Abstract
Dietary diversity often varies inversely with prey resource abundance. This pattern, although typically measured at the population level, is usually assumed to also characterize the behavior of individual animals within the population. However, the pattern might also be produced by changes in the degree of variation among individuals. Here we report on dietary and associated behavioral changes that occurred with the experimental translocation of sea otters from a food-poor to a food-rich environment. Although the diets of all individuals were broadly similar in the food-rich environment, a behaviorally based dietary polymorphism existed in the food-poor environment. Higher dietary diversity under low resource abundance was largely driven by greater variation among individuals. We further show that the dietary polymorphism in the food-poor environment included a broad suite of correlated behavioral variables and that the individuals that comprised specific behavioral clusters benefited from improved foraging efficiency on their individually preferred prey. Our findings add to the growing list of examples of extreme individuality in behavior and prey choice within populations and suggest that this phenomenon can emerge as a behavioral manifestation of increased population density. Individuality in foraging behavior adds complexity to both the fitness consequences of prey selection and food web dynamics, and it may figure prominently as a diversifying process over evolutionary timescales.
Keywords: foraging efficiency, niche width, polymorphism, prey handling
A large and long-standing body of theoretical and empirical research has led to the well established view that the dietary diversity of consumers increases as food becomes limiting (1–4). The implicit assumption underlying both the theory and observation is that individuals within populations are responding to changing prey availability in broadly similar ways. However, the commonly observed population-level pattern of increased dietary diversity with reduced prey abundance could also occur via individual diversification (5, 6), in which case the dietary breadth of any given individual in response to prey limitation might change very little. We subsequently refer to the former process as the within-individual diversity hypothesis (WIDH) and the latter as the among-individual diversity hypothesis (AIDH), recognizing that the two processes are not mutually exclusive. The WIDH and AIDH involve fundamentally different mechanisms that have very different implications for population, community, and evolutionary ecology (7), yet the relative importance of these two processes remains largely unevaluated for most free-living consumers, particularly large vertebrates. This lack of attention likely reflects the fact that long-term dietary records from specific individuals are difficult to obtain, and comparable samples of individual behavior and diet under conditions of high and low resource abundance are seldom available.
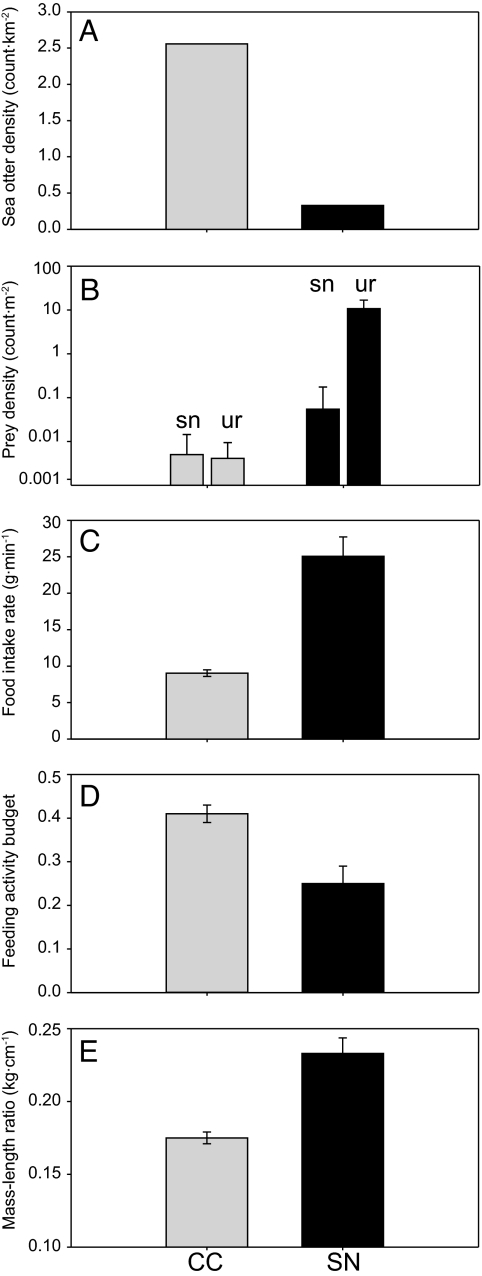
California sea otters (Enhydra lutris nereis) provide a unique opportunity to explore the WIDH–AIDH dichotomy. Diet in this species is easily determined from shore-based observations because sea otters invariably return to the surface to consume their prey, and we have obtained longitudinal records of sea otter diet and foraging behavior from tagged individuals that span multiple years (8). Moreover, the experimental translocation of sea otters from central California (henceforth CC) to San Nicolas Island (henceforth SN) in the southern California Bight in 1987–1990 established a second population in a comparatively food-rich environment where the diversity of potential invertebrate prey is similar or slightly greater than that at CC (9). After an initial posttranslocation decline (resulting largely from dispersal back to CC), the SN population stabilized in the early 1990s and then began growing at ≈9% per year. This growth trend is expected to continue because the current population size (≈40 individuals) is well below the estimated equilibrium density (Fig. 1A). The abundance of commonly consumed sea otter prey at SN exceeds that at CC by as much as three orders of magnitude (Fig. 1B), a difference that results from the sea otter's well known ability to limit its invertebrate prey populations (10) and the comparative low density and long period of absence of sea otters from the SN system.
