 × 1U/1 |
| 2A4U | h−/h− his1∷padh1-loxP-kanMX6/his1+ lys4∷loxP-ura4-kanMX6/lys4+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 2A/1 × 4U/2 |
| 2A5U | h−/h− his1∷padh1-loxP-kanMX6/his1+ arg1∷loxP-ura4-kanMX6/arg1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 2A/1 × 5U/2 |
| 3A1U | h−/h− arg7∷padh1-loxP-kanMX6/arg7+ lys3∷loxP-ura4-kanMX6/lys3+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 3A/1 × 1U/2 |
| 3A2U | h−/h− arg7∷padh1-loxP-kanMX6/arg7+ his1∷loxP-ura4-kanMX6/his1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 3A/1 × 2U/2 |
| 3A4U cis | h−/h− arg7∷padh1-loxP-kanMX6/arg7+ lys4∷loxP-ura4-kanMX6/lys4+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 3A4U × C1 |
| 3A4U trans | h−/h− arg7∷padh1-loxP-kanMX6/arg7+ lys4∷loxP-ura4-kanMX6/lys4+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 3A/1 × 4U/2 |
| 3A5U | h−/h− arg7∷padh1-loxP-kanMX6/arg7+ arg1∷loxP-ura4-kanMX6/arg1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 3A/1 × 5U/2 |
| 4A1U | h−/h− lys4∷padh1-loxP-kanMX6/lys4+ lys3∷loxP-ura4-kanMX6/lys3+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 4A/1 × 1U/2 |
| 4A2U | h−/h− lys4∷padh1-loxP-kanMX6/lys4+ his1∷loxP-ura4-kanMX6/his1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 4A/1 × 2U/2 |
| 4A3U cis | h−/h− lys4∷padh1-loxP-kanMX6/lys4+ arg7∷loxP-ura4-kanMX6/arg7+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 4A3U × C2 |
| 4A3U trans | h−-/h− lys4∷padh1-loxP-kanMX6/lys4+ arg7∷loxP-ura4-kanMX6/arg7+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 4A/1 × 3U/2 |
| 4A5U | h−/h− lys4∷padh1-loxP-kanMX6/lys4+ arg1∷loxP-ura4-kanMX6/arg1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 4A/1 × 5U/2 |
| 5A1U | h−/h− arg1∷padh1-loxP-kanMX6/arg1+ lys3∷loxP-ura4-kanMX6/lys3+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 5A/2 × 1U/1 |
| 5A2U | h−/h− arg1∷padh1-loxP-kanMX6/arg1+ his1∷loxP-ura4-kanMX6/his1+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 5A/2 × 1U/1 |
| 5A3U | h−/h− arg1∷padh1-loxP-kanMX6/arg1+ arg7∷loxP-ura4-kanMX6/arg7+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 5A/2 × 3U/1 |
| 5A4U | h−/h− arg1∷padh1-loxP-kanMX6/arg1+ lys4∷loxP-ura4-kanMX6/lys4+ leu1-32/leu1-32 ura4-D18/ura4-D18 ade6-M210/ade6-M216 | Fusion: 5A/2 × 4U/1 |
The promoter construct and the gene construct are located on the same chromosome.
The promoter construct and the gene construct are on different homologous chromosomes.
Construction of strains and an expression vector:
General molecular procedures were performed as described by Sambrook et al. (1989). To construct strains carrying the padh1-loxP-kanMX6 insert, first the sequence of the adh1 promoter (McLeod et al. 1987) was amplified by PCR with primers: GTTGGATCCGCATGCCCTACAACAACTAAGAAAATGGC (forward) and GTTGGATCCATAACTTCGTATAATGTATGCTATACGAAGTTATGGAATTCTCTTGCTTAAAGAAAAGCG (reverse), using genomic DNA as template. Both primers contained a BamHI restriction site (italics) and the reverse primer contained the 34-bp long loxP sequence of bacteriophage P1 (underlined). This fragment was inserted into the BamHI site of plasmid pFA6a-kanMX6 (Bähler et al. 1998) which is situated right upstream of the kanMX6 cassette, and its correctness was verified by sequencing. The resulting plasmid was digested with restriction enzymes PvuII, EcoRV, and XmnI; the PvuII–EcoRV fragment carrying the padh1-loxp-kanMX6 construct was purified from gel and used for blunt-end ligation (see below).
Five S. pombe genes were cloned in pGEM-T Easy vector (Promega, Madison, WI) by amplifying their sequences with PCR. The genes and the corresponding forward and reverse primers were as follows. lys3: ATGGTTGCTCCTCATCTTTGGTTACGTGC and CACTAAATGCTTCGGAAGATTCCCTTGGC; his1: ATGGATTTGGTTAACC ACTTGGAAGATCGG and GAGAAAGCTTATCCATCACTTGGGCC; arg7: CTATGGGGAGGTAGATTTTCAGGAGC and CTTGAACACAATGCTTAGCAGTA CCACC; lys4: ATGTCTGTGTCCGAAGCTAATGGTACT and TTAAGCAGACGCTT CTTTGGTGATTCTATC; arg1: CTTACCCACAAAGATCCAACTCC and TTCAAAGTGCTCTCCATGATATCC. With a second set of gene-specific primers PCR reactions were carried out on these pGEM-T Easy vectors carrying the different genes. The primers were designed such that, in the resulting fragments, ∼200-bp long sequences of the 5′ and 3′ end of the corresponding gene flanked the sequence of the vector. The primers were as follows. For the pGEM-T Easy-lys3 vector: GTTGTCGACATGTACGCGTCCTTAGGTGC and GTTACTAGTAATTCTGTACCG TCGAGTCG; pGEM-T Easy-his1: GTTGTCGACCCTTTAAGACATTCACGCAGG and GTTACTAGTTGGTTATATCATCGCCCAGC; pGEM-T Easy-lys4: GTTGTCGACCACCAAAGTTGTCCAATGCC and GTTACTAGTCGTTTGACTGG TTGGAATGC; pGEM-T Easy-arg1: GTTGTCGACACTTCAGGATGAGCATGACC and GTTACTAGTATTCGTGGATATGCTGGTCG. To the pGEM-T Easy-arg7 vector two primer pairs were applied; the fragment resulting from primers CTCTTTGAATTTGTTCCAATCC and ACTCATCATATTTCGGGATCC was used to create the padh1-loxP-kanMX6 construct, and the fragment resulting from primers GTTGTCGACACTTC GGATGAGCATGACC and GTTACTAGTATTCGTGGATATGCTGGTCG was used to get the ura4-loxP-kanMX6 construct. SalI (GTCGAC) and SpeI (ACTAGT) restriction sites are indicated in italics. The 5′gene pGEM-T Easy-3′gene fragments were blunt-end ligated with the padh1-loxP-kanMX6 construct, the orientation of the cassette was checked by PCR, and those with correct orientation (see Figure 1B) were chosen for yeast transformation.
