Abstract
Ca2+ influx induced by membrane depolarization triggers the exocytosis of secretory vesicles in various cell types such as endocrine cells and neurons. Peptidyl growth factors enhance Ca2+-evoked release, an effect that may underlie important adaptive responses such as the long-term potentiation of synaptic transmission induced by growth factors. Here, we show that activation of the c-Jun N-terminal kinase (JNK) plays an essential role in nerve growth factor (NGF) enhancement of Ca2+-evoked release in PC12 neuroendocrine cells. Moreover, JNK associated with phosphorylated synaptotagmin-4 (Syt 4), a key mediator of NGF enhancement of Ca2+-evoked release in this system. NGF treatment led to phosphorylation of endogenous Syt 4 at Ser135 and translocation of Syt 4 from immature to mature secretory vesicles in a JNK-dependent manner. Furthermore, mutation of Ser135 abrogated enhancement of Ca2+-evoked release by Syt 4. These results provide a molecular basis for the effect of growth factors on Ca2+-mediated secretion.
Keywords: JNK, NGF, PC12 cells, secretion, synaptotagmin-4
Introduction
Hormonal secretion and neurotransmitter release are highly regulated processes triggered by Ca2+ influx through voltage-gated Ca2+ channels (VGCCs). Ca2+ influx following depolarization induces Ca2+-dependent fusion of secretory vesicles with the plasma membrane and exocytosis of signal molecules. Accumulating evidence has indicated that the efficacy of this Ca2+-evoked release can be modulated by peptidyl factors (growth factors, neurotrophins, etc.). For instance, insulin secretion from pancreatic beta cells is enhanced by incretins (Kashima et al, 2001), and histamine secretion from mast cells is enhanced by substance P treatment, contributing to inflammatory reactions (Stempelj and Ferjan, 2005). In neurons, neurotrophic factors such as nerve growth factor (NGF), neurotrophin 3 and brain-derived neurotrophic factor have been shown to increase the neurotransmitter release in response to synaptic activation and depolarization in various systems (Lohof et al, 1993; Kang and Schuman, 1995; Thoenen, 1995). The effects of neurotrophic factors on neurotransmitter release might account in part for synaptic plasticity and some forms of learning and memory (Zakharenko et al, 2003 and the references therein). However, the mechanisms by which these peptidyl factors enhance the Ca2+-evoked release remain largely unknown.
Numerous proteins have been implicated in the regulation and execution of exocytosis, including SNARE proteins, Rab proteins and synaptotagmins. Synaptotagmins (Syts) constitute a family of putative membrane-trafficking proteins that are characterized by an N-terminal transmembrane domain, a variable spacer and two C-terminal Ca2+-binding C2 domains (C2A and C2B) (Yoshihara and Montana, 2004; Fukuda, 2006). Synaptotagmin-1 (Syt 1) is proposed as a potential Ca2+ sensor for Ca2+-evoked release (Geppert et al, 1994; Fernandez-Chacon et al, 2001; Yoshihara and Littleton, 2002), but the function of other Syts remains unclear. Syt 4 is of particular interest because its null mutants exhibit deficits in hippocampus-dependent memory and fine motor coordination (Ferguson et al, 2000, 2004). Syt 4 has been isolated as an immediately early gene induced by depolarization in PC12 pheochromocytoma cells and by seizure in the hippocampus and piriform cortex (Vician et al, 1995; Berton et al, 2000; Ibata et al, 2000). Unlike Syt 1, Syt 4 cannot bind Ca2+ effectively due to an amino-acid substitution within the C2A domain (von Poser et al, 1997). Syt 4 has also been proposed as a potential Ca2+ sensor based on its capacity to bind to phospholipids in the presence of Ca2+ (Fukuda et al, 1996). Several studies have recently suggested that Syt 4 does not work as a presynaptic Ca2+ sensor like Syt 1 (Adolfsen et al, 2004; Ting et al, 2006), but rather works as a postsynaptic or glial Ca2+ sensor (Ibata et al, 2002; Zhang et al, 2004; Yoshihara et al, 2005). Syt 4 has been implicated in enhancement (Thomas et al, 1999; Zhang et al, 2004), inhibition (Eaton et al, 2000; Machado et al, 2004) and modulation (Wang et al, 2001; Tsuboi and Rutter, 2003; Wang et al, 2003) of Ca2+-evoked release in a context- and cell type-dependent manner. Thus far, little is known about regulation of Syt 4, although it seems to be a key regulator of Ca2+-evoked release (Yoshihara and Montana, 2004; Fukuda, 2006).
The c-Jun N-terminal kinase (JNK) represents a group of mitogen-activated protein kinases that are activated by a wide variety of cellular stresses and peptidyl factors (Minden et al, 1994; Davis, 2000). In Caenorhabditis elegans, the JNK pathway is involved in motor coordination via regulation of synaptic vesicle localization (Kawasaki et al, 1999; Byrd et al, 2001). It is unclear, however, whether mammalian JNK regulates secretion processes. Here, we show that JNK is responsible for the enhancement of Ca2+-evoked release by NGF treatment in PC12 cells. We found that JNK directly associates with and phosphorylates Syt 4 at Ser135. Importantly, in this system, Syt 4 plays an essential role for NGF enhancement of Ca2+-evoked release, as revealed by RNA interference experiments. The JNK-mediated phosphorylation of Syt 4 at Ser135 appears to be important for the localization of Syt 4 at mature secretory vesicles, and for enhancement of Ca2+-evoked release. These results have revealed a previously unrecognized molecular link between peptidyl growth factors and secretory machinery.
Results
JNK mediates NGF enhancement of Ca2+-evoked release
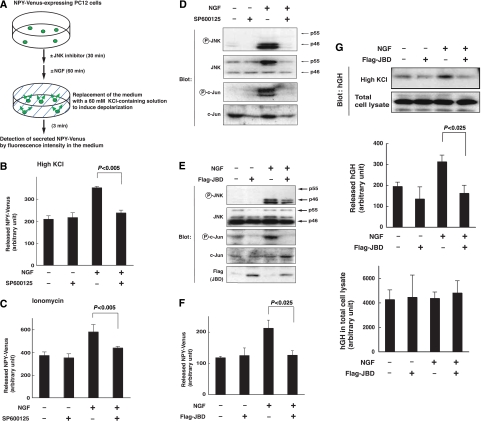
In the neuroendocrine cell line PC12 pheochromocytoma, growth factors such as NGF, epidermal growth factor and insulin-like growth factor-1 facilitate depolarization-induced release of dopamine, acetylcholine and other peptides (Amino et al, 2002; Itakura et al, 2005). To investigate the mechanisms underlying growth factor enhancement of exocytosis, we utilized PC12 cells as a model system, and two secretory molecules to monitor exocytosis: a fusion protein between neuropeptide Y and a modified yellow fluorescence protein relatively resistant to low pH (NPY-Venus) (Nagai et al, 2002) and human growth hormone (hGH) (Wick et al, 1993; Oishi et al, 1998). For our first line of experiments, we established a stable line of PC12 cells expressing NPY-Venus, and confirmed that bath application of a high concentration (60 mM), but not a low concentration (4.7 mM), of KCl to the medium for 3 min resulted in the release of NPY-Venus into the medium (Figure 1A and Supplementary Figure S1). This depolarization-induced secretion of NPY-Venus was inhibited by the addition of the Ca2+ chelator EGTA at 10 mM or the L-type VGCC inhibitor nifedipine at 100 nM in the medium (Supplementary Figures S1 and S2), indicating that this release was induced by depolarization-mediated Ca2+ influx through VGCC. We then compared levels of secretion following depolarization in the presence and absence of NGF. Pretreatment of PC12 cells with NGF for 60 min significantly enhanced the level of depolarization-induced secretion of NPY-Venus without affecting the total amount of NPY-Venus expressed, reflecting an NGF enhancement of Ca2+-evoked release (Figure 1B and data not shown). Pretreatment with NGF also enhanced the level of ionomycin-induced secretion of NPY-Venus, further indicating the enhancing effect of NGF on the Ca2+-evoked release (Figure 1C).