Fig. 1.
A comparison of summary statistics that together characterize sea otter population status and prey abundance at two study sites in California, CC (gray bars) and SN (black bars). (A) Sea otter population density (km−2). (B) Estimates of the benthic density (m−2) of two commonly consumed sea otter prey taxa: sn, marine snails (Astraea sp.), and ur, sea urchins (Strongylocentrotus spp.). (C) Estimated mean rate of biomass intake (g·min−1) by foraging sea otters. (D) The mean proportion of time spent feeding. (E) Body condition of adult sea otters (mass/length ratios). Error bars plotted in B–E represent ± 1 SE.
Between 2003 and 2005, we collected detailed information on diet and foraging behavior from 11 radio-tagged animals at SN, and we collected similar data from 34 animals at CC between 2001 and 2004. At each site, the samples consisted of adult animals (≥3 years of age) of both sexes with largely overlapping home ranges, and individuals at both sites foraged in generally similar mixed rocky and sand-bottom habitats. The average food intake rate was more than twice as great at SN than at CC (Fig. 1C); animals at SN thus spent only half as much time foraging (Fig. 1D) and were in better body condition (Fig. 1E) compared with animals at CC. The difference in prey abundance between CC and SN is typical of other areas with and without sea otters (11), and the associated interpopulation differences in body condition, rate of food intake, and daily activity budget occur at the extremes of the recorded values for each metric in this species (12–15). Taken together, these data suggest that food resources are effectively unlimited for sea otters at SN but are strongly limiting to the CC population. The SN–CC comparison thus provides an experimental means of evaluating the behavioral responses of individuals in a wild predator population to release from food limitation.
Results
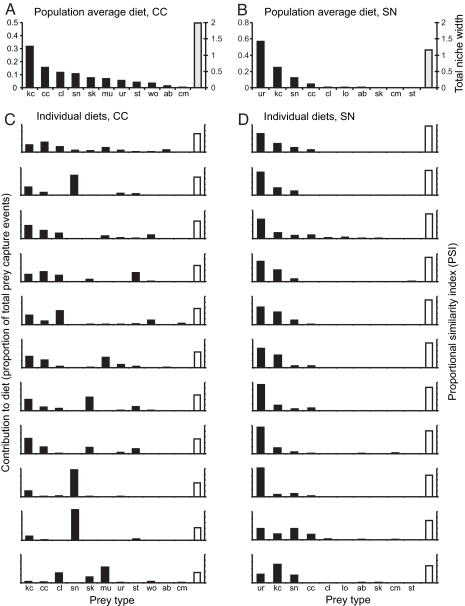
On the basis of extensive foraging observations (n = 37,255 observed feeding dives and 23,339 prey captures for CC; n = 5,341 observed feeding dives and 2,361 prey captures for SN) collected across all seasons for each study animal, we found strong support at the population level for the classical prediction of greater dietary diversity in the food-limiting environment; the total niche width at CC was almost twice that at SN (Fig. 2 A and B). The WIDH predicts that the greater dietary diversity at CC is primarily a reflection of individual diet breadth, and thus the ratio of within-individual diversity to total niche width should be close to one. Alternatively, the AIDH implies greater dietary variation among individuals. On average, individual niche widths at CC (1.25 ± 0.34) were slightly greater than those at SN (1.05 ± 0.25); however, the ratio of within-individual diversity to total dietary niche width was substantially lower at CC (0.7 ± 0.06) than at SN (0.9 ± 0.02).
Fig. 2.