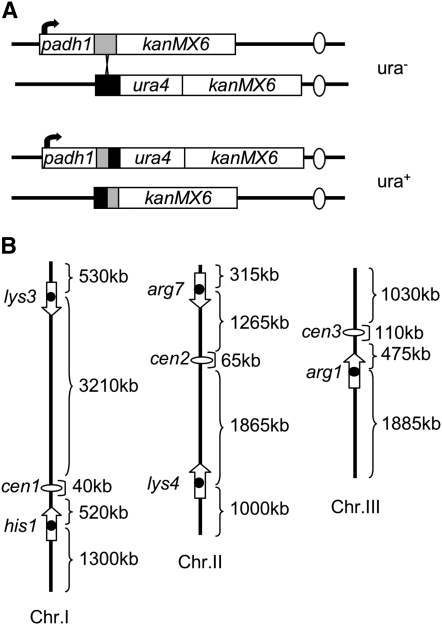
Figure 1.—
Cre/loxP recombination assay. (A) Recombination between the promoter construct (padh1-loxP-kanMX6) and the gene construct (loxP-ura4-kanMX6) results in ura4+ phenotype. The shaded and solid boxes represent loxP sequences, the ovals indicate centromeres. (B) The position and orientation of the loxP sites integrated into the fission yeast genome are indicated by arrows.
To construct strains with the loxP-ura4-kanMX6 insert the ORF of the ura4 gene with 52-bp upstream sequence was amplified with primers: GTTGGATCC ATAACTTCGTATAGCATACATTATACGAAGTTATCCAAGAACCTCTTTTTTGCTTGGATCG (forward) and GTTGGATCCTTAATGCTGAGAAAGTCTTTGCTGATATGC (reverse) on plasmid pCG1 (Grimm et al. 1988) as template. The primers carried a BamHI restriction site (italics) and the forward primer contained the loxP sequence (underlined). The construct was inserted into the BamHI site of pFA6a-kanMX6 and checked by sequencing. The resulting plasmid was digested with PvuII, Ecl136II, and BanI; the PvuII–Ecl136II fragment carrying the ura4-loxP-kanMX6 construct was purified from gel and used for blunt-end ligation with the 5′gene pGEM-T Easy-3′gene fragments described above. The orientation of the constructs was checked by PCR.
The pGEM-T Easy vectors carrying the padh1-loxP-kanMX6 or the ura4-loxP-kanMX6 construct flanked by gene specific sequences were digested with NotI and used to transform strain C1 or C4 (Table 1) according to the method of Bähler et al. (1998). Transformants were selected on YEA + 6 plates supplemented with 100 mg/liter Geneticin/G418 (Invitrogen, Carlsbad, CA). Integration of the constructs at the proper site was confirmed by PCR. Finally, the strains carrying the padh1-loxP-kanMX6 or ura4-loxP-kanMX6 construct were crossed with a partner of opposite mating type (C2 or C3, Table1), and single integration of the appropriate construct was confirmed by the regular segregation (2:0) of G418-resistant clones in tetrad analysis.
To express the Cre recombinase of bacteriophage P1 in fission yeast, first its sequence was amplified by PCR on plasmid pNK553 (a pUC18-based plasmid carrying the GAL1-Cre sequence; plasmid collection, N. Kleckner laboratory). The forward and reverse primers containing an NdeI and a SalI site (italics), respectively, were: GTTTCATATGTCCAATTTACTGACCG and GTTTGTCGACCTAATCGCCATCTTCCAGC. The fragment was cloned into the corresponding restriction sites of plasmid pREP81 (Basi et al. 1993) and the sequence was confirmed by PCR. This plasmid carries a weak version of the thiamine repressible nmt1 promoter.
Measurement of recombination frequencies:
Diploid strains carrying a promoter and a gene construct were created by protoplast fusion and then transformed with plasmid pREP81-Cre according to the lithium acetate method (Ito et al. 1983). Transformants grew on MMA + 4 (supplemented with leucine, lysine, histidine, and arginine) for 7 days. Individual colonies were isolated and cultivated in MML + 4 (supplemented with nutrients as above) for 1 day. MMA contains thiamine, therefore it allowed only weak expression of the Cre protein. Edinburg minimal medium (EMM2) designed for nmt1 promoter studies for fission yeast was not chosen because diploid strains showed worse growth on this medium. Transformant colonies were picked directly from primary selective plates, suspended in MML + 4 medium, grown to a cell density of 5 × 106 to 1–2 × 107, and replated on YEA + 5 to determine viable cell number and on appropriately supplemented MMA plates to detect ura4+ recombinants. Recombination frequencies were expressed as number of ura4+ recombinants per 103 colony-forming units (CPU).
Generally eight measurements (on eight transformant colonies) were carried out with each pair of loxP sites. In this assay the actual recombination frequency determined for a cell clone depends on the time of occurrence of recombination between the loxP constructs. Analogously to a fluctuation test, early recombination of the loxP sites occasionally resulted in unusually high recombination frequency (jackpot clone). Involvement of jackpot clones in the calculation of average recombination frequencies would distort the result; therefore for each pair of loxP sites, the highest and the lowest recombination frequencies were systematically excluded from the calculation.
RESULTS
The recombination assay:
Cre recombinase promotes site-specific recombination between two 34-bp loxP sequences of bacteriophage P1 (Hoess et al. 1982; Ambremski et al. 1983). For this reaction, the probability of recombination over time is a reflection of the local concentrations of two sites with respect to one another. Correspondingly, the relative frequencies of recombination between different pairs of loxP sites reflects differences in relative spatial disposition within the nucleus, with pairs of sites that tend to be closer together giving higher recombination frequencies than pairs of sites that tend to be farther apart. In constructs where recombination results in an appropriately selectable functional readout, such as turning on expression of a gene, recombination rates (and thus relative spatial dispositions) can be determined genetically. Such a system has been used previously to probe genome disposition in budding yeast (Burgess and Kleckner 1999). An analogous system involving the Flp recombination reaction has been used to probe the genome of Drosophila (Golic et al. 1997).
In this study we apply this approach to fission yeast. For this purpose, two constructs containing the loxP sequence were created (Figure 1A). In the promoter construct (padh1-loxP) the loxP site is downstream of padh1, a strong, constitutive promoter (McLeod et al. 1987). In the gene construct (loxP-ura4) it is upstream of a promoterless ura4 gene. Cre-promoted crossing over between two sites results in a padh1-loxP-ura4 fusion and prototroph phenotype. The frequency of ura+ prototrophs reflects the frequency of collision of the loci and recombination between the two loxP sites.