Figure 1.
Inhibition of JNK abrogates NGF enhancement of Ca2+-evoked release in PC12 cells. (A) Scheme of the procedure to examine the effects of NGF and JNK inhibitor SP600125 on depolarization-evoked release of NPY-Venus from PC12 cells. (B–D) PC12 cells stably expressing NPY-Venus were preincubated with 20 μM SP600125 (+) or DMSO (−) for 30 min, followed by a further 1 h incubation in the presence or absence of 100 ng/ml NGF. (B) The culture medium was replaced with 60 mM KCl-containing physiological salt solution (high KCl) and incubated for 3 min. The resulting medium was collected for detection of secreted NPY-Venus by fluorescence intensity. Results are presented as mean±s.d. from triplicate samples. Essentially the same results were obtained in three independent experiments. (C) The culture medium was replaced with 5 μM ionomycin-containing physiological salt solution (ionomycin) and incubated for 3 min. (D) The cells were lysed and subjected to immunoblot analysis with antibodies to phosphorylated JNK (P-JNK), to JNK, to phosphorylated c-Jun (P-c-Jun) or to c-Jun. (E–G) PC12 cells stably expressing NPY-Venus were infected with retroviruses encoding either Flag-tagged JNK binding domain (JBD) of JNK-interacting protein 1 (JIP1), or with corresponding control retrovirus (−). Infected cells were incubated for 1 h in the presence or absence of 100 ng/ml NGF. (E) Infected cells were lysed and subjected to immunoblot analysis with antibodies to phosphorylated JNK, JNK, phosphorylated c-Jun, c-Jun or Flag epitope. (F) The culture medium was replaced with 60 mM KCl-containing salt solution and incubated for 3 min. The resulting medium was collected for detection of secreted NPY-Venus by fluorescence intensity. Results are presented as mean±s.d. from triplicate samples. Essentially, the same results were obtained in three independent experiments. (G) PC12 cells were transfected with an expression vector encoding hGH and with an expression vector encoding Flag-JBD or its corresponding control vector. After 72 h, the cells were incubated for 1 h in the presence or absence of 100 ng/ml of NGF. The culture medium was then replaced its 60 mM KCl-containing salt solution for 3 min. The resulting medium was collected for detection of secreted hGH by immunoblot analysis. Results are presented as mean±s.d. from triplicate samples. Essentially, the same results were obtained in three independent experiments.
To investigate which downstream effectors of NGF might be involved in this enhanced secretion, we tested several kinase inhibitors and found that the JNK inhibitor SP600125 (20 μM) effectively canceled the NGF enhancement of depolarization-induced secretion of NPY-Venus (81.2±15.5% inhibition of NGF enhancement, and 32.6±4.5% inhibition for the absolute value, n=3, P<0.005, Student's t-test: Figure 1B), whereas the MEK inhibitor U0126 or p38 inhibitor SB202190 did not (data not shown). The addition of SP600125 also suppressed NGF enhancement of ionomycin-induced secretion of NPY-Venus (Figure 1C).
To determine the activity of JNK under conditions of NGF-enhanced secretion, we examined the expression and phosphorylation levels of proteins in the JNK signaling pathway. We detected both the p55 and p46 forms of JNK and the JNK substrate c-Jun in PC12 cells. The JNK forms correspond to spliced isoforms of JNK1, JNK2 and JNK3 (alpha1 and beta1 for p46; alpha2 and beta2 for p55) (Gupta et al, 1996). Interestingly, phosphorylation of p46 was selectively induced by NGF treatment (Figure 1D), whereas both p55 and p46 forms were equivalently phosphorylated in response to anisomycin treatment of PC12 cells (data not shown). NGF also induced c-Jun phosphorylation. Pretreatment of PC12 cells with 20 μM of SP600125 effectively suppressed NGF-induced phosphorylation of both p46 JNK and its substrate c-Jun (Figure 1D), suggesting that phosphorylation of these proteins depends on JNK activation. We also utilized JNK-binding domain (JBD) of JNK-interacting protein 1 (JIP1) to specifically inhibit endogenous JNK (Dickens et al, 1997). Expression of JBD by a retroviral expression vector slightly suppressed NGF-induced phosphorylation of p46 JNK, and almost completely suppressed that of c-Jun (Figure 1E). Under this condition, JBD expression markedly canceled NGF enhancement of depolarization-induced secretion (91.5±7% reduction of NGF enhancement) (Figure 1F) and ionomycin-induced secretion (data not shown) of NPY-Venus. On the other hand, neither SP600125 treatment nor JBD expression reduced depolarization-induced secretion of NPY-Venus in the absence of NGF (Figure 1B and F). These results together suggest that JNK is specifically involved in the NGF enhancement of Ca2+-evoked release, and not in Ca2+-evoked release itself.
We also examined the Ca2+-evoked release of hGH. hGH was introduced into PC12 cells by transient transfection. Treatment with a high concentration of KCl released around 5% of hGH expressed in PC12 cells into the medium. Pretreatment with NGF increased this depolarization-induced hGH secretion by 53.2%, whereas it did not change the total amount of hGH expressed in PC12 cells (Figure 1G). Importantly, co-expression of JBD with hGH markedly suppressed the NGF enhancement of KCl-induced hGH secretion (Figure 1G). This again suggests that JNK activation is necessary for NGF enhancement of Ca2+-evoked release.
We then asked whether activation of the JNK pathway by itself might enhance Ca2+-evoked release. To examine this, we could not express a potent activator of the JNK pathway since its expression induces apoptotic cell death (Tsuruta et al, 2004). We instead expressed a moderate activator of JNK, MKK7-DED (the kinase MKK7 harboring mutations of S287D, T291E and S293D), and found that it induced a moderate phosphorylation of endogenous JNK and slightly but significantly enhanced depolarization-induced secretion of NPY-Venus into the medium, whereas an inactive mutant of MKK7 (MKK7 harboring mutations of S287A, T291A and S293A) did not (Supplementary Figure S3). This result supports the hypothesis that activation of JNK enhances Ca2+-evoked release.
JNK associates with Syt 4 in vitro and in vivo
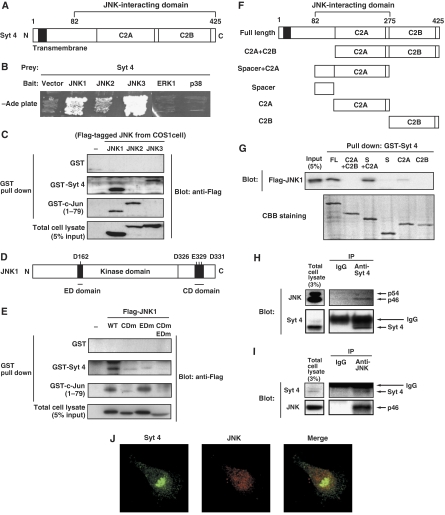
In an attempt to identify JNK targets, we performed a yeast two-hybrid screen of a human embryonic brain library with human JNK2 as bait. We isolated a fragment of Syt 4 (amino-acid residues 82–425) as an interactor of JNK2 (Figure 2A). Using directed yeast two-hybrid analysis, we found that this fragment of Syt 4 interacted strongly with JNK1 and JNK3 and moderately with JNK2, and did not interact with ERK1 or p38, other members of the MAP kinase family (Figure 2B).
Figure 2.
