Abstract
Recent histological studies suggest relatively rapid growth in dinosaurs. However, the timing of reproductive maturity (RM) in dinosaurs is poorly known because unambiguous indicators of RM are rare. One exception is medullary bone (MB), which is an ephemeral bony tissue that forms before ovulation in the marrow cavities of birds as a calcium source for eggshelling. Recently, MB also was described in a single specimen of the saurischian dinosaur Tyrannosaurus rex. Here, we report two other occurrences of MB: in another saurischian dinosaur, Allosaurus, and in the ornithischian dinosaur Tenontosaurus. We show by counting lines of arrested growth and performing growth curve reconstructions that Tenontosaurus, Allosaurus, and Tyrannosaurus were reproductively mature by 8, 10, and 18 years, respectively. RM in these dinosaurs coincided with a transition from growth acceleration to deceleration. It also far precedes predictions based on the growth rates of living reptiles scaled to similar size. Despite relatively rapid growth, dinosaurs were similar to reptiles in that RM developed before reaching asymptotic size. However, this reproductive strategy also occurs in medium- to large-sized mammals and correlates with a strategy of prolonged multiyear growth. RM in actively growing individuals suggests that these dinosaurs were born relatively precocial and experienced high adult mortality. The origin of the modern avian reproductive strategy in ornithuran birds likely coincided with their extreme elevations in growth rate and truncations to growth duration.
Keywords: life history, bone histology, medullary bone, bird, reproductive strategy
Efforts to understand the growth strategies of dinosaurs have been controversial, and some studies suggest that nonavian dinosaurs grew like living reptiles scaled to equivalent size (1, 2). If so, dinosaurs would have become reproductively mature relatively late in life (>20 years) (1, 2). However, recent skeletochronological and histological analyses of dinosaur growth show that dinosaurs grew faster and formed more densely vascularized bone tissues than living reptiles (3–8). Rapid growth, which in living vertebrates occurs only in birds and mammals, generally predicts an earlier onset of reproductive maturity (RM) (9, 10), so according to their growth strategies, dinosaurs should show RM at ages far younger than those predicted by models of reptilian growth.
Three advances allow the assessment of RM in dinosaurs. First, skeletochronology provides the means to estimate the age at death of extinct taxa. Bones of extant and extinct taxa record lines of arrested growth (LAGs), which are likely annual markers. LAGs allow age estimation of individuals and reconstruction of growth curves (3, 7, 11, 12).
Second, growth curve reconstruction fits size and estimated age data to sigmoidal models of growth, which allows the calculation of important life-history traits of a taxon. Such traits include maximum growth rate, asymptotic size, and age at growth inflection (13). The growth inflection marks the transition from growth acceleration to deceleration and is of physiological interest because it generally coincides with the onset of RM in extant animals (14, 15).
Third, RM now can be identified independently in dinosaurs by a histological proxy. Recent histological examination of the long bones of a single specimen of the theropod Tyrannosaurus rex [Museum of the Rockies (MOR) 1125] revealed an endosteal tissue called medullary bone (MB) (16), which forms in extant female birds before ovulation as a calcium reservoir for eggshell production (17, 18). MB does not form in all birds (17) and is resorbed rapidly in those that do (18), so it is not surprising that two decades of intensive histological sampling of dinosaurs has revealed only a single specimen with MB.
Our study reports two additional occurrences of endosteally derived bone tissue corresponding to MB in another theropod, Allosaurus fragilis (Utah Museum of Natural History UUVP 5300; Late Jurassic, Morrison Family, Cleveland-Lloyd Quarry, Utah), and in the ornithopod Tenontosaurus tilletti [Sam Noble Oklahoma Museum of Natural History (OMNH) 34784; Early Cretaceous, Cloverly Family, OMNH locality V1044, Montana]. We use MB and skeletochronology to infer when Tyrannosaurus, Allosaurus, and Tenontosaurus likely reached RM. Using those ages at RM, we test whether dinosaurs grew and matured reproductively like living reptiles scaled to similar size. Finally, we assess the relationship between growth and reproductive strategies in dinosaurs and draw comparisons to reptiles, birds, and mammals.