Each loxP construct was integrated at five different loci of the fission yeast genome (Figure 1B). Stable diploid strains (h−/h−) carrying a promoter and a gene construct were created by protoplast fusion (Table 1). Sites were chosen to represent different chromosomes and different absolute and relative positions from centromeres and telomeres. All loxP constructs are oriented in such a way that recombination between sites located on two chromosomes results in reciprocal translocation of chromosome arms, thus ensuring that recombination never creates aberrant situations with dicentric or acentric chromosomes. This requirement was met by using orientations in which the direction arrow of recombination always points away from the centromere (Figure 1B). An additional benefit of this convention is that recombination between loxP sites located on the same chromosome inverts the region between the loci. Cre recombinase is expressed from the nmt1 promoter of plasmid pREP81-Cre, which is very weak under the repressed conditions chosen for the current study. Recombination frequencies were determined in individual clones by plating on selective and nonselective media and expressed as the number of ura4+ recombinants per viable colony-forming units (see materials and methods). The observed frequencies represent recombination over ∼25 generations of exponential growth. It is not known whether recombination is constant among different cell-cycle stages. However, in actively growing S. pombe, ∼80% of the generation time is G2, suggesting that most recombination events are occurring during this period.
Formation of ura+ prototrophs in this system is dependent on the Cre protein and two loxP sites (Table 2). In the absence of Cre recombinase, no prototroph colonies were detected. Elimination of the ura4-loxP construct prevents prototroph formation in the diploid strains completely, due to their ura4-D18 background (full deletion of ura4, Grimm et al. 1988). Elimination of the promoter construct reduced the number of ura+ prototrophs severely, likely due to low levels of recombination with pseudo-loxP sites. In budding yeast such sites occur downstream of promoter regions (Sauer 1992). Similar sites may exist in fission yeast as well, with recombination between the loxP-ura4 insert and a suitably positioned endogenous pseudo-loxP site accounting for the low residual recombination detectable in strains lacking the promoter construct.
TABLE 2.
Formation of ura+ recombinants depends on Cre recombinase and two loxP inserts
| Position of loxP insert
|
Recombination frequencya
|
||
|---|---|---|---|
| padh1-loxP | loxP-ura4 | −Cre | +Cre |
| arg1 | arg1 | <0.00022 | 79 ± 16.5 |
| arg1 | lys4 | <0.00017 | 4.5 ± 1.5 |
| None | arg1 | 0.16 ± 0.16 | |
| None | lys4 | 0.06 ± 0.02 | |
| None | arg7 | 0.03 ± 0.02 | |
| None | his1 | 0.05 ± 0.01 | |
| None | lys3 | <0.006 | |
Each value represents the average of three measurements.
Expressed as the number of ura+ recombinants/103 CPU.
Data set and evaluation of insert-specific effects:
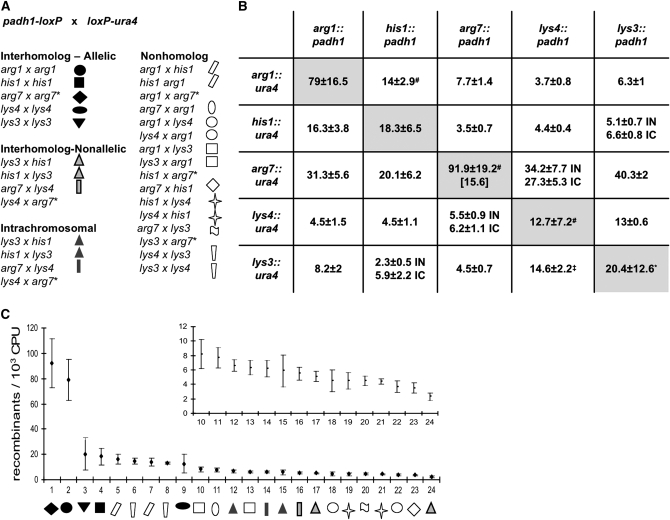
Recombination frequencies were determined between each possible pairwise combination of promoter and gene construct. These 29 combinations represent four different types of interactions (Figure 2A).
Type I: Interhomolog allelic. In five strains the loxP constructs are situated at allelic positions of homologous chromosomes.
Type II: Interhomolog nonallelic. In four strains, the two interacting sites are located on homologous chromosomes but at nonallelic positions.
Type III: Intrachromosomal. In four strains, the two loxP sites are situated at different positions along the same physical chromosome.
Type IV: Nonhomolog. In the remaining 16 strains, the loxP sites are present on nonhomologous chromosomes.
Figure 2.—
Recombination frequencies among 29 pairs of loxP constructs. (A) Recombination frequencies were detected in four different types of interactions: (1) interhomolog allelic, the loxP sites are located at allelic positions of homologous chromosomes; (2) interhomolog nonallelic, loxP sites at nonallelic positions of homologous chromosomes; (3) intrachromosomal, loxP sites on the same chromosome; and (4) nonhomolog, loxP sites positioned on nonhomologous chromosomes. Reciprocal pairs are denoted by the same symbol. (*) The arg7∷ura4 construct shows insert-specific aberrancy (see text). (B) Summary of recombination frequencies and their standard deviations. padh1 represents the promoter construct and ura4 stands for the gene construct integrated at different loci. Recombination frequencies of allelic interactions are shaded. IN, interhomolog nonallelic interaction; IC, intrachomosomal interaction. Values represent the average and standard deviation of six measurements, unless otherwise indicated. (*) Fourteen measurements, (#) 5 measurements, (‡) 4 measurements. Value in brackets represents the measured recombination frequency of the arg7 allelic interaction that was corrected for the hyperrecombinogenic effect of arg7∷ura4 by dividing by six, which is the average hyperrecombinogenic effect of this locus seen in nonallelic interactions. (C) Plot of recombination frequencies arrayed in descending rank order. Symbols are assigned to the different chromosomal interactions as in A. Inset: the part of the plot showing the lowest recombination frequencies (strains 10–24) is magnified for better visibility and thus easier comparison of the values.
The recombination frequencies and their standard deviations observed in these 29 strains are summarized in Figure 2B.
In principle, recombination frequencies might be affected not only by the relative spatial positions of the interacting loci but by peculiarities of a particular construct and its context. The possibility of such effects can be examined using the 24 strains in which the promoter construct and the gene construct are situated at nonallelic positions (types II–IV above). These strains comprise 12 different interlocus interactions, each represented by two reciprocal construct configurations according to which locus has the promoter construct and which has the gene construct. The members of each pair differ only with respect to which loxP construct is located at which of the two loci. Thus, if the inserts themselves do not cause any specific effect, the recombination frequencies of a reciprocal pair are expected to be close to each other. Conversely, a construct-specific effect should be revealed as a systematic difference for recombination frequencies involving that construct.