JNK interacts with Syt 4 in vitro and in vivo. (A) Schematic structure of Syt 4. (B) The yeast strain PJ69-4A was transformed with an expression vector encoding a kinase-negative form of either JNK1, JNK2, JNK3, ERK1 or p38 fused with the DNA-binding domain of GAL4, or corresponding empty vector, together with an expression vector encoding Syt 4 (82–425) fused to the transcriptional activation domain of GAL4 or corresponding empty vector. (C) COS1 cells were transfected with an expression vector for Flag-tagged JNK1, JNK2 or JNK3, or corresponding empty vector. The lysate of transfected cells was incubated with GST, GST-Syt 4 or GST-c-Jun (1–79) immobilized to glutathione-sepharose beads. The co-precipitated JNK was detected by an immunoblot analysis with antibodies to Flag. (D) Schematic diagram of the JNK1. (E) COS1 cells were transfected with an expression vector for Flag-tagged JNK1 (wild-type, CDm (D326N, E329N, E331N), EDm (D162T) or, CDm EDm (D162T, D326N, E329N, E331N)), or corresponding control vector. The lysate of transfected cells was incubated with GST, GST-Syt 4, GST-c-Jun (1–79) immobilized to glutathione-sepharose beads. The co-precipitated JNK was detected by immunoblot analysis. (F) Schematic diagram of the Syt 4 deletion mutants used for the GST pull-down assay. (G) COS1 cells were transfected with an expression vector for Flag-tagged JNK1. The lysate of transfected cells was incubated with GST-tagged full length Syt 4, C2A and C2B domain of Syt 4 (resides 148–425), spacer and C2A domain of Syt 4 (resides 82–275), spacer domain of Syt 4 (resides 82–158), C2A domain of Syt 4 (resides 148–275) or C2B domain of Syt 4 (resides 285–425) immobilized to glutathione-sepharose beads. The co-precipitated JNK was detected by immunoblot analysis. (H, I) PC12 cells were lysed and subjected to immunoprecipitation with antibodies to Syt 4, to JNK or with rabbit control IgG. The immunoprecipitates were subjected to immunoblot analysis with antibodies to JNK, or to Syt 4. (J) PC12 cells were fixed and subjected to immunocytochemistry with antibodies to Syt 4, and to JNK. Fluorescence images were recorded by a confocal laser-scanning microscope and a typical image is shown.
We next examined the interaction between JNK and Syt 4 proteins using pull-down assays. Either GST alone, GST fused to the full-length Syt 4 (GST-Syt 4) or to Syt 4 lacking the transmembrane region (amino-acid residues 39–425, GST-Syt 4 TMΔ) was immobilized on glutathione-sepharose and incubated with recombinant JNK1. We found that JNK1 associated with GST-Syt 4 and GST-Syt 4 TMΔ, but not with GST alone (data not shown). This result suggests that JNK1 directly binds to Syt 4.
We then compared JNK isoforms in their capacity to associate with Syt 4 and c-Jun. Either GST alone, GST-Syt 4 or GST-c-Jun (amino-acid residues 1–79) was immobilized on glutathione-sepharose and incubated with the extracts prepared from COS1 cells expressing either JNK1, 2 or 3. The association of JNK1 with GST-Syt 4 was found to be remarkably strong, comparable to the association of JNK1 with GST-c-Jun (amino-acid residues 1–79) that harbors a high-affinity docking domain (Kallunki et al, 1996) (Figure 2C). JNK3 also associated with GST-Syt 4, whereas JNK2 barely did so (Figure 2C). This isoform specificity of JNKs for Syt 4 binding (JNK1>JNK3>JNK2) was different from that for c-Jun binding, which prefers JNK2>JNK1>JNK3 as previously reported (Gupta et al, 1996). This result is of particular interest considering functional differences between JNK isoforms shown in the previous studies (Davis, 2000).
Given the strong association of JNK1 with Syt 4, we examined whether the docking domains within JNK1 are involved in this association. It has been shown that MAP kinase family members including JNK1 contain two distinct docking domains, called the ED and CD domains, that are involved in specific and efficient associations of the MAP kinases with their substrates, activators, phosphatases and scaffold proteins (Tanoue et al, 2001; Mooney and Whitmarsh, 2004). Whereas GST-c-Jun (1–79) binding to JNK1 was reduced by mutation of the CD domain but not of the ED domain, GST-Syt 4 binding to JNK1 was reduced by mutation of either the CD or the ED domain (Figure 2D and E). Syt 4 thus appears to share docking domains within JNK1 with other JNK substrates and modulators. The requirement for two docking sites on JNK1 for binding to Syt 4 underscores the strength of interaction and specificity for JNK rather than other MAP kinases.
We then used pull-down assays to determine which domains within Syt 4 are responsible for its association with JNK1. Syt 4 consists of an N-terminal transmembrane region, a spacer and two C-terminal C2 domains C2A and C2B. We found that Flag-tagged JNK1 was co-precipitated by GST fused to the full-length Syt 4 or to the spacer and C2A domains together (S+C2A) (Figure 2F and G), but it barely associated with either a part of the spacer domain (amino-acid residues 82–158) alone or C2A domain alone (Figure 2G). JNK1 therefore associates with a region between amino-acid residues 82–275 of Syt 4.
To investigate interactions between the native, endogenous proteins, we performed co-immunoprecipitation assays of JNK and Syt 4. When endogenous Syt 4 was immunoprecipitated from the PC12 cell extracts, p46 form of endogenous JNK was co-precipitated, whereas it was not found in control IgG immunoprecipitates (Figure 2H). Immunoprecipitation of endogenous JNK also resulted in co-precipitation of endogenous Syt 4 (Figure 2I). Moreover, an immunocytochemical analysis revealed that Syt 4 colocalized with JNK at intracellular puncta (Figure 2J). Collectively, these results strongly suggest that Syt 4 interacts directly with JNK in vivo and in vitro.
JNK phosphorylates Syt 4 in vitro and in vivo
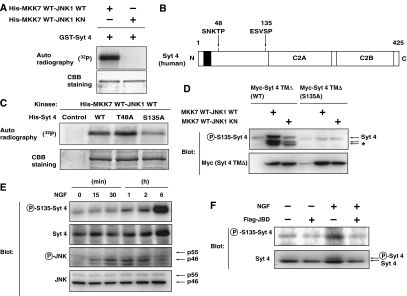
Given that Syt 4 binds to the CD and ED domains of JNK1, we hypothesized that Syt 4 might serve as a JNK substrate. To investigate whether JNK can phosphorylate Syt 4, we performed in vitro kinase assays using His-tagged JNK1. We indeed found that active JNK (MKK7 WT-JNK1 WT), but not JNK with an inactive kinase domain (MKK7 WT-JNK1 KN), phosphorylated recombinant GST-tagged Syt 4 (Figure 3A). To identify JNK phosphorylation site on Syt 4, we have mutated potential phosphorylation sites (Ser-Pro or Thr-Pro sequences) on Syt 4, as JNK is a proline-directed kinase. Among the five Ser-Pro or Thr-Pro sequences in human Syt 4, we focused on Thr48 and Ser135 that are evolutionally conserved from zebrafish to human (Figure 3B). We found that mutation of Ser135 into Ala (S135A) reduced the level of Syt 4 phosphorylation by active JNK in vitro, whereas mutation of Thr48 into Ala (T48A) had little effect (Figure 3C), suggesting that Ser135 is a JNK phosphorylation site on Syt 4.
Figure 3.