Population-level and individual diet histograms for sea otters at CC (Left) and SN (Right), illustrating differences in total niche width and individual diet specialization between the two populations. The left vertical axis of each graph indicates the dietary prevalence of various prey types (measured as the proportion of total prey capture events). A and B show the mean diet composition for otters at CC and SN, respectively, with the rightmost bar in each graph indicating the total niche width for each population as measured by the Shannon–Weiner index (right vertical axes). Prey types consumed at each site are indicated by two letter codes on the horizontal axis: ur, urchins; kc, kelp crabs; sn, marine snails; cc, Cancer crabs; cl, clams, scallops, and other infaunal bivalve mollusks; lo, lobster; ab, abalone; sk, small unidentified kelp-dwelling invertebrates; cm, cephalopod mollusks; st, sea stars; mu, mussels; wo, worms; sd, sand dollars. C and D depict the corresponding diet histograms for 11 individuals at each site, with the rightmost bar in each graph indicating the proportional similarity index (PSI, right vertical axes), or the degree to which each individual's diet matches the population-level diet. Tick marks on both the left and right vertical axes in C and D are at intervals of 0.2.
To rule out the potentially confounding effects of sample size and environmental variability in the CC–SN comparison, we selected the subsample of 11 individuals from CC whose spatial concurrence most closely matched that of the SN sample (mean pairwise overlap of home-range polygons = 42%) and contrasted the degree of dietary variation between these two groups. The degree of similarity between individual diets and the population “average” diet (the proportional similarity index, or PSI) was relatively low for the 11 animals at CC (PSI = 0.547) compared with the 11 animals at SN (PSI = 0.819), indicating that individuals specialized to a much greater extent at CC. We found the same pattern when we repeated the analysis using bootstrapped samples from each location: the average PSI value at CC was 0.565 [95% bootstrap confidence level (CL95) = 0.467–0.664], whereas the average PSI value at SN was 0.824 (CL95 = 0.769–0.879). These data indicate a clear, qualitative difference between study sites: plots of diet composition were generally similar among the individuals at SN but differed greatly between individuals at CC, with almost no overlap among certain individuals (Fig. 2 C and D). Thus, the sea otter population at CC is best characterized as a diversified mixture of individual specialists, in contrast to SN where individual diets are similar and generally reflect the population average. These findings provide strong support for the AIDH.
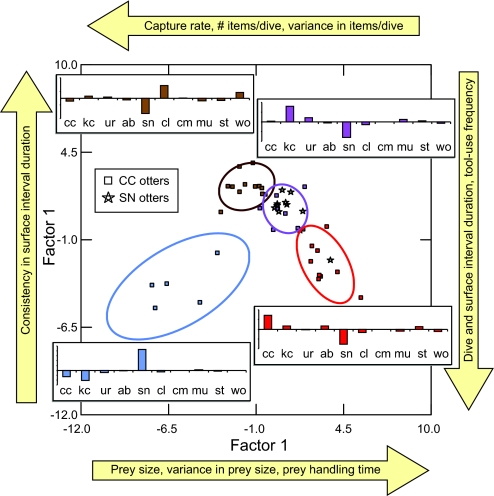
Predators often exhibit distinct patterns of search and handling behavior when using different prey types (16), and this is particularly likely to be the case for a predator such as the sea otter, whose diet spans a taxonomically and phenotypically diverse array of prey (8). The AIDH thus leads to an expectation of greater among-individual behavioral variation in the resource-limited population. To test this prediction, we recorded a suite of behavioral variables during each observed foraging bout. After characterizing each otter's modal foraging behavior in multivariate space, we found CC otters to be 3.6 times more variable in their behavior than SN otters (bootstrapped CL95 = 1.91–6.02). Moreover, variation in foraging behavior across the entire SN + CC dataset aggregated into four clusters, or behavioral modes, that were statistically distinct (Wilks' λ = 0.0074, F36,89 = 10.561, P < 0.0001, jackknifed classification accuracy = 89%) and readily discriminated along two canonical axes summarizing behavioral variation (Fig. 3). All but one of the SN individuals fell into a single cluster, whereas CC individuals were more evenly divided among clusters, and animals that exhibited different behavioral modes also tended to specialize on different prey types (Fisher's exact test, P = 0.0002). Interestingly, the one SN individual grouped apart from the others was a 16-year-old female that had been part of the original translocation, suggesting that she may have retained some characteristics of her pre-translocation feeding behavior.
Fig. 3.