Results
Homology of MB.
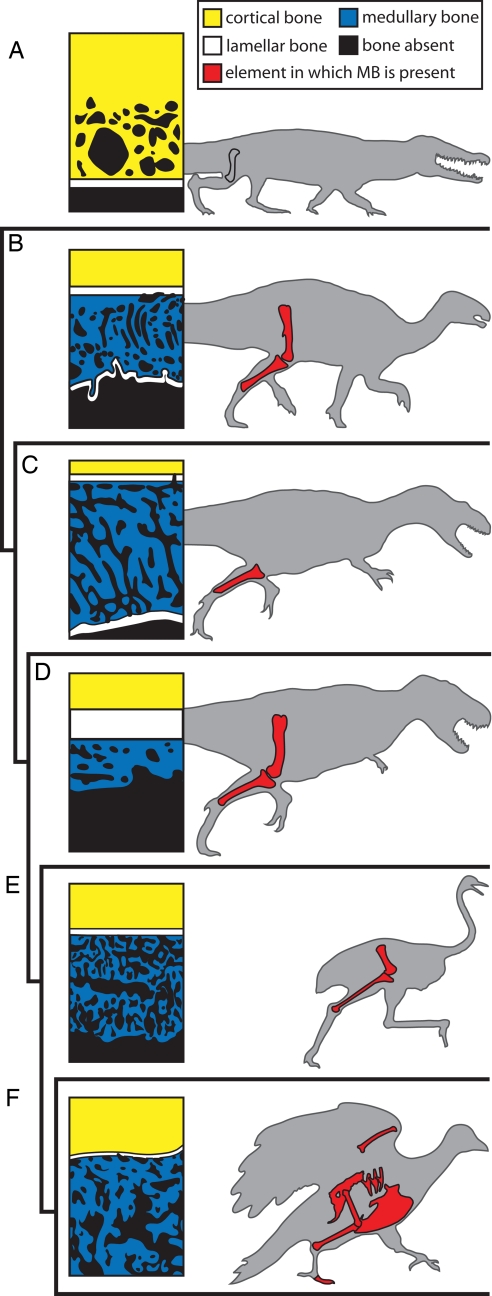
We found endosteally derived bone tissue in the mid-diaphyses of an associated femur and tibia from a single individual of Tenontosaurus (OMNH 34784) and in the middiaphysis of a tibia from disarticulated remains of Allosaurus (UUVP 5300). Associated fossil eggs would strengthen the likelihood that the endosteal tissue is MB. To our knowledge, however, none have been found associated with any specimen of Tenontosaurus. A single fossil egg was located in the same quarry as UUVP 5300, but unique shell morphology and indistinguishable embryonic remains preclude assignment to Allosaurus (19). Irrespective of eggs, we link the endosteal bone tissue to MB (and consequently to RM) by using three classically accepted criteria of homology: histology, position, and development [see details in supporting information (SI) Text and SI Figs. 4–8]. In summary, the structure of bony spicules, broad skeletal distribution (at least in OMNH 34784), and intramembranous development by the endosteum correspond to the MB of Tyrannosaurus and living birds. Thus, the tissue in question in OMNH 34784 and UUVP 5300 is almost certainly homologous to MB. Because alligators apparently do not form MB during ovulation (20), whereas both ornithischian and saurischian dinosaurs (including living birds) appear capable of MB formation, our findings further suggest that MB was common to all dinosaurs and originated in ornithodiran archosaurs by the earliest Late Triassic Period (21) (Fig. 1).
Fig. 1.
Endosteally derived bone tissues in archosaurs. Schematics on the left represent the bone histology of actively shelling females. (A) Alligator resorbs cortical bone as a source of calcium for eggs and does not deposit MB internal to the endosteal lamellae. (B and C) Tenontosaurus (B) and Allosaurus (C) deposit MB internal to endosteal lamellae and later deposit a second layer of lamellae on the internal-most edge of the MB tissues. (D–F) Tyrannosaurus (D), Struthio (ostrich) (E), and Columba (pigeon) (F) do not deposit a second set of lamellae internal to the MB. Highlighted elements on the right (A: gray; B–F: red) indicate those sampled in this or previous studies (16, 18, 20, 44). Some histology schematics have been modified from published images: Alligator (44), Tyrannosaurus (16), Struthio (16), and Columba (45).