JNK phosphorylates Syt 4 at Ser135 in vitro and in vivo. (A) Bacterially expressed GST-fused recombinant Syt 4 proteins were incubated with bacterially expressed His-tagged recombinant MKK7-JNK1 fusion proteins (fusion of wild-type MKK7 and wild-type or kinase-negative JNK) in a kinase reaction buffer in the presence of [γ-32P]ATP for 30 min. Phosphorylation of proteins was detected by autoradiography. (B) Potential JNK phosphorylation sites in Syt 4. (C) Bacterially expressed His-tagged Syt 4 proteins (wild-type (WT), T48A or S135A mutants) were incubated with bacterially expressed His-tagged MKK7-JNK1 fusion proteins in a kinase reaction buffer in the presence of [γ-32P]ATP. (D) COS1 cells were transfected with an expression vector for either wild-type or S135A Myc-tagged Syt 4 TMΔ (lacking the transmembrane domain), together with an expression vector for MKK7-JNK1. After 24 h, transfected cells were lysed and subjected to immunoblot analysis with antibodies specific for Syt 4 phosphorylated on S135 (P-S135-Syt 4) or antibodies to Myc epitope. The asterisk indicates the position of degradation products of Myc-tagged Syt 4. Note that the amount of Myc-tagged Syt 4 protein was increased in MKK7-JNK1-expressing cells. (E) PC12 cells were cultured for periods as indicated in the presence of 100 ng/ml NGF. The cells were lysed and subjected to immunoblot analysis with antibodies specific for Syt 4 phosphorylated on S135, antibodies to Syt 4, phosphorylated JNK or JNK. (F) PC12 cells were infected with retrovirus encoding JBD or with corresponding control retrovirus (−). Infected cells were incubated for 2 h in the presence or absence of 100 ng/ml NGF. The cells were lysed and subjected to immunoblot analysis with antibodies specific for Syt 4 phosphorylated on S135, or for Syt 4.
To further determine whether JNK is capable of phosphorylating Ser135 of Syt 4, we raised an antibody that specifically reacts with this site, by immunizing rabbits with a Syt 4 polypeptide containing phosphorylated Ser135. This anti-phospho-Ser135 antibody recognized a band of the appropriate molecular weight in extracts from COS1 cells co-expressing myc-tagged Syt 4 lacking the transmembrane region (amino-acid residues 41–425) and active JNK1 (MKK7 WT-JNK1 WT) (Figure 3D). Co-expression of inactive JNK was much less effective in inducing this phosphorylation (Figure 3D). Importantly, the antibody did not recognize Syt 4 harboring S135A mutation, even when the protein was co-expressed with active JNK. These results indicate that active JNK can phosphorylate Syt 4 at Ser135, and that our anti-phospho-Ser135 antibody can recognize this event specifically.
The next key question we asked was whether phosphorylation of endogenous Syt 4 at Ser135 is induced in response to NGF in a JNK-dependent manner. Immunoblot analysis with anti-phospho-Ser135 Syt 4 antibody showed that Syt 4 phosphorylation at Ser135 was increased in response to NGF treatment in PC12 cells, reaching maximal level in 6 h after NGF treatment (Figure 3E). The level of Syt 4 protein did not dramatically change by NGF treatment, consistent with a previous report (Vician et al, 1995). Phosphorylation of the p46 form of JNK, used as a reporter of JNK activity, was evident after 15 min of NGF treatment and reached maximal level after around 30 min (Figure 3E).
Finally, we examined whether NGF-dependent phosphorylation of Syt 4 requires JNK activation, by introducing the JNK inhibitor JBD using a retroviral vector. Expression of JBD effectively suppressed the basal and NGF-stimulated phosphorylation of Syt 4 at Ser135 (Figure 3F), indicating that JNK activation is essential for NGF stimulation of Syt 4 phosphorylation.
Syt 4 mediates NGF-stimulated, but not basal, Ca2+-evoked release
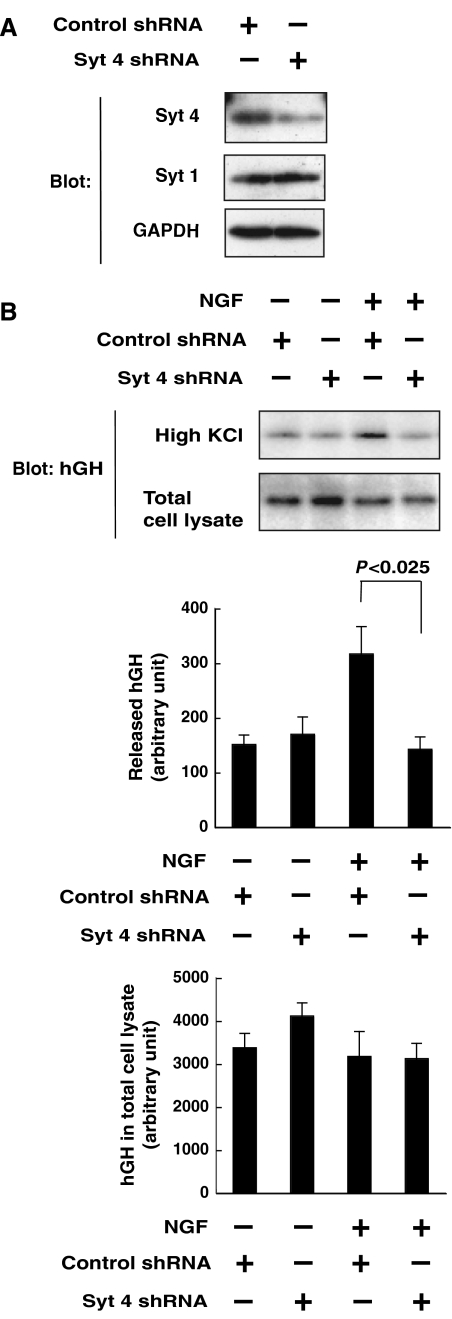
It has been shown that Syt 4 plays both stimulatory and inhibitory roles in the regulation of Ca2+-evoked release, depending on the cellular context. Before examining the role of Ser135 phosphorylation in PC12 cells, we wished to clarify the role of Syt 4 in NGF regulation of Ca2+-evoked release in these cells. Introduction of short hairpin RNA (shRNA) targeted for Syt 4 reduced the amount of Syt 4 protein in PC12 cells (Figure 4A). Under this condition, NGF stimulation of depolarization-induced hGH secretion was markedly inhibited by Syt 4 knockdown, whereas the basal level of depolarization-induced hGH release was unchanged (Figure 4B). This suggests that Syt 4 is involved only in growth factor modulation of Ca2+-evoked release, and not in Ca2+-evoked release itself, which appears similar to the role of JNK mentioned above.
Figure 4.
Requirement of Syt 4 for NGF enhancement of Ca2+-evoked release in PC12 cells. (A) PC12 cells were transfected with an expression vector encoding either Syt 4 shRNA or control shRNA. After 72 h, transfected cells were lysed and subjected to immunoblot analysis with antibodies to Syt 4, Syt 1 or GAPDH. (B) PC12 cells were transfected with expression vectors encoding hGH and either a Syt 4 siRNA or a control siRNA. After 72 h, the cells were incubated for 60 min in the presence or absence of 100 ng/ml of NGF. The culture medium was then replaced with 60 mM KCl-containing salt solution for 3 min. The resulting medium was collected for detection of secreted hGH by immunoblot analysis. Results are presented as mean±s.d. from triplicate samples. Essentially, the same results were obtained in three independent experiments.