An ordination of the first two factors from a discriminant analysis conducted to evaluate the classification of individual sea otters into one of four clusters on the basis of foraging behavior. Each factor represents a canonical function of 14 parameters recorded from study animals at two study sites, CC and SN, and factor scores for individual otters are plotted in this multivariate space. To aid in interpretation, the qualitative relationship between key behavioral variables and the discriminant factors is indicated by arrows and text outside the ordination. Individuals are color-coded on the basis of the behavioral cluster to which they were initially assigned, and the 95% bivariate confidence ellipses for each cluster are also plotted. Histogram panels are superimposed adjacent each cluster (color-coded as above) to depict the mean relative abundance of 10 key prey types in the diets of the CC otters within that cluster, expressed as proportional discrepancies from the population average (two-letter prey codes are defined in Fig. 2, and vertical axis tick marks are at intervals of 0.05).
Why would individuals specialize rather than becoming dietary generalists when resources are limiting? Theory suggests that this specialization will occur if preferred prey are relatively easy to capture and handle, whereas less preferred prey require complex foraging skills or specific phenotypic traits for efficient utilization (5, 17). In accordance with this theory, we predicted that those sea otters at CC that specialized on a particular prey type (i.e., consumed proportionally more than most conspecifics; see Methods) should benefit from improved handling efficiency for that prey. Nine prey types were consumed in sufficiently large numbers to contrast handling efficiency between specialists and nonspecialists. Five of the 9 contrasts differed significantly, all favoring improved foraging efficiency by specialists over nonspecialists (Table 1). Despite the low statistical power associated with the small number of contrasts, the probability of this pattern resulting from chance alone is 0.031. Overall, individuals that specialized on a particular prey type were able to process 25% more prey items per unit of time than were nonspecialists.
Table 1.
Handling efficiency (amount of time required to handle and consume one prey item) of sea otters at CC that specialize on nine different prey types, contrasted with the handling efficiency of nonspecialists
| Prey type | Prey diameter, cm | Specialists, mean per-item handling time (SD), s | n | Nonspecialists, mean per-item handling time (SD), s | n | % increase in efficiency | Student's t value | P |
|---|---|---|---|---|---|---|---|---|
| Marine snails | 1–5 | 9.41 (1.578) | 5 | 18.916 (8.222) | 6 | 101.0 | 2.771 | 0.036 |
| Kelp crabs | 6–10 | 84.092 (13.829) | 5 | 108.674 (40.68) | 29 | 29.2 | 2.518 | 0.021 |
| Urchins | 1–5 | 20.575 (3.933) | 6 | 25.978 (7.615) | 18 | 26.3 | 2.243 | 0.038 |
| Clams | 6–10 | 47.785 (10.208) | 5 | 67.994 (30.083) | 15 | 42.3 | 2.243 | 0.038 |
| Worms | 11–15 | 8.32 (0.601) | 4 | 11.223 (2.649) | 8 | 34.9 | 2.952 | 0.018 |
| Average % increase in efficiency for prey types with significant differences: | 46.7 | |||||||
| Cancer crabs | 11–15 | 183.217 (75.074) | 5 | 188.323 (91.386) | 25 | 2.8 | 0.134 | 0.898 |
| Abalone | 11–15 | 166.667 (43.501) | 3 | 111.75 (24.865) | 4 | −33.0 | 1.96 | 0.145 |
| Sea stars | 11–15 | 44.606 (8.685) | 5 | 58.521 (40.432) | 18 | 31.2 | 1.352 | 0.191 |
| Mussels | 1–5 | 24.968 (4.167) | 5 | 22.493 (6.042) | 11 | −9.9 | 0.95 | 0.362 |
| Average % increase in efficiency for prey types without significant differences: | −2.2 | |||||||
| Average % increase in efficiency for all prey types: | 25.0 | |||||||
Handling time data were filtered to include only the most commonly captured size class for each prey type. Sample sizes (n) indicate the number of animals classified as specialists and nonspecialists, respectively (the mean handling time for a given prey type by one animal represents the sample unit for statistical comparison). The relative difference between specialists and nonspecialists is presented as the percent increase in handling efficiency for specialists (number of items handled per unit time), followed by the statistical significance (based on a two-tailed t test) of this difference.
Discussion
Our results indicate that, for southern sea otters, higher dietary diversity under low resource abundance is largely driven by variation among individuals. Within-individual diet diversity was also slightly higher in the food-limiting environment, demonstrating that both within- and among-individual variation can contribute to population-level patterns; however, the qualitative differences between the two sites with respect to individual specialization are most consistent with the AIDH. Conceivably, these differences are related to some factor other than food abundance [the most plausible alternative hypotheses, and the reasons we have discounted them, are summarized in the supporting information (SI) Text], but this is the simplest and most parsimonious explanation.