In Figure 2B the 12 reciprocal pairs are located symmetrically to either side of the diagonal line of allelic interactions. For all combinations involving inserts at four of the test loci, his1, arg1, lys3, and lys4, the ratios of the recombination frequencies of the reciprocal pairs are close to one, with the highest difference of approximately twofold observed only in one case (lys3 × his1 and his1 × lys3) (Table 3A). However, for every combination involving the fifth locus, arg7, reciprocity is not observed (Table 3B). Here there is at least a fourfold, and on average a sixfold, difference between the recombination frequencies of the reciprocal pairs. This asymmetry appears to be specifically attributable to the arg7∷ura4 construct. Frequencies measured for combi-nations between this construct and constructs at other loci are very high; in contrast, recombination frequencies measured with the arg7∷padh1 construct are comparable to those measured with inserts at the other four loci. These data suggest that the arg7∷ura4 construct exhibits a hyperrecombinogenic effect which is an insert-specific aberrancy, whereas the other nine inserts lack any insert-specific peculiarity. For this reason, all of the data for the arg7∷ura4 construct combination with nonallelic loci (five strains in total) were excluded from further analysis. However, for the allelic interaction involving this construct, arg7∷ura4 × arg7∷padh1, the recombination frequency observed is typical of other allelic interactions, even if the hyperrecombinogenic effect of arg7∷ura4 is taken into account (below). This interaction is therefore still included in our analysis.
TABLE 3.
Comparison of recombination frequencies of reciprocal interactions
| Reciprocal interactions compared padh1-loxP × ura4-loxP | Recombinants/103 CPU | Ratios of recombination frequencies |
|---|---|---|
| A. Not involving arg7 | ||
| arg1 × his1; his1 × arg1 | 16.3 vs. 14 | 1.16 |
| arg1 × lys4; lys4 × arg1 | 4.5 vs. 3.7 | 1.22 |
| arg1 × lys3; lys3 × arg1 | 8.2 vs. 6.3 | 1.3 |
| his1 × lys4; lys4 × his1 | 4.5 vs. 4.4 | 1.02 |
| lys4 × lys3; lys3 × lys4 | 14.6 vs. 13 | 1.12 |
| lys3 × his1; his1 × lys3 transa | 5.1 vs. 2.3 | 2.22 |
| lys3 × his1; his1 × lys3 cisb | 6.6 vs. 5.9 | 1.12 |
| B. Involving arg7 | ||
| arg1 × arg7; arg7 × arg1 | 31.3 vs. 7.7 | 4.06 |
| his1 × arg7; arg7 × his1 | 20.1 vs. 3.5 | 5.74 |
| lys3 × arg7; arg7 × lys3 | 40.3 vs. 4.5 | 8.96 |
| lys4 × arg7; arg7 × lys4 transa | 34.2 vs. 5.5 | 6.22 |
| lys4 × arg7; arg7 × lys4 cisb | 27.3 vs. 6.2 | 4.4 |
trans, interhomolog trans interaction.
cis, intrachromosomal interaction.
General variations among the four categories of interactions:
Data for the (29 − 5 = 24) strains under consideration is presented graphically in Figure 2C. Strains are arrayed in rank order from highest to lowest levels of recombination. This presentation makes it clear that recombination frequencies vary over a total range of ∼34-fold (strains 2 vs. 24). The standard deviations of measured recombination frequencies for the examined strains vary but, on average, correspond to ∼25% of the base recombination value (23.4%).
With regard to the four types of interactions examined, several general trends are recognizable. First, allelic interactions generally show the highest recombination frequencies. This trend implies a tendency for homologous chromosomes to be generally coaligned. Second, intrachromosomal interactions exhibit higher recombination frequencies than interhomolog nonallelic interactions. This trend suggests that, irrespective of the relative positions of homologs, each chromosome tends to occupy its own territory. Third, additional features of the data show that centromeres and telomeres play particularly important roles in chromosome disposition, via the combined effects of nonspecific and homology-based interactions.
Allelic interhomolog recombination frequencies are higher than related nonhomolog recombination frequencies:
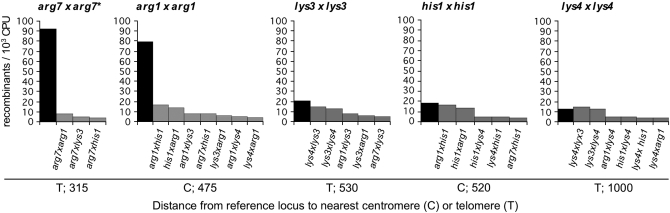
Four data of the five allelic interactions represent the highest recombination frequencies in the rank order (Figure 2C). An overall comparison of allelic vs. nonhomolog interactions reveals that the mean recombination frequency of allelic interactions is 5.5-fold greater than that of the nonhomolog interactions. (The difference is still 4-fold in favor of the allelic interactions if the arg7 locus, that is the allelic interaction which involves the aberrant arg7∷ura4 insert, is not considered.) When each allelic interaction is compared to the relevant subset of related nonhomolog data, i.e., to nonhomolog interactions that involve the same locus, a preference for allelic interactions is again clear (Figure 3): for four of the five loci, the allelic interaction is significantly higher than any of the related nonallelic interactions while, for the fifth (lys4 × lys4), the allelic interaction and one nonallelic interaction are the two highest in the set. These data suggest that homologs generally tend to be colocalized.
Figure 3.—
Comparison of recombination frequencies of allelic interactions to related nonhomologous interactions. In each interaction the first gene symbol represents the padh1-loxP construct and the second symbol the loxP-ura4 construct inserted at the indicated gene. (*) arg7∷ura4 exhibits insert-specific aberrancy.
Upon further inspection, a more sophisticated pattern also emerges (Figure 3): the difference between the allelic and related nonhomolog interactions varies systematically with the distance of the reference locus from its centromere or its telomere, whichever is closest. This difference is highest for the most centromere-proximal and the most telomere-proximal loci (arg1 and arg7, respectively), next highest for the next most centromere- and telomere-proximal loci (his1 and lys3, respectively), and hardly discernible for lys4, which is located far from both its telomere and centromere. Quantitatively, the difference between the allelic interaction and the highest related nonhomolog interaction is 4.8- and 2-fold for the most centromere- and telomere-proximal loci, respectively. (In this calculation an average of 6-fold hyperrecombinogenic effect of the arg7∷ura4 locus was considered.) For the his1 and lys3 loci the difference is 1.1- and 1.4-fold, respectively. For the lys4 locus the two highest related nonhomologous data actually slightly exceed the allelic value. These patterns suggest that centromere and telomere effects play an important role in interactions between allelic loci.
In considering the detailed basis for these effects, two additional findings are relevant. First, the nonhomolog recombination frequency between the two telomere-proximal loci, arg7 and lys3, is low (Figure 3), implying that higher allelic interactions near telomeres do not result from nonspecific telomere clustering. Thus, bias seen at these two loci can be inferred to reflect effects on homology-based interactions at or near the telomeres. Second, in contrast, nonhomolog recombination frequencies between the centromere-proximal arg1 and his1 loci are very high, even approaching the his1 allelic value (Figure 3). Thus, nonspecific centromere clustering could be a prominent contributor to interaction probabilities. These centromere and telomere effects might comprise the entire basis for allelic bias; alternatively, direct interhomolog interactions between allelic loci may also be occurring (discussion).