Essential role of Ser135 and JNK in Syt 4-mediated secretion
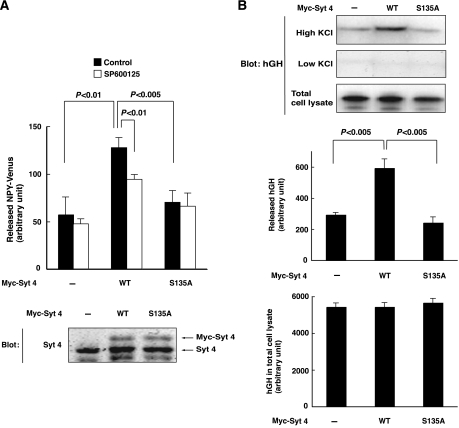
Since both Syt 4 and JNK are found to be necessary for NGF enhancement of Ca2+-evoked release, we asked whether JNK phosphorylation of Syt 4 on Ser135 plays a role in the regulation of secretion. We found that ectopic expression of Myc-tagged Syt 4 enhanced depolarization-induced secretion of NPY-Venus (Figure 5A), as well as that of hGH (Figure 5B). Significantly, this enhancement of Ca2+-evoked release did not take place when Ser135 was mutated into Ala, in both systems (Figure 5A and B). The JNK inhibitor SP600125 also suppressed the stimulatory effect of Syt 4 overexpression on Ca2+-evoked release (Figure 5A). It thus appears that overexpression of Syt 4 may in part be able to replace the stimulatory effect of NGF on Ca2+-evoked release, and that this enhancement of secretion by Syt 4 requires JNK-dependent phosphorylation of Syt 4 on Ser135.
Figure 5.
Syt 4 enhances Ca2+-evoked release in a Ser-135-dependent manner in PC12 cells. (A, B) PC12 cells stably expressing NPY-Venus were infected with retrovirus encoding Myc-tagged wild-type Syt 4, S135A Syt 4, or corresponding control retrovirus (−). (A) Infected cells were preincubated with 20 μM SP600125 (open bars) or DMSO (filled bars) for 30 min, incubated for 3 min in 60 mM KCl-containing salt solution and the resulting culture medium was collected for detection of secreted NPY-Venus. Results are presented as mean±s.d. from triplicate samples. Essentially, the same results were obtained in three independent experiments (upper panel). Infected cells were lysed and subjected to immunoblot analysis with antibodies to Syt 4 (lower panel). (B) PC12 cells were transfected with expression vectors encoding hGH and either Myc-tagged wild-type Syt 4 or S135A Syt 4. After 72 h, the cells were incubated with 60 mM KCl-containing salt solution for 3 min. The resulting medium was collected for detection of secreted hGH by immunoblot analysis. Results are presented as mean±s.d. from triplicate samples. Essentially, the same results were obtained in three independent experiments.
NGF-dependent translocation of Syt 4 to the mature secretory vesicles via JNK-mediated phosphorylation
Our results thus far have indicated that NGF enhances Ca2+-evoked release via activation of JNK, and that JNK facilitates Ca2+-evoked release via phosphorylation of Syt 4 at Ser135. What then might be the role of Ser135 phosphorylation on the function of Syt 4? It has been shown that Syt 4 is localized in various subcellular compartments including the Golgi apparatus, large dense-core vesicles, immature secretory vesicles and distal parts of neurites in PC12 cells (Ibata et al, 2000; Fukuda et al, 2001, 2003). Syt 4 was found to be localized in Syt 1-containing vesicles (Ferguson et al, 1999; Thomas et al, 1999; Wang et al, 2003; Ting et al, 2006), whereas it was localized to Syt 1-free vesicles (Berton et al, 2000; Ibata et al, 2002), depending on the cellular context. A previous report has shown that Syt 4 is normally localized at Golgi membranes and immature secretory vesicles in PC12 cells, but translocates to mature dense-core vesicles in response to NGF stimulation (Fukuda et al, 2003). We therefore tested the hypothesis that JNK-mediated Ser135 phosphorylation might regulate the localization of Syt 4 in response of NGF.
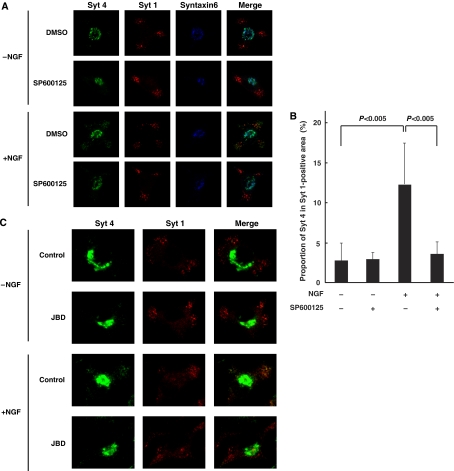
We first examined the localization of Syt 4 by immunocytochemistry. In control PC12 cells, the majority of Syt 4 appears to be colocalized with Syntaxin6, a marker for immature secretory vesicles and trans-Golgi network (Ahras et al, 2006). In contrast, as previously reported, NGF treatment for 4 h resulted in translocation of a fraction of Syt 4 away from the Golgi apparatus, causing Syt 4 staining to partially overlap with the staining of Syt 1, a marker for mature secretory vesicles (Figure 6A). The proportion of Syt 4 in Syt 1-positive area was increased by NGF treatment from 2.7 to 12.3% (n=20, P<0.005). However, when cells were treated with the JNK inhibitor SP600125, the translocation of Syt 4 was markedly inhibited (3.5% in the presence of NGF and SP600125 treatment; n=20, P<0.005) (Figure 6A and B and Supplementary Figure S4). Expression of JBD also inhibited the translocation of Syt 4 by NGF (Figure 6C). These results indicate that JNK is involved in regulating the NGF-mediated redistribution of Syt 4 to mature vesicles, which may underlie the NGF enhancement of Ca2+-evoked release. It is still unclear whether NGF activation of JNK affects the abundance of mature vesicles in addition to the redistribution of Syt 4.
Figure 6.
Requirement of JNK for NGF-dependent localization of Syt 4 to mature dense-core vesicles in PC12 cells. (A, B) PC12 cells were preincubated with 20 μM SP600125 (+) or DMSO (−) for 30 min, followed by a further 4 h incubation in the presence or absence of 100 ng/ml NGF. (C) PC12 cells were infected with retrovirus encoding JBD, or with corresponding control retrovirus (control). Infected cells were incubated for 4 h in the presence or absence of 100 ng/ml NGF. (A, C) The cells were fixed and subjected to immunocytochemistry with antibodies to Syt 4, Syt 1 (a marker for mature dense-core vesicle) and Syntaxin6 (a marker for Golgi body and immature secretory vesicle). Fluorescence images were recorded by a confocal laser-scanning microscope and typical images of cells are shown. (B) Quantification of the intracellular distribution of Syt 4. The cells were fixed and subjected to immunocytochemistry with antibodies to Syt 4, and Syt 1. The integrated fluorescence intensities of Syt 4 within the whole cell (a) and of Syt 1-positive (b) areas were detected by a confocal laser-scanning microscope. The proportion of (a) to (b) was calculated from 20 independent images by MetaMorph software. Data are means±s.d. P<0.005, Student's t-test.
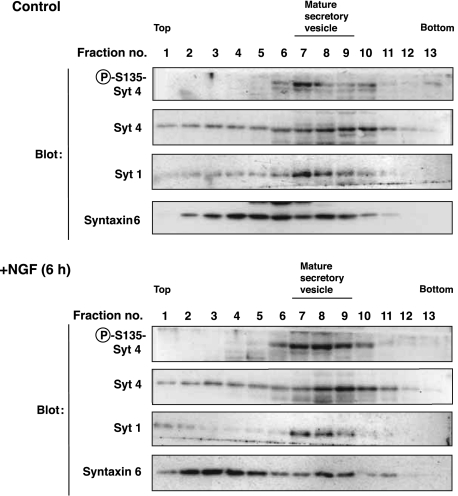
To ask whether phosphorylation of Syt 4 might have an impact on its localization, we performed cell fractionation by sucrose-density gradient centrifugation. The overall elution pattern of Syt 4 protein did not change largely by NGF treatment, probably reflecting that the proportion of phosphorylated Syt 4 among total Syt 4 was small. Importantly, Syt 4 phosphorylated on Ser135 was co-eluted with Syt 1, a marker for mature secretory vesicles, whereas total Syt 4 was broadly eluted with Syntaxin6, a marker for immature vesicles and Golgi apparatus (Figure 7). This result suggests that phosphorylated Syt 4 localized at mature secretory vesicles.