Individuality in animal behavior is now well established and widely reported (7). Alternate foraging modes associated with physical polymorphisms provide some of the most well known examples (18–20), but individual differences in prey selection and foraging behavior also have been reported that appear to be unrelated to either environmental context or morphological correlates in the consumers (21–25). Behavioral polymorphisms probably occur widely in nature but generally go unnoticed in the absence of longitudinal data from individuals. As a result, little attention has been paid to the existence or significance of individual diet specialization, and the exact mechanisms underlying this phenomenon are poorly understood. Our findings provide evidence for both plasticity and context dependency in the expression of dietary polymorphisms. A similar phenomenon was recently demonstrated for sticklebacks (6), although in that case the behaviorally driven niche diversification mapped onto preexisting phenotypical differences; we have shown that context-dependent diet specialization can occur even in the absence of morphological correlates.
The key elements of context dependency in diet specialization are consumer population density, the associated changes in prey availability, and the economics of prey choice (26, 27). As sea otter numbers increase and the abundance of their preferred prey is reduced by predation, the consumption of less preferred prey types apparently becomes profitable (28), thus explaining an increased dietary breadth at the level of the population. Some of these prey types require complex capture or handling techniques for efficient utilization: This requirement, along with increased competition for food, will tend to place a premium on the acquisition of prey-specific foraging skills (5, 17). Such skills can be learned and honed through experience (29), but because of the costs associated with skill acquisition and the limited ability of individuals to maintain a repertoire of skills for all prey types at high performance levels (16), individuals may become more or less proficient in different prey-specific foraging techniques. It is clear how this scenario would lead to density-dependent diet specialization and behavioral diversification over the short term, and the predictions are consistent with our results (Fig. 3 and Table 1), but what prevents convergence (over multiple generations) on one optimal diet? The same mechanisms responsible for morphological character divergence—namely, correlational selection on multiple traits and frequency-dependent trade-offs—are equally capable of maintaining behavioral polymorphisms within a population (30), especially when there is matrilineal cultural transmission of learned foraging skills or prey preferences (8, 31).
Extreme individuality in diet and behavior such as that we describe for sea otters has a wide range of implications and potential consequences. For example, it probably leads to increased variation in the fitness of individuals (21), may serve as an important diversifying mechanism on evolutionary timescales (5, 7), and adds complexity to consumer–prey interactions (32). Food webs, defined by the suite of all trophic linkages, are invariably described at the level of species, or even groups of species, but intraspecific diet specialization could introduce substantial variability to food web topology, thereby affecting community dynamics and stability (33).
The interindividual differences in diet and foraging behavior we report for sea otters in central California are far greater than those that have been used to characterize many competing species based on population-level averages (8). It is possible that behavioral polymorphisms such as those we describe here, especially those that are maintained along matrilines (8, 31), represent an important substrate for the tempo and mode of evolutionary diversification. At very least, it would appear that macroevolutionary drivers of diversity among species (i.e., within-guild competition and niche diversification) are also evident in extreme form at the level of individuals within populations (6).
We suspect that the patterns reported in this paper are not unique to sea otters but occur in many other species, including other large, wide-ranging vertebrates for which longitudinal data on individual diets and feeding behavior have been logistically difficult to acquire. Recent advances such as molecular techniques for diet characterization and bio-logging technology provide new opportunities for the detection and measurement of individual specialization in diet and foraging behavior (34). We hope that these results and our interpretations of them will encourage others to look for similar patterns in other species and ecosystems, and to begin a more systematic and rigorous search for their causes and consequences.
Methods
Community Metrics.
The CC study site consisted of nearshore waters between Pt. Piedras Blancas and Pt. Estero along the central coast of California; the SN study site consisted of nearshore waters surrounding San Nicolas Island in the southern California Bight (SI Fig. 4). Sea otter densities at each site (km−2) were measured as the number of animals counted during annual spring censuses (2001–2003 average counts for CC, 2003–2005 average counts for SN) divided by the area of suitable habitat (subtidal benthos between the mean low tide line and the 40-m isobath) (35). Relative prey availability at each study site was evaluated by using standardized scuba-based sampling protocols (36), as described in SI Text.
Study Animal Sampling.