Overall chromosome disposition as reflected by nonhomolog interactions:
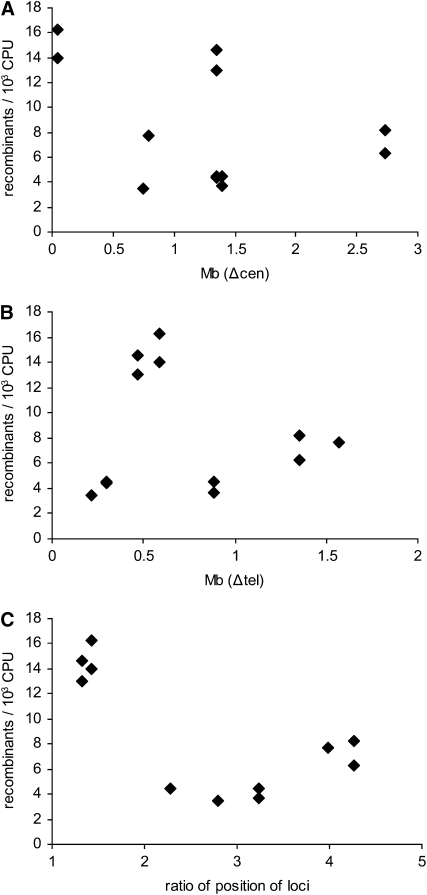
Interactions between nonhomologous loci are expected to more or less reflect the overall organization of chromosomes within the nucleus. In the interphase nuclei of fission yeast, all centromeres cluster at the spindle pole body (Funabiki et al. 1993). This Rabl-like configuration of centromeres tends to colocalize those loci which lie at the same distance from their centromeres. If this effect were reflected in the recombination frequencies of loci on nonhomologous chromosomes, recombination values would be higher when the loci are located at similar distances from their centromeres and gradually decrease with the more disparate locus-to-centromere distances. To examine this possibility, nonhomolog recombination frequencies were plotted against the difference of the locus-to-centromere distance of the two loci (Figure 4A). Interactions between the two loci that are very close to their respective centromeres (his1 × arg1) do show the highest recombination frequency. However, the data for more centromere-distal loci do not exhibit the anticipated correlation.
Figure 4.—
Analysis of nonhomologous interactions. (A) Recombination frequencies of nonhomologous interactions are plotted against the difference of the respective locus-to-centromere distances. (B) Recombination frequencies are plotted against the difference of the respective locus-to-telomere distances. (C) Recombination frequencies are plotted against the ratio of the positions of the interacting loci. (For determination of the positions of loci see text.)
Cytological studies also show that telomeres exhibit partial clustering, less pronounced than that observed for centromeres, at several places of the nuclear periphery (Scherthan et al. 1994). We therefore also examined the relationship between the nonhomolog Cre/loxP recombination values and the difference of locus-to-telomere distances (Figure 4B). Again, no clear correlation could be established. This might reflect the fact that clustering occurs only, or primarily, between the telomeres of homologous chromosomes (see discussion).
A possible reason for the absence of any regularity in these comparisons is that the two factors should be considered simultaneously, i.e., that the probability of interaction at a given locus is a function of both centromere and telomere colocalization. To assess this possibility, we first examined the simplest case in which predicted interaction frequencies would represent the average, or perhaps the “weighted average,” of the two effects considered separately above. A plot of the difference in locus-to-centromere distance vs. the difference in locus-to-telomere distance for each of the eight nonhomolog interactions fails to reveal any such pattern (not shown).
We therefore tried a second approach which instead considers the relative position of loci along their chromosome arms, irrespective of absolute distance from the centromere or nearest telomere. First, the position of each locus was defined as the ratio of its locus-to-centromere distance and the length of its chromosome arm. The decimal fraction obtained this way expresses the relative position of the locus within its chromosome arm, i.e., to both its centromere and its telomere. The relative positions of the loci can be compared simply by dividing the greater decimal fraction by the smaller one to create their ratio. This approach reveals a provocative correlation: as shown in Figure 4C, the two pairs of loci that exhibit the highest nonhomolog interaction frequencies, arg1 × his1 and lys3 × lys4, are also the two loci whose relative positions within the corresponding chromosome arms are most similar; in contrast, other pairs of loci exhibit lower interaction frequencies irrespective of position. Interestingly, this correlation holds despite the fact that the arg1 and his1 loci are located ∼25% of the way from the centromere to the telomere along their chromosome arms while the lys3 and lys4 loci are located ∼75% of the way along. These findings suggest that additional factors which influence chromosome contacts should be considered (see discussion).
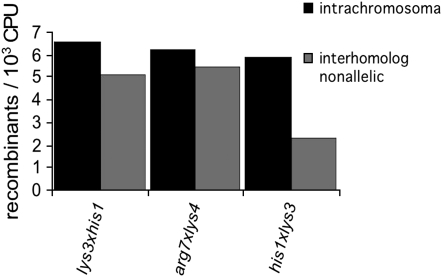
Intrachromosomal recombination frequencies are higher than the corresponding “interhomolog trans” recombination frequencies:
In six of the analyzed strains, the loxP sites are located at different genomic positions on the same chromosome. In three cases, the two loci are located in “cis,” i.e., on the same physical chromosome; in three cases they are located in “trans,” i.e., on different chromosomes. In the categories above, interactions of the former type were called intrachromosomal; the latter one was named interhomolog nonallelic (see Figure 2A; arg7∷ura4 strains excluded). A comparison of the two types of interactions reveals that the frequency of intrachromosomal interactions always exceeds the frequency of interhomolog nonallelic interactions. This relationship holds not only in each of the three individual pairwise comparisons (Figure 5) but also overall, with all three intrachromosomal (cis) values being higher than all of the corresponding interchromosomal (trans) values (Figure 2C). These data suggest that interactions along the length of a single physical chromosome occur more frequently than interaction of the same two loci when they are located on the two homologs, which in turn implies that the tendency of a single chromosome to occupy its own territory is stronger than the tendency for homologs to be colocalized (discussion).
Figure 5.—
Comparison of intrachromosomal and interhomolog nonallelic interactions.
DISCUSSION
In this study we applied the Cre/loxP site-specific recombination system to fission yeast to analyze, by a functional assay, interlocus interactions, and thus chromosomal positioning, in living diploid vegetative cells.
Main tendencies of chromosomal interactions in vegetatively cycling fission yeast cells:
Four main interaction tendencies are directly revealed:
Homologs are colocalized, most strongly at centromeres and telomeres. This feature is shown by the fact that allelic interaction frequencies are higher than interaction frequencies for nonallelic loci on homologs and exceed the majority of nonhomolog interactions as well. Especially high levels of allelic interactions were also observed at the two most centromere-proximal sites (his1 and arg1) and the two most telomere-proximal sites (lys3 and arg7). The preference for allelic interactions at the one examined “interstitial” locus (lys4) is weak or nonexistent. Thus, homologs are closely colocalized at their centromeres and at their telomeres but more loosely colocalized all along their lengths. This general picture is in accord with the finding that painting of whole chromosomes in spread preparations (chromosomes I and II) show that homologous chromosomes occupy joint territories in >90% of diploid fission yeast nuclei (Scherthan et al. 1994).