Figure 7.
Phosphorylated Syt 4 localizes at mature dense-core vesicles in PC12 cells. PC12 cells were incubated for 6 h in the presence or absence of 100 ng/ml NGF. The cells were homogenized and the post-nuclear supernatants loaded on a discontinuous 0.8–1.8 M sucrose gradient. Fractions were collected after centrifugation. Samples were subjected to immunoblot analysis with antibodies specific for Syt 4 phosphorylated on S135, antibodies to Syt 4, Syt 1 (a marker for mature dense-core vesicle) and Syntaxin6 (a marker for Golgi body and immature secretary vesicle).
Discussion
Growth factors such as NGF stimulate Ca2+-evoked release in various cellular contexts. In this study, we have shown that JNK plays an important role in NGF enhancement of Ca2+-evoked release in PC12 neuroendocrine cells. Moreover, our results suggest that Syt 4 is a major target for JNK in enhancing Ca2+-evoked release, based on several lines of evidence: (1) JNK associates with Syt 4 in vitro and in PC12 cells; (2) JNK phosphorylates Syt 4 at Ser135 in vitro and in PC12 cells in response to NGF stimulation; (3) both JNK and Syt 4 are necessary for NGF enhancement of Ca2+-evoked release, but not for basal Ca2+-evoked release; (4) expression of wild-type Syt 4, but not S135A Syt 4, promoted Ca2+-evoked release, and this promotion was in part suppressed by the JNK inhibitor and (5) NGF stimulation results in the translocation of Syt 4 to mature secretory vesicles in a Ser135- and JNK-dependent manner. These results strongly suggest a functional interaction of JNK with Syt 4, demonstrating a connection between growth factor signaling and secretory machinery.
The role of Syt 4 in regulation of Ca2+-evoked release can be stimulatory or inhibitory, depending on the cellular contexts or systems, although the molecular basis for these differences in Syt 4-mediated responses is not clear. In our study, Syt 4 was found to be necessary for NGF enhancement of Ca2+-evoked release, but not for basal Ca2+-evoked release. Moreover, overexpression of wild-type Syt 4, but not S135A-mutated Syt 4, promoted Ca2+-evoked release. Therefore, it is possible that Syt 4 promotes exocytosis only when phosphorylated upon growth factor stimulation. It would be of interest to examine whether the non-phosphorylated form of Syt 4 has a different function from the phosphorylated form, which might in part account for the context-dependent functions of Syt 4.
The localization of Syt 4 in cells is also controversial. Some reports have shown that Syt 4 is localized mainly at immature secretory vesicles and the trans-Golgi network (Ibata et al, 2000; Fukuda et al, 2001, 2003), and others have shown that Syt 4 colocalizes with Syt 1-containing vesicles (Ferguson et al, 1999; Thomas et al, 1999; Wang et al, 2003; Ting et al, 2006). In this study, we found that phosphorylated form of Syt 4 is clearly localized at different compartments from its non-phosphorylated form. Phosphorylated form of Syt 4 is co-eluted with Syt 1, a marker of mature secretory vesicles, whereas the majority of Syt 4 is co-eluted with Syntaxin6, a marker of immature secretory vesicles or Golgi apparatus. This phosphorylation-dependent translocation might explain distinct localization of Syt 4 among the systems in previous reports. Previous reports have indicated that Syt 4 can be regulated by kinases at a transcription level (Ibata et al, 2000), but this, to our knowledge, is the first evidence to show direct regulation of Syt 4 by phosphorylation.
What is the role of Ser135 phosphorylation at the molecular level? Ser135 resides within the spacer domain of Syt 4, which is flanked by the transmembrane and C2A domains. One possibility is that Ser135 phosphorylation affects phosphatidylserine or Ca2+ binding to C2A domain. There is precedence for phosphorylation to affect phospholipid binding of Syt: phosphorylation of Syt 2 by WNK1 increases the Ca2+ requirement for its phospholipid binding (Lee et al, 2004). Moreover, the spacer domain of Syt 4 has been shown to be involved in Golgi apparatus localization (Fukuda et al, 2001). It is thus possible that phosphorylation of Syt 4 on Ser135 results in its dissociation from an anchoring protein at the Golgi apparatus/immature vesicles and facilitates relocation to mature vesicles. It will be important to determine the identity of Syt 4-anchoring proteins at the Golgi apparatus (and immature vesicles) to prove this hypothesis.
The members of the MAP kinase family often utilize ‘docking' to their substrates to ensure specific and efficient phosphorylation. The requirement of the docking domains (the CD domain and ED site) within JNK for its binding to Syt 4 further supports the notion that Syt 4 is a good substrate of JNK. Indeed, JNK association with Syt 4 is comparable in strength to its association with c-Jun in a GST pull-down assay. Since the docking domains are shared by scaffolding proteins, activators and phosphatases of JNK (Tanoue et al, 2001; Mooney and Whitmarsh, 2004), it may be worthwhile to examine possible functions of Syt 4 in the regulation of JNK pathway. Interestingly, in our preliminary experiments, MKK7 and Ask1 appear to associate with Syt 4 in a GST pull-down assay (Supplementary Figure S5), implicating a scaffolding function of this molecule.
In neurons, synapse-specific enhancement of synaptic strength has been suggested to require retrograde signals for its induction. Syt 4 is localized at postsynaptic vesicles (Ibata et al, 2002), and Yoshihara et al (2005) have recently revealed that postsynaptic Syt 4 is involved in retrograde signaling that enhances presynaptic activation in Drosophila neuromuscular junction. Neurotrophic factors as well as global synaptic activation (such as by seizure) have also been implicated in enhancing synaptic strength, although the underlying mechanisms are still largely unknown (Koyama et al, 2004 and the references therein). Since Syt 4 gene deletion in mouse results in defective hippocampus-dependent memory and fine motor coordination (Ferguson et al, 2004), it would be particularly interesting to investigate whether JNK activation following neurotrophic factor stimulation or other stimuli (e.g. seizure) has any role on Syt 4 in enhancing synaptic strength at either pre- or postsynaptic level in relation to memory formation.
Materials and methods
Materials
SP600125 was obtained from BIOMOL (Plymouth Meeting, PA), ionomycin was from Sigma-Aldrich (St Louis, MO), nifedipine, G418 and puromycin were from Nacalai (Kyoto, Japan), NGF was from Promega (Madison, WI), blasticidin S was from ICN Biomedicals (Aurora, OH) and U0126 and SB202190 were from Calbiochem (San Diego, CA).
Cell culture and transfection
PC12 cells or PC12 cells stably expressing NPY-Venus (Mori et al, 2004) were maintained in DMEM (Sigma-Aldrich) supplemented with 10% FBS, 5% HS and 1% penicillin/streptomycin. COS1 cells were maintained in DMEM with 10% FBS, 1% penicillin/streptomycin. Ecotropic virus-packaging (PLAT-E) cells (a kind gift of Dr T Kitamura, University of Tokyo, Tokyo, Japan) were maintained in DMEM with 10% FBS, 1% penicillin/streptomycin, 8 μg/ml blasticidin S and 0.8 μg/ml puromycin. COS1 cells and PLAT-E cells were transfected with plasmids by the use of Fugene6 (Roche Diagnostics, Indianapolis, IN), and PC12 cells were transfected with LipofectAMINE 2000 (Invitrogen, Carlsbad, CA).