Captures and radio-tagging of study animals were conducted in 2001–2003 at CC (n = 48) and in 2003–2005 at SN (n = 13). Sea otters were captured by using scuba-based methods (37), and then anesthetized, flipper-tagged, and surgically implanted with VHF radio transmitters by using standardized procedures (38). Body condition was measured as the weight/length ratio (kg·m−1), with total length (snout to tail) measured on a flat surface. Details of the capture, anesthetics, tagging, and subsequent monitoring by radio telemetry of sea otters included in this study are provided elsewhere (39). All activities were covered by an institutional permit issued by University of California, Santa Cruz, to J.A.E. and M.T.T. and a Federal Permit (MA672624) issued by the U.S. Fish and Wildlife Service to J.A.E. To ensure representative dietary composition data for each individual, we restricted further analyses to those animals from which we were able to record ≥10 independent feeding bouts spanning at least 1 year, and comprising ≥300 known-outcome feeding dives (n = 34 at CC and n = 11 at SN). The male/female ratios of the two samples, 0.21 at CC and 0.36 at SN, were not significantly different (Fisher's exact test, P = 0.421).
Activity Budgets and Home Ranges.
Activity was measured by sampling instantaneous behavior at 10-min intervals throughout continuous 12- to 24-hour focal animal monitoring sessions (15). These data were available for 28 of the 34 animals at CC and 7 of the 11 animals at SN, and they were used to estimate the average proportion of a 24-h period allotted to feeding behavior at each site. To quantify individual home ranges, each study animal was located three to seven times per week, and its precise geographic position, recorded by using GPS, was entered into a Geographic Information System (GIS) database. We collected a minimum of 200 daily locations per animal and used fixed kernel density estimation techniques (40) to delineate annual home-range polygons for each animal (see SI Text).
Diet Composition, Diversity, and Rate of Food Intake.
Observational data on foraging behavior and diet composition were recorded by using standardized methods (41, 42). The relative frequency of capture of each prey type was used to calculate diet composition for each study animal (SI Table 2) and to estimate diet diversity (measured as the Shannon–Weiner index) at both the individual and population levels. Using estimation procedures described elsewhere (43), we calculated the ratio of within-individual diet diversity to total population niche width, and the PSI, where PSI values ≪ 1 indicate nonverlapping diets, or individual specialization. These metrics were estimated for the 11 animals at SN and for the 11 animals at CC whose degree of home-range overlap most closely matched the SN sample. We then repeated the analyses using 5,000 bootstrap samples drawn from each study group (11 animals were selected randomly with replacement for each iteration), thereby allowing us to estimate the mean, standard deviation and CL95 limits for each parameter based on comparable sample sizes. Prey biomass intake (g·min−1) was estimated as the product of the number of prey items captured (and consumed) and the estimated edible biomass of each item, summed across all dives in a foraging bout and then divided by the total duration of the bout. Intake rates were averaged across bouts for each individual and then across individuals at each study site.
Analysis of Foraging Behavior.
In addition to quantifying diet composition, observational data were used to characterize individual foraging behavior. We used principal components analysis to collapse 14 behavioral variables (SI Table 3) into a smaller number of orthogonal factors before contrasting behavioral variation at SN and CC. Examination of eigenvalues indicated that five principal components captured the majority (90%) of the variance among animals. We calculated the among-individual variance in PCA scores at each study site, averaging variance estimates for the five principal components (weighted by their associated eigenvalues) to obtain a single representative index of individual behavioral variability at CC (varCC) and at SN (varSN). To account for the differing sample sizes, we repeated this entire analysis for 5,000 bootstrap samples of 11 animals from each study site and, for each sample pair, calculated the ratio of variance indices (varCC/varSN), reasoning that if individual behavioral variation did not differ between sites, the average ratio for all 5,000 samples should be approximately equal to one.
We next analyzed the 14 behavioral parameters, using hierarchical cluster analysis, to determine whether individuals exhibited consistent and distinct patterns of foraging behavior. Ward's minimum variance method was used to link similar individuals based on standardized Euclidean distances (44), and examination of the resulting dendrogram and scree-plot of internode distances (SI Fig. 5) indicated four clusters. We used linear discriminant analysis to assess (i) the efficacy of the behavioral classification scheme, (ii) which behavioral parameters were most useful for distinguishing between clusters, and (iii) how individuals from each study site were distributed among clusters. Wilks' λ was used to test for multivariate differences among clusters (45), and we also measured the proportion of individuals consistently assigned to the same cluster using jackknife resampling of the classification matrix (44). We then plotted all individual otters on an ordination of the first two discriminant factors, interpreting each axis on the basis of the standardized canonical discriminant functions and the mean parameter values for animals in each cluster (SI Table 4). To examine the relationship between behavioral mode and diet composition for CC study animals, we calculated the mean proportional contribution of each prey type to the diets of animals in each cluster, and graphically contrasted the resulting diet histograms. We used a numerical approximation to Fisher's exact test (46) to evaluate the null hypothesis that specialists for each prey type (as defined below) were distributed randomly among clusters.