-
Centromeres are nonspecifically clustered. This feature is shown by the fact that by far the highest nonhomolog interaction frequency was seen between his1 and arg1, the two most centromere-proximal loci and is in accord with the robust general clustering of centromeres observed cytologically (Funabiki et al. 1993). In this context it is interesting to note that fission yeast centromeres are large. They consist of a central region which is unique to each centromere, and the surrounding outer repeats with highly repetitive motifs common to all centromeres. It was hypothesized that the outer repetitive regions were required for interactions with the corresponding regions of other centromeres and thus they form higher order complex structures (Takahashi et al. 1992). Association of the large heterochromatic centromere regions may facilitate chromosomal contacts over a relatively long distance. It could thus promote high levels of interactions among loci located at similar distances from the centromeres even when they are on nonhomolog chromosomes.
Nonspecific clustering may also account for the unusually high levels of allelic interactions seen at centromere-proximal loci (above); on the other hand, these effects could reflect a combination of direct (homology-based) interactions that are also enhanced by centromere-related effects, either nonspecific clustering and/or an intrinsically higher activity of heterochromatin for true homologous interactions (Dernburg et al. 1996). Interestingly, the arg1 locus shows much higher interallelic interaction frequencies than his1 (Figures 2C and 3), even though the two loci are located at similar distances from their respective centromeres. Similarly, the centromere of the arg1 chromosome (III) has an almost thrice-larger centromere than that of the his1 chromosome I (see Figure 1). This correspondence points to an important role for centromeric heterochromatin in allelic interactions, be it direct or indirect. Among several specific possibilities, a larger heterochromatic region might increase the stiffness of the chromosome arm with the result that a centric interaction will have a bigger effect on a nearby locus.
-
Direct homology-based pairing at (or near) telomeres. High allelic interaction levels were detected at the telomere-proximal lys3 and arg7 loci and, in contrast to centromere-proximal loci, these allelic interactions significantly exceed the level of related nonhomologous interaction (lys3 × arg7). We infer that chromosome pairing at the telomere proximal regions is homology directed and that homology-based interactions override any tendency for nonspecific telomere–telomere interactions. Suggestive cytological observations are in accord with this view. FISH analysis of diploid cells using a probe that recognized both ends of two chromosomes (I and II) monitors the positions of eight chromosome ends. However, not more than four signals were detected in whole diploid cells (Funabiki et al. 1993) or in 40% of the spread diploid nuclei (Scherthan et al. 1994). Though it is not demonstrated directly, a plausible interpretation of these cytological data is that homologous telomeres tend to associate at the nuclear envelope of fission yeast (for a precedent, see Church 1981). Additionally, for chromosome arms of similar length, association might be less dependent upon nuclear envelope association. More generally, homologous telomere pairing is a well-documented phenomenon in somatic plant cells (e.g., Fussell 1975, 1977).
The simplest possibility is that homology recognition detected for telomere-proximal loci occurs directly in telomeric regions per se. However, it cannot be rigorously excluded that homology sensing occurs only, or also, at near-telomere loci, with telomeres exerting a cis effect that increases the efficiency or stability of such interactions (e.g., via altered chromatin structure).
Each chromosome tends to occupy its own territory. This feature is shown by the fact that intrachromosomal interactions are higher than interactions between nonallelic loci on homologs. If two homologs were totally intertwined with one another, a locus on one homolog would not know the difference between whether the other locus is on the same chromosome or the homolog. Thus, it appears that homologs, even though generally coaligned, are still far enough apart that they are “effectively” each in its own individual territory. However, the overall difference between the levels of intrachromosomal and interhomolog nonallelic recombination is low. This is an expected consequence of the picture described above. Given that homologs are held together tightly at centromere and telomere regions, and loosely in interstitial regions, the distance between two (marked) loci will be very similar regardless of whether they both occur on the same physical chromosome or on the two different members of a homolog pair.
Transient nonspecific telomere interactions and their implications for nonhomolog chromosomal interactions:
Cytological data suggest that some degree of nonspecific telomere clustering does occur. In the FISH data discussed above, this is shown by observation of fewer than four signals in many nuclei. However, no strong tendency for such clustering was obvious in the current study. In this context, the current data suggest that nonspecific telomere clustering is variable, transient, and/or limited to certain stages of the cell cycle.
Another aspect of the current interaction data provides a second line of evidence for such “occasional” nonspecific telomere colocalization, as follows. With the exception of the two loci that are linked to their centromeres (arg1 and his1), nonhomolog interaction frequencies detected by Cre/loxP recombination do not obviously reflect either the Rabl orientation or any nonspecific clustering of telomeres. On the other hand, the two pairs of loci that exhibit the highest interaction frequencies (arg1 × his1 and lys3 × lys4) have in common that, for each pair, the component loci occur at similar relative positions along their respective chromosome arms, i.e., similar fractions of the distance between the flanking centromere and telomere regions. While it is possible that this correspondence is fortuitous, it is intriguing to consider that it might have some significance for in vivo chromosome disposition. Occasional nonspecific telomere clustering could be the missing piece in the puzzle. When a pair of heterologous telomeres happen to associate, the unrelated chromosome arms whose ends are involved in the association are now spatially linked at both their centromeres and their telomeres. In this situation, interaction frequencies for loci located internally within the two chromosome arms, well away from either point of stable linkage, will be determined by their relative position between the two linkage points, rather than the absolute distance to either point.
Telomere mobility:
It is likely that telomeres change their positions around the nuclear envelope in vegetatively growing cells. Telomere movement was detected in budding yeast (Hediger et al. 2002), and dynamic association and dissociation of telomeres was demonstrated in human cells as well (Molenaar et al. 2003). In the current study, the dynamic nature of the nucleus could explain the generally low interaction probabilities among all pairs of loci (even in homologous position). Motion would greatly increase the range of possible interlocus dispositions, thus reducing the period of colocalization for any particular locus pair; similarly, colocalization times may usually be too short to permit stable recombinase-mediated association. Low interaction frequencies could also explain the high standard deviation of recombination frequencies detected throughout this study (on average 23.4%, and data not shown), exactly as observed for mutation frequencies in small cell populations (Luria and Delbruck 1943).