In vitro kinase assay
Kinase assays were performed for 30 min at 37°C in a reaction mixture (final volume of 25 μl) containing purified His6-MKK7-JNK protein, GST-Syt 4 protein or His6-Syt 4, 5 μCi of [γ-32P]ATP, 100 μM unlabeled ATP, 20 mM Tris-HCl (pH 7.5) and 15 mM MgCl2. Proteins were then resolved by SDS–polyacrylamide gel electrophoresis and subjected to autoradiography.
GST pull-down assay
The cell lysates prepared from COS1 cells expressing Flag-tagged JNK1/2/3 or recombinant JNK1 were incubated with equal molar amount of either GST, GST-Syt 4 or GST-c-Jun-binding glutathione-sepharose containing 10 mM Hepes-NaOH (pH 7.5), 100 mM NaCl, 0.1% NP-40 and protease and phosphatase inhibitors for 2 h at 4°C. The bead-bound proteins were subjected to immunoblot analysis with antibodies to Flag or to JNK.
Co-immunoprecipitation analysis
PC12 cells were washed with PBS, and then lysed in an extraction buffer containing 50 mM Tris--HCl (pH 7.2), 500 mM NaCl, 0.5% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 10 mM MgCl2 and protease inhibitors. The lysates were centrifuged at 20 400 g for 20 min, and the resulting supernatant was subjected to immunoprecipitation with antibodies to Syt 4. The immunoprecipitates were subjected to immunoblot analysis with antibodies to JNK or Syt 4.
Immunofluorescence analysis
PC12 cells were grown on poly-D-lysine-coated coverslips and cultured for 48 h. Cells were fixed in PBS containing 4% PFA for 10 min at 37°C. The fixed coverslips were permeabilized in PBS containing 0.3% Triton X-100 for 3 min, washed twice in PBS and incubated in a blocking solution (PBS containing 2% BSA) for 30 min. For triple staining, the samples were incubated with anti-Syt 4 antibody overnight, and then incubated with Alexa Fluor488 anti-rabbit IgG antibody (Molecular Probes, Eugene, OR), anti-Syt 1 antibody labeled with Alexa Fluor546 using Zenon Mouse IgG2b-labeling kit (Molecular Probes) and anti-Syntaxin6 antibody labeled with Alexa Fluor647 using Zenon Mouse IgG1-labeling kit (Molecular Probes) for 1 h. For quantification, the samples were incubated with anti-Syt 4 and anti-Syt 1 antibody overnight, and then incubated with Alexa Fluor488 anti-rabbit IgG antibody and Alexa Fluor594 anti-mouse IgG antibody for 1 h. Syt 1-positive areas contained both Syt 1-positive and -negative vesicles, and were used to locate mature vesicles. Fluorescence images were recorded by a confocal laser-scanning microscope (model LSM-510 and LSM-510 META; Carl Zeiss MicroImaging Inc.). All images were captured at × 63 by using objective lenses (1.4 NA; Plan-Apochromat). Pictures were analyzed by using LSM5 Image Browser (Carl Zeiss MicroImaging Inc.).
Cell fractionation
PC12 cells were washed with PBS, scraped into 400 μl of ice-cold homogenization buffer (0.8 M sucrose, 1 mM MgCl2, 1 mM EDTA, 10 mM Hepes--NaOH, pH 7.5) supplemented with the protease inhibitors described above, and homogenized with a Potter-Elvehjem homogenizer (model Mazela Z; Eyela). Nuclei and unbroken cells were removed by centrifugation at 800 g for 10 min. A post-nuclear supernatant was loaded on a 1.6 ml discontinuous sucrose step gradient (from the bottom 0.2 ml 1.8 M, 0.2 ml 1.6 M, 0.3 ml 1.4 M, 0.3 ml 1.3 M, 0.3 ml 1.2 M and 0.3 ml 1.1 M). After centrifugation for 24 h at 200 000 g, 13 samples were collected from the top on the tube.
Statistical analysis
Results were expressed as the mean±s.d. and were analyzed by using the unpaired t test.
Supplementary Material
Supplementary Information
Supplementary Figures
Acknowledgments
We thank Drs Elisabeth Nigh, Motojiro Yoshihara and Jonathan A Cooper for critical reading of the manuscript, Drs Akitsugu Yamamoto, Kumiko Sogawa, Michio Hiroshima and Makio Tokunaga for valuable experimental supports and Drs Mikiko Sodeoka, Keisuke Onishi, Hiroshi Minoshima, Myeonghwan Kim and the members of Gotoh's laboratory for help and discussions. This work was supported in part by Grants-in-Aid from the Ministry of Education, Science, Sports and Culture of Japan, and by PRESTO21 and SORST of the Japan Science and Technology Corporation.
References
- Adolfsen B, Saraswati S, Yoshihara M, Littleton JT (2004) Synaptotagmins are trafficked to distinct subcellular domains including the postsynaptic compartment. J Cell Biol 166: 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahras M, Otto GP, Tooze SA (2006) Synaptotagmin IV is necessary for the maturation of secretory granules in PC12 cells. J Cell Biol 173: 241–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amino S, Itakura M, Ohnishi H, Tsujimura J, Koizumi S, Takei N, Takahashi M (2002) Nerve growth factor enhances neurotransmitter release from PC12 cells by increasing Ca(2+)-responsible secretory vesicles through the activation of mitogen-activated protein kinase and phosphatidylinositol 3-kinase. J Biochem (Tokyo) 131: 887–894 [DOI] [PubMed] [Google Scholar]
- Berton F, Cornet V, Iborra C, Garrido J, Dargent B, Fukuda M, Seagar M, Marqueze B (2000) Synaptotagmin I and IV define distinct populations of neuronal transport vesicles. Eur J Neurosci 12: 1294–1302 [DOI] [PubMed] [Google Scholar]
- Byrd DT, Kawasaki M, Walcoff M, Hisamoto N, Matsumoto K, Jin Y (2001) UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron 32: 787–800 [DOI] [PubMed] [Google Scholar]
- Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103: 239–252 [DOI] [PubMed] [Google Scholar]
- Dickens M, Rogers JS, Cavanagh J, Raitano A, Xia Z, Halpern JR, Greenberg ME, Sawyers CL, Davis RJ (1997) A cytoplasmic inhibitor of the JNK signal transduction pathway. Science 277: 693–696 [DOI] [PubMed] [Google Scholar]
- Eaton BA, Haugwitz M, Lau D, Moore HP (2000) Biogenesis of regulated exocytotic carriers in neuroendocrine cells. J Neurosci 20: 7334–7344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GD, Anagnostaras SG, Silva AJ, Herschman HR (2000) Deficits in memory and motor performance in synaptotagmin IV mutant mice. Proc Natl Acad Sci USA 97: 5598–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson GD, Thomas DM, Elferink LA, Herschman HR (1999) Synthesis degradation, and subcellular localization of synaptotagmin IV, a neuronal immediate early gene product. J Neurochem 72: 1821–1831 [DOI] [PubMed] [Google Scholar]
- Ferguson GD, Wang H, Herschman HR, Storm DR (2004) Altered hippocampal short-term plasticity and associative memory in synaptotagmin IV (−/−) mice. Hippocampus 14: 964–974 [DOI] [PubMed] [Google Scholar]
- Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC (2001) Synaptotagmin I functions as a calcium regulator of release probability. Nature 410: 41–49 [DOI] [PubMed] [Google Scholar]
- Fukuda M (2006) The role of synaptotagmin and synaptotagmin-like protein (Slp) in regulated exocytosis. In Molecular Mechanisms of Exocytosis, Regazzi R (ed), pp 42–61. Austin, TX, USA: Landes Bioscience [Google Scholar]