Handling Efficiency.
We measured prey-specific handling efficiency as the number of items handled at the surface per unit time (subsurface prey handling was excluded from this analysis as it was impossible to measure), and for each study animal at CC, we calculated mean handling efficiency for every prey type used. To avoid the confounding effects of prey size (larger items take longer to handle and consume than small items), we limited analysis to the most frequently captured size class of each prey type. We identified specialists for each prey type as those animals for which the proportional contribution to the diet of the prey exceeded the 80th percentile value measured across all individuals. We compared handling efficiency between specialists and nonspecialists for the nine predominant prey types by using two-tailed t tests and adjusting for separate variances. The sample unit for this contrast was a single individual's mean handling efficiency for a particular prey type, and we limited analysis to those individuals having at least three records of prey capture with known handling times for that prey type. We expressed significant differences as the average proportional increase (or decrease) in handling efficiency for specialists as compared with nonspecialists.
Supplementary Material
ACKNOWLEDGMENTS.
We are grateful to the dozens of collaborating researchers and volunteers who contributed to fieldwork and particularly to the Partnership for Interdisciplinary Studies of Coastal Oceans (PISCO) program and M. Kenner for prey sampling at CC and SN. We thank G. Smith and the U.S. Navy for support at SN and D. Canestro and the University of California Natural Reserve System for support at CC. The manuscript benefited from reviews by D. Bolnick, C. Hays, B. Lyon and K. Ralls. Primary financial support for fieldwork activities was provided by a grant from the Minerals Management Service and by funding from the U.S. Geological Survey, the California Department of Fish and Game, the Monterey Bay Aquarium, and the Friends of Long Marine Laboratory.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709263105/DC1.
References
- 1.Roughgarden J. Am Nat. 1972;106:683–718. [Google Scholar]
- 2.Schoener TW. Annu Rev Ecol Syst. 1971;2:369–404. [Google Scholar]
- 3.Krebs JR, Erichsen JT, Webber MI, Charnov EL. Anim Behav. 1977;25:30–38. [Google Scholar]
- 4.Thompson ID, Colgan PW. Oecologia (Berlin) 1990;83:443–451. doi: 10.1007/BF00317193. [DOI] [PubMed] [Google Scholar]
- 5.Svanback R, Bolnick DI. Evol Ecol Res. 2005;7:993–1012. [Google Scholar]
- 6.Svanback R, Bolnick DI. Proc R Soc Biol Sci Ser B. 2007;274:839–844. doi: 10.1098/rspb.2006.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolnick DI, Svanback R, Fordyce JA, Yang LH, Davis JM, Hulsey CD, Forister ML. Am Nat. 2003;161:1–28. doi: 10.1086/343878. [DOI] [PubMed] [Google Scholar]
- 8.Estes JA, Riedman ML, Staedler MM, Tinker MT, Lyon BE. J Anim Ecol. 2003;72:144–155. [Google Scholar]
- 9.Carol AB, Bernardo RB, Steven DG. Mar Biol. 2006;149:689–701. [Google Scholar]
- 10.Riedman ML, Estes JA. US Fish Wildl Serv Biol Rep. 1990;90(I–III):1–126. [Google Scholar]
- 11.Estes JA, Duggins DO. Ecol Monogr. 1995;65:75–100. [Google Scholar]
- 12.Garshelis DL, Garshelis JA, Kimker AT. J Wildl Manage. 1986;50:637–647. [Google Scholar]
- 13.Laidre KL, Estes JA, Tinker MT, Bodkin J, Monson D, Schneider K. J Anim Ecol. 2006;75:978–989. doi: 10.1111/j.1365-2656.2006.01117.x. [DOI] [PubMed] [Google Scholar]