Somatic pairing in S. pombe:
The current study provides good evidence for homology-based interactions in or near telomeres and further supports the notion that homologs tend to be colocalized. However, it leaves unanswered the question of whether homology-based interactions do or do not occur in other regions, i.e., in centromeres or arm regions. Given a clear influence of centromere and telomere interactions on the frequency of allelic interactions, it is possible that homology recognition occurs exclusively in telomeric regions, with allelic interactions of nearby loci increased as an indirect consequence of this primary effect in combination with nonspecific clustering of telomeres. In this case, the strong colocalization of homologs to joint territories seen by FISH (above) might reflect the fact that spreading of chromosomes for analysis tends to stretch the chromosomes out in one direction, with the result that specific homolog pairing in telomere regions might lead to general colocalization even in the absence of direct homolog–homolog interactions.
On the other hand, there are credible reasons to suspect that direct homologous interactions in regions other than telomeres might occur but be providing only a small contribution to interhomolog interaction frequencies in the current in vivo whole cell analysis. As discussed in a previous Cre/loxP analysis in budding yeast: the nucleus is small, the chromosomes are flexible, and interactions between homologs detected by FISH are separated by ≥50 kb. These factors in combination predict that the difference in relative local concentrations of homologous and nonhomologous sequences will always be quite small, even despite the occurrence of centromere clustering and telomere-specific homolog pairing (Gotta et al. 1996; Burgess and Kleckner 1999). The same situation could apply in S. pombe.
Synthesis:
The Cre/loxP analysis used in the current study reports actual in vivo chromosome disposition as reflected in interlocus recombination frequencies. The observed patterns show that centromeres and telomeres play by far the most predominant determining role. Three types of effects are identified as being functionally important: stable nonspecific centromere colocalization; robust homology-directed interactions between telomeres; and variable, transient, and/or occasional nonspecific colocalization of telomeres (presumably on the nuclear envelope). Our results further show that intrachromosomal interactions predominate over interchromosomal interactions, even those between homologs, in accord with a dominant role for chromosome territories. We had hoped to obtain additional evidence regarding direct homology-based pairing outside of telomeric regions. However, we find that the situation in S. pombe is as ambiguous as in Saccharomyces cerevisiae, with effects of direct interstitial pairing (if any) obscured by the combined effects of chromosome size and chromosome mobility and/or centromere and telomere effects.
Chromosome disposition and ectopic recombination:
Chromosome disposition within the nucleus is suspected to be a primary factor in determining the frequency of homologous recombination, as seen, e.g., in studies of ectopic recombination in budding yeast meiosis (Goldman and Lichten 2000; Schlecht et al. 2004) and of mouse double-strand-break dynamics (Soutoglou et al. 2007). We are now in a position to assess this possibility for S. pombe by comparing the results of previously presented mitotic recombination data (Virgin et al. 2001) with the current data obtained by Cre/loxP analysis.
The homologous recombination data set comprises allelic and ectopic recombination frequencies for pairwise combinations of three usable test loci. (We have ignored homologous recombination data involving a fourth locus, ura4, because it is near the rDNA repeat, specifically avoided in our study because of potential atypical effects.) Significant similarities emerge. In both cases, allelic interactions are higher than ectopic interactions, with an average 8.7-fold and 5.5-fold (above), respectively, with most or all allelic interactions higher than any ectopic interaction. Moreover, the hierarchy of interactions among different loci for homologous recombination corresponds to that observed here for Cre/loxP: the highest at ade6, which is very near its centromere; and the second highest at z15, which is telomere-proximal, and at z7, which is interstitial. With respect to ectopic interactions, the highest recombination ratio from the three relevant measurements is obtained for z7 × z15. Interestingly, and in accord with Cre/loxP data, these two loci occupy corresponding relative positions along their respective chromosome arms.
The current data also bear on a second issue. Ectopic mitotic recombination frequencies in budding yeast are 5–10% those of allelic recombination while, in fission yeast, ectopic interactions tend to be somewhat lower (Virgin et al. 2001). Such a difference might, in principle, reflect differences in chromosome disposition within the nucleus, i.e., a stronger tendency for colocalization of homologs in the latter case. The current results suggest that the difference probably lies elsewhere: differences between allelic and nonallelic Cre/loxP recombination frequencies in fission yeast as defined by the current analysis are similar to or, if anything, less than those observed for budding yeast as analogously defined (Burgess and Kleckner 1999).
Acknowledgments
We are grateful to members of the Kleckner laboratory for their input in experimentation and analysis and to Jim Henle and Kate Mirkin for reading of the manuscript in preparation. This study was supported by a grant to N.K. from the National Institutes of Health RO1-GM 44794.
References
- Ambremski, K., R. Hoess and N. Sternberg, 1983. Studies on the properties on P1 site-specific recombination: evidence for topologically unlinked products following recombination. Cell 32: 1301–1311. [DOI] [PubMed] [Google Scholar]
- Aragon-Alcaide, L., S. Reader, A. Beven, P. Shaw, T. Miller et al., 1997. Association of homologous chromosomes during floral development. Curr. Biol. 7: 905–908. [DOI] [PubMed] [Google Scholar]
- Arnoldus, E. P. J., A. C. B. Peters, G. T. A. M. Bots, A. K. Raap and M. van der Ploeg, 1989. Somatic pairing of chromosome 1 centromeres in interphase nuclei of human cerebellum. Hum. Genet. 83: 231–234. [DOI] [PubMed] [Google Scholar]
- Bähler, J., J.-Q. Wu, M. S. Longtine, N. G. Shah, A. McKenzie III et al., 1998. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951. [DOI] [PubMed] [Google Scholar]
- Basi, G., E. Schmid and K. Maundrell, 1993. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcription efficiency but not the transcription start point or thiamine repressibility. Gene 123: 131–136. [DOI] [PubMed] [Google Scholar]
- Burgess, S. M., and N. Kleckner, 1999. Collisions between yeast chromosomal loci in vivo are governed by three layers of organization. Genes Dev. 13: 1871–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess, S. M., N. Kleckner and B. M. Weiner, 1999. Somatic pairing in budding yeast: existence and modulation. Genes Dev. 13: 1627–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church, K., 1981. The architecture of chromosome movements within the premeiotic interphase nucleus pp. 83–102 in Mitosis/Cytokinesis, edited by A. M. Zimmerman and A. Forer. Academic Press, New York.