RM Estimated by Skeletochronology.
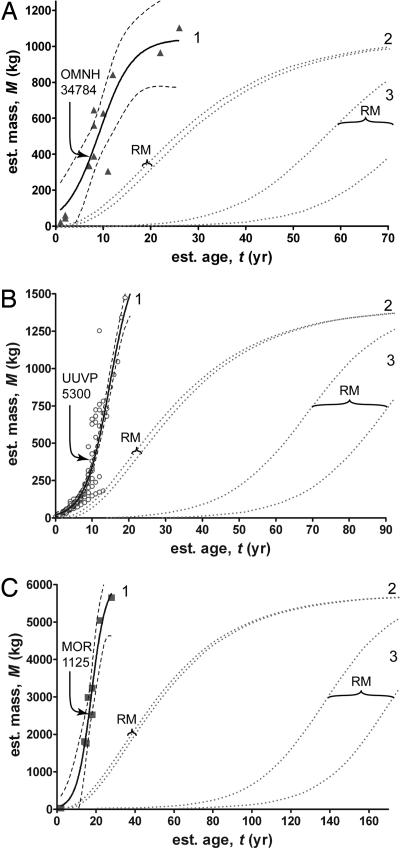
Although previous analyses place the age at death of OMNH 34784, UUVP 5300, and MOR 1125 at 7, 15, and 18 years, respectively (7, 12, 22), age estimates are sensitive to the method of estimation (7). To assess the degree of uncertainty involved with age estimation, we reanalyzed the specimens using several reasonable methods of age estimation (see SI Text) and present the medians and bootstrapped 95% confidence intervals (C.I.) of those estimates. For OMNH 34784, UUVP 5300, and MOR 1125, age at death likely occurred at 8 (7, 8 C.I.) years, 10 (7, 13 C.I.) years, and 18 (14, 21 C.I.) years, respectively. The presence of MB in these specimens suggests that the ages at death represent an upper bound to estimates of RM. None of these specimens show histological evidence of asymptotic growth (e.g., the presence of an external fundamental system or tightly spaced growth lines near the periosteal surface), and their position on the growth curves (see SI Table 2 for data used for growth curve reconstruction) significantly precedes the growth asymptote (Fig. 2, curves labeled 1), which indicates that the individuals with MB were reproductively mature while still growing actively.
Fig. 2.
Age of RM and mass growth curves for Tenontosaurus (A), Allosaurus (B), and Tyrannosaurus (C). Using skeletochronology and nonlinear regression, logistic growth curves (labeled 1) were determined empirically with 95% confidence bands (dashed lines) for each species (12, 22, 23). Arrows point to specimens with MB and represent an upper bound for age and size at RM. Empirical growth curves were compared with alternative models of growth, which use the growth rates of living reptiles scaled to dinosaurian size and the von Bertalanffy (curves labeled 2) and logistic (curves labeled 3) models of mass accumulation. The effect of neonate mass on RM is not large for the von Bertalanffy models of growth in the three dinosaurs, but assuming a large neonate mass does decrease the estimated age of RM for the logistic models. Regardless, RM estimated from scaled reptilian growth rates, von Bertalanffy, and logistic models always produces much older estimates than do skeletochronological methods, which strongly suggests that dinosaurs did not grow like scaled-up living reptiles.