- Fukuda M, Ibata K, Mikoshiba K (2001) A unique spacer domain of synaptotagmin IV is essential for Golgi localization. J Neurochem 77: 730–740 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kanno E, Ogata Y, Saegusa C, Kim T, Loh YP, Yamamoto A (2003) Nerve growth factor-dependent sorting of synaptotagmin IV protein to mature dense-core vesicles that undergo calcium-dependent exocytosis in PC12 cells. J Biol Chem 278: 3220–3226 [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kojima T, Mikoshiba K (1996) Phospholipid composition dependence of Ca2+-dependent phospholipid binding to the C2A domain of synaptotagmin IV. J Biol Chem 271: 8430–8434 [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC (1994) Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell 79: 717–727 [DOI] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ (1996) Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 15: 2760–2770 [PMC free article] [PubMed] [Google Scholar]
- Ibata K, Fukuda M, Hamada T, Kabayama H, Mikoshiba K (2000) Synaptotagmin IV is present at the Golgi and distal parts of neurites. J Neurochem 74: 518–526 [DOI] [PubMed] [Google Scholar]
- Ibata K, Hashikawa T, Tsuboi T, Terakawa S, Liang F, Mizutani A, Fukuda M, Mikoshiba K (2002) Non-polarized distribution of synaptotagmin IV in neurons: evidence that synaptotagmin IV is not a synaptic vesicle protein. Neurosci Res 43: 401–406 [DOI] [PubMed] [Google Scholar]
- Itakura M, Yamamori S, Kuwahara R, Sekiguchi M, Takahashi M (2005) Two distinct regulatory mechanisms of neurotransmitter release by phosphatidylinositol 3-kinase. J Neurochem 94: 502–509 [DOI] [PubMed] [Google Scholar]
- Kallunki T, Deng T, Hibi M, Karin M (1996) c-Jun can recruit JNK to phosphorylate dimerization partners via specific docking interactions. Cell 87: 929–939 [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM (1995) Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science 267: 1658–1662 [DOI] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S (2001) Critical role of cAMP-GEFII-Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem 276: 46046–46053 [DOI] [PubMed] [Google Scholar]
- Kawasaki M, Hisamoto N, Iino Y, Yamamoto M, Ninomiya-Tsuji J, Matsumoto K (1999) A Caenorhabditis elegans JNK signal transduction pathway regulates coordinated movement via type-D GABAergic motor neurons. EMBO J 18: 3604–3615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama R, Yamada MK, Fujisawa S, Katoh-Semba R, Matsuki N, Ikegaya Y (2004) Brain-derived neurotrophic factor induces hyperexcitable reentrant circuits in the dentate gyrus. J Neurosci 24: 7215–7224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BH, Min X, Heise CJ, Xu BE, Chen S, Shu H, Luby-Phelps K, Goldsmith EJ, Cobb MH (2004) WNK1 phosphorylates synaptotagmin 2 and modulates its membrane binding. Mol Cell 15: 741–751 [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo MM (1993) Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature 363: 350–353 [DOI] [PubMed] [Google Scholar]
- Machado HB, Liu W, Vician LJ, Herschman HR (2004) Synaptotagmin IV overexpression inhibits depolarization-induced exocytosis in PC12 cells. J Neurosci Res 76: 334–341 [DOI] [PubMed] [Google Scholar]
- Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis RJ, Johnson GL, Karin M (1994) Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science 266: 1719–1723 [DOI] [PubMed] [Google Scholar]
- Mooney LM, Whitmarsh AJ (2004) Docking interactions in the c-Jun N-terminal kinase pathway. J Biol Chem 279: 11843–11852 [DOI] [PubMed] [Google Scholar]
- Mori Y, Higuchi M, Masuyama N, Gotoh Y (2004) Adenosine A2A receptor facilitates calcium-dependent protein secretion through the activation of protein kinase A and phosphatidylinositol-3 kinase in PC12 cells. Cell Struct Funct 29: 101–110 [DOI] [PubMed] [Google Scholar]
- Nagai T, Ibata K, Park ES, Kubota M, Mikoshiba K, Miyawaki A (2002) A variant of yellow fluorescent protein with fast and efficient maturation for cell-biological applications. Nat Biotechnol 20: 87–90 [DOI] [PubMed] [Google Scholar]
- Oishi H, Sasaki T, Nagano F, Ikeda W, Ohya T, Wada M, Ide N, Nakanishi H, Takai Y (1998) Localization of the Rab3 small G protein regulators in nerve terminals and their involvement in Ca2+-dependent exocytosis. J Biol Chem 273: 34580–34585 [DOI] [PubMed] [Google Scholar]
- Stempelj M, Ferjan I (2005) Signaling pathway in nerve growth factor induced histamine release from rat mast cells. Inflamm Res 54: 344–349 [DOI] [PubMed] [Google Scholar]
- Tanoue T, Maeda R, Adachi M, Nishida E (2001) Identification of a docking groove on ERK and p38 MAP kinases that regulates the specificity of docking interactions. EMBO J 20: 466–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H (1995) Neurotrophins and neuronal plasticity. Science 270: 593–598 [DOI] [PubMed] [Google Scholar]
- Thomas DM, Ferguson GD, Herschman HR, Elferink LA (1999) Functional and biochemical analysis of the C2 domains of synaptotagmin IV. Mol Biol Cell 10: 2285–2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JT, Kelley BG, Sullivan JM (2006) Synaptotagmin IV does not alter excitatory fast synaptic transmission or fusion pore kinetics in mammalian CNS neurons. J Neurosci 26: 372–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Rutter GA (2003) Multiple forms of ‘kiss-and-run' exocytosis revealed by evanescent wave microscopy. Curr Biol 13: 563–567 [DOI] [PubMed] [Google Scholar]
- Tsuruta F, Sunayama J, Mori Y, Hattori S, Shimizu S, Tsujimoto Y, Yoshioka K, Masuyama N, Gotoh Y (2004) JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J 23: 1889–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vician L, Lim IK, Ferguson G, Tocco G, Baudry M, Herschman HR (1995) Synaptotagmin IV is an immediate early gene induced by depolarization in PC12 cells and in brain. Proc Natl Acad Sci USA 92: 2164–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Poser C, Ichtchenko K, Shao X, Rizo J, Sudhof TC (1997) The evolutionary pressure to inactivate. A subclass of synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. J Biol Chem 272: 14314–14319 [DOI] [PubMed] [Google Scholar]
- Wang CT, Grishanin R, Earles CA, Chang PY, Martin TF, Chapman ER, Jackson MB (2001) Synaptotagmin modulation of fusion pore kinetics in regulated exocytosis of dense-core vesicles. Science 294: 1111–1115 [DOI] [PubMed] [Google Scholar]
- Wang CT, Lu JC, Bai J, Chang PY, Martin TF, Chapman ER, Jackson MB (2003) Different domains of synaptotagmin control the choice between kiss-and-run and full fusion. Nature 424: 943–947 [DOI] [PubMed] [Google Scholar]
- Wick PF, Senter RA, Parsels LA, Uhler MD, Holz RW (1993) Transient transfection studies of secretion in bovine chromaffin cells and PC12 cells. Generation of kainate-sensitive chromaffin cells. J Biol Chem 268: 10983–10989 [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Galle KT, Littleton JT (2005) Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science 310: 858–863 [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Littleton JT (2002) Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron 36: 897–908 [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Montana ES (2004) The synaptotagmins: calcium sensors for vesicular trafficking. Neuroscientist 10: 566–574 [DOI] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, Morozov A (2003) Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1–CA3 synapses. Neuron 39: 975–990 [DOI] [PubMed] [Google Scholar]
- Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG (2004) Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci USA 101: 9441–9446 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information
Supplementary Figures