- Dernburg, A. F., J. W. Sedat and R. S. Hawley, 1996. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. [DOI] [PubMed] [Google Scholar]
- Egel, R., 1973. Commitment to meiosis in fission yeast. Mol. Gen. Genet. 121: 277–284. [Google Scholar]
- Egel, R., and M. Egel-Mitani, 1974. Premeiotic DNA synthesis in fission yeast. Exp. Cell Res. 88: 127–134. [DOI] [PubMed] [Google Scholar]
- Fransz, P., J. H. De Jong, M. Lysak, M. R. Castiglione and I. Schubert, 2002. Interphase chromosomes in Arabidopsis are organized as well defined chromocenteres from which euchromatin loops emanate. Proc. Natl. Acad. Sci. USA 99: 14584–14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki, H., I. Hagan, S. Uzava and M. Yanagida, 1993. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J. Cell Biol. 121: 961–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung, J. C., W. F. Marshall, A. Dernburg, D. A. Agard and J. W. Sedat, 1998. Homologous chromosome pairing in Drosophila melanogaster proceeds through multiple independent initiations. J. Cell Biol. 141: 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fussell, C. P., 1975. The position of interphase chromosomes and late replicating DNA in centromere and telomere regions of Allium cepa. Chromosoma 50: 201–210. [Google Scholar]
- Fussell, C. P., 1977. Telomere association in interphase nuclei of Allium cepa demonstrated by C-banding. Exp. Cell Res. 110: 111–117. [DOI] [PubMed] [Google Scholar]
- Gemkow, M. J., P. J. Verveer and D. J. Arndt-Jovin, 1998. Homologous association of the Bithorax-complex during embryogenesis: consequences for transvection in Drosophila melanogaster. Development 125: 4541–4552. [DOI] [PubMed] [Google Scholar]
- Goldman, A. S. H., and M. Lichten, 2000. Restriction of ectopic recombination by interhomolog interactions during Saccharomyces cerevisiae meiosis. Proc. Natl. Acad. Sci. USA 97: 9537–9542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic, M. M., Y. S. Rong, R. B. Petersen, S. L. Lindquist and K. G. Golic, 1997. FLP-mediated DNA mobilization to specific target sites in Drosophila chromosomes. Nucleic Acid Res. 25: 3665–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotta, M., T. Laroche, A. Formenton, L. Maillen, H. Scherthan et al., 1996. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J. Cell Biol. 134: 1349–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm, C., J. Kohli, J. Murray and K. Maundrell, 1988. Genetic engineering of Schizosaccharomyces pombe: a system for gene disruption and replacement using the ura4 gene as a selectable marker. Mol. Gen. Genet. 215: 81–86. [DOI] [PubMed] [Google Scholar]
- Gutz, H., H. Heslot, U. Leupold and N. Loprieno, 1974. Schizosaccharomyces pombe, pp. 395–446 in Handbook of Genetics, Vol. 1, edited by R. C. King. Plenum, New York.
- Hediger, F., F. R. Neumann, G. van Houwe, K. Dubrana and S. M. Gasser, 2002. Live imaging of telomeres: yKu and Sir proteins define redundant telomere anchoring pathways in yeast. Curr. Biol. 12: 2076–2089. [DOI] [PubMed] [Google Scholar]
- Hiraoka, Y., A. F. Dernburg, S. J. Parmelee, M. C. Rykowski, D. A. Agard et al., 1993. The onset of homologous chromosome pairing during Drosophila melanogaster embryogenesis. J. Cell Biol. 120: 591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser, M., D. Mathog, Y. Gruenbaum, H. Saumweber and J. W. Sedat, 1986. Spatial organization of chromosomes in the salivary gland nuclei of Drosophila melanogaster. J. Cell Biol. 102: 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoess, R. H., M. Ziese and N. Sternberg, 1982. P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc. Natl. Acad. Sci. USA 79: 3398–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H., Y. Fukuda, K. Murata and A. Kimura, 1983. Transformation of intact cells treated with alkali cations. J. Bacteriol. 153: 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaSalle, J. M., and M. Lalande, 1996. Homologous association of oppositely imprinted chromosomal domains. Science 272: 725–728. [DOI] [PubMed] [Google Scholar]
- Lorenz, A., J. Fuchs, R. Burger and J. Loidl, 2003. Chromosome pairing does not contribute to nuclear architecture in vegetative yeast cells. Eukaryot. Cell 2: 856–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria, S. E., and M. Delbruck, 1943. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28: 491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall, W. F., A. F. Dernburg, B. Harmon, D. A. Agard and J. W. Sedat, 1996. Specific interaction of the chromatin with the nuclear envelope: positional determination within the nucleus of Drosophila melanogaster. Mol. Biol. Cell 7: 825–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod, M., M. Stein and D. Beach, 1987. The product of the mei3+ gene, expressed under control of the mating-type locus, induces meiosis and sporulation in fission yeast. EMBO J. 6: 729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar, C., K. Wiesmeijer, N. P. Verwoerd, S. Khazen, R. Eils et al., 2003. Vizualizing telomere dynamics in living mammalian cells using PNA probes. EMBO J. 22: 6631–6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto, P., A. P. Santos, G. Moore and P. Shaw, 2004. Chromosomes associate premeiotically and in xylem vessel cells via their telomeres and centromeres in diploid rice (Oryza sativa). Chromosoma 112: 300–307. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E. F. Fritsch and T. Maniatis, 1989. Molecular Cloning: A Laboratory Manual, Ed. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sauer, B., 1992. Identification of cryptic lox sites in the yeast genome by selection for Cre-mediated chromosome translocations that confer multiple drug resistance. J. Mol. Biol. 223: 911–928. [DOI] [PubMed] [Google Scholar]
- Scherthan, H., J. Bähler and J. Kohli, 1994. Dynamics of chromosome organization and pairing during meiotic prophase in fission yeast. J. Cell Biol. 127: 273–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlecht, H. B., M. Lichten and A. S. Goldman, 2004. Compartmentalization of the yeast meiotic nucleus revealed by analysis of ectopic recombination. Genetics 168: 1189–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipiczki, M., and L. Ferenczy, 1977. Protoplast fusion of Schizosaccharomyces pombe auxotrophic mutants of identical mating-type. Mol. Gen. Genet. 151: 77–81. [DOI] [PubMed] [Google Scholar]
- Soutoglou, E., J. F. Dorn, K. Sengupta, M. Jasin, A. Nussenzweig et al., 2007. Positional stability of single double-strand breaks in mammalian cells. Nat. Cell Biol. 9: 675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack, S. M., and W. V. Brown, 1969. Somatic pairing, reduction and recombination: an evolutionary hypothesis of meiosis. Nature 222: 1275–1276. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., S. Murakami, Y. Chikashige, H. Funabiki, O. Niwa et al., 1992. A low copy number central sequence with strict symmetry and unusual chromatin structure in fission yeast centromere. Mol. Biol. Cell 3: 819–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin, J. B., J. P. Bailey, F. Hasteh, J. Neville, A. Cole et al., 2001. Crossing over is rarely associated with mitotic intragenic recombination in Schizosaccharomyces pombe. Genetics 157: 63–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner, B. M., and N. Kleckner, 1994. Chromosome pairing via multiple interstitial interactions before and during meiosis in yeast. Cell 77: 977–991. [DOI] [PubMed] [Google Scholar]
- Xu, N., C. L. Tsai and J. T. Lee, 2006. Transient homologous chromosome pairing marks the onset of X-inactivation. Science 311: 1149–1152. [DOI] [PubMed] [Google Scholar]
- Zickler, D., 2006. From early homolog recognition to synaptonemal complex formation. Chromosoma 115: 158–174. [DOI] [PubMed] [Google Scholar]