- 14.Monson DH, Estes JA, Bodkin JL, Siniff DB. Oikos. 2000;90:457–468. [Google Scholar]
- 15.Ralls K, Siniff DB. J Wildl Manage. 1990;54:251–259. [Google Scholar]
- 16.Cunningham PN, Hughes RN. Mar Ecol Prog Ser. 1984;16:21–26. [Google Scholar]
- 17.Robinson BW, Wilson DS. Am Nat. 1998;151:223–235. doi: 10.1086/286113. [DOI] [PubMed] [Google Scholar]
- 18.Hori M. Science. 1993;260:216–219. doi: 10.1126/science.260.5105.216. [DOI] [PubMed] [Google Scholar]
- 19.Sutherland WJ. Nature. 1987;325:483–484. [Google Scholar]
- 20.Schluter D, Price TD, Grant PR. Science. 1985;227:1056–1059. doi: 10.1126/science.227.4690.1056. [DOI] [PubMed] [Google Scholar]
- 21.Annett CA, Pierotti R. Ecology. 1999;80:288–297. [Google Scholar]
- 22.Schindler D, Hodgson JR, Kitchell JF. Oecologia. 1997;110:592–600. doi: 10.1007/s004420050200. [DOI] [PubMed] [Google Scholar]
- 23.Werner TK, Sherry TW. Proc Natl Acad Sci USA. 1987;84:5506–5510. doi: 10.1073/pnas.84.15.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West L. Ecology. 1986;67:798–809. [Google Scholar]
- 25.Sargeant BL, Mann J, Berggren P, Krutzen M. Can J Zool. 2005;83:1400–1410. [Google Scholar]
- 26.Glasser JW. Am Nat. 1982;119:250–262. [Google Scholar]
- 27.Svanback R, Persson L. J Anim Ecol. 2004;73:973–982. [Google Scholar]
- 28.Ostfeld RS. Oecologia. 1982;53:170–178. doi: 10.1007/BF00545660. [DOI] [PubMed] [Google Scholar]
- 29.Mackney PA, Hughes RN. Behaviour. 1995;132:1241–1253. [Google Scholar]
- 30.Sinervo B, Calsbeek R. Annu Rev Ecol Evol Syst. 2006;37:581–610. [Google Scholar]
- 31.Kruetzen M, Mann J, Heithaus MR, Connor RC, Bejder L, Sherwin WB. Proc Natl Acad Sci USA. 2005;102:8939–8943. doi: 10.1073/pnas.0500232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Werner EE. Am Nat. 1992;140:S5–S32. [Google Scholar]
- 33.Kondoh M. Science. 2003;299:1388–1391. doi: 10.1126/science.1079154. [DOI] [PubMed] [Google Scholar]
- 34.Tinker MT, Costa DP, Estes James A, Wieringa N. Deep–Sea Res Part II. 2007;54:330–342. [Google Scholar]
- 35.Laidre KL, Jameson RJ, DeMaster DP. Mar Mamm Sci. 2001;17:294–309. [Google Scholar]
- 36.Foster MS, Dean TA, Deysher LE. In: Handbook of Phycological Methods: Ecological Field Methods: Macroalgae. Littler MM, Littler DS, editors. Vol 4. New York: Cambridge Univ Press; 1985. pp. 199–232. [Google Scholar]
- 37.McCleneghan K, Ames JA. J Mammal. 1976;57:410–412. [Google Scholar]
- 38.Siniff DB, Ralls K. Mar Mamm Sci. 1991;7:211–229. [Google Scholar]
- 39.Tinker MT, Doak DF, Estes JA, Hatfield BB, Staedler MM, Bodkin JL. Ecol Appl. 2006;16:2293–2312. doi: 10.1890/1051-0761(2006)016[2293:iddarc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 40.Worton BJ. Ecology. 1989;70:164–168. [Google Scholar]
- 41.Ralls K, Hatfield BB, Siniff DB. Can J Zool. 1995;73:523–531. [Google Scholar]
- 42.Watt J, Siniff DB, Estes JA. Oecologia (Berlin) 2000;124:289–298. doi: 10.1007/s004420000373. [DOI] [PubMed] [Google Scholar]
- 43.Bolnick DI, Yang LH, Fordyce JA, Davis JM, Svanback R. Ecology. 2002;83:2936–2941. [Google Scholar]
- 44.McGarigal K, Cushman S, Stafford S. Multivariate Statistics for Wildlife and Ecology Research. New York: Springer; 2000. [Google Scholar]
- 45.Everitt BS, Dunn G. Applied Multivariate Data Analysis. New York: Oxford Univ Press; 2001. [Google Scholar]
- 46.Mehta CR, Patel NR. ACM Trans Math Soft. 1986;12:154–161. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.