Two additional results suggest that the skeletochronological estimates of RM are biologically reasonable. First, the ages of the reproductively mature specimens coincide with the inflection points calculated from previously reported growth curves (refs. 12, 22, and 23 and Fig. 2, curves labeled 1) for each species. Second, the skeletochronological estimates of RM coincide with predictions of RM based on the scaling of maturation time across a range of taxa spanning in size from viruses to whales (24) (Eq. 2 and SI Table 3). Note that the two smaller estimates of adult mass in Tyrannosaurus predicted slightly earlier, but significantly different, onsets of RM compared with the skeletochronological estimates (SI Table 3), which is not surprising because the presence of MB does not necessarily indicate the earliest onset of RM. Thus, our skeletochronological estimates should be treated as upper bounds on the age at first RM. Regardless, RM determined by skeletochronology and a scaling equation strongly suggests that dinosaurs were capable of reproduction before they reached asymptotic size.
Modeling Reptilian Growth and RM for Dinosaurs.
Because larger animals grow absolutely faster than their smaller relatives, rapid dinosaurian growth rates may be solely a function of size. If so, dinosaurian growth rates could be extrapolated from growth rates of living reptiles (1, 2). We show that if dinosaurs grew like “scaled-up” living reptiles (Eq. 5), RM would have occurred years (if not decades) after the skeletochronological estimates (Table 1 and Fig. 2). There is uncertainty in estimating the (i) neonate body mass, (ii) adult body mass, and (iii) pattern of mass accumulation in extinct taxa, so we used several estimates of body mass and two models of growth (Eqs. 6 and 7) to calculate a range of ages of scaled-up “reptilian” RM. Lower-end predictions of reptilian RM suggest that Tenontosaurus, Allosaurus, and Tyrannosaurus would reach RM by 10, 16, and 31 years, respectively (Table 1 and Fig. 2, curves labeled 2). Although these predicted ages resemble the skeletochronological estimates, they are problematic because they require high neonate masses [based on volumetric reconstructions of sauropod eggs (1)] and predict prolonged growth to asymptotic size. For example, in Tyrannosaurus, the lower-end prediction of RM [and the predicted longevity (Eq. 12)] exceeds the known skeletochronological ages of adult individuals (7, 23). Upper-end predictions of scaled reptilian RM suggest that Tenontosaurus, Allosaurus, and Tyrannosaurus were mature reproductively by 82, 87, and 218 years, respectively (Table 1 and Fig. 2, curves labeled 3). These upper-end predictions, including other predictions based on the logistic model, are decades to centuries older than the skeletochronologically reconstructed age of any analyzed individual from these species (refs. 7, 12, 22, and 23 and Fig. 2, curves labeled 1).
Table 1.
Predictions of reproductive maturity based on scaled growth rates of living reptiles
| Model | Taxon | Estimated mass, kg | Ref. | Maximum growth rate, kg/yr | K, yr−1 | Lower end of RM, yr | Upper end of RM, yr | Minimum longevity, yr |
|---|---|---|---|---|---|---|---|---|
| von Bertalanffy | Tenontosaurus | 243 | 46 | 10 | 0.097 | 10 | 12 | 42 |
| 600 | 47 | 19 | 0.070 | 15 | 17 | 57 | ||
| 1,034 | 22 | 27 | 0.058 | 18 | 20 | 70 | ||
| Allosaurus | 700 | 48 | 21 | 0.067 | 16 | 17 | 61 | |
| 952 | 46 | 25 | 0.060 | 17 | 19 | 68 | ||
| 1,400 | 25 | 32 | 0.052 | 20 | 22 | 78 | ||
| Tyrannosaurus | 4,500 | 25 | 69 | 0.035 | 31 | 33 | 118 | |
| 5,654 | 23 | 80 | 0.032 | 34 | 36 | 128 | ||
| 6,650 | 46 | 89 | 0.030 | 36 | 38 | 135 | ||
| 10,200 | 48 | 118 | 0.026 | 42 | 43 | 157 | ||
| Logistic | Tenontosaurus | 243 | 46 | 10 | 0.172 | 26 | 37 | 43 |
| 600 | 47 | 19 | 0.125 | 43 | 58 | 67 | ||
| 1,034 | 22 | 27 | 0.103 | 57 | 75 | 86 | ||
| Allosaurus | 700 | 48 | 21 | 0.118 | 46 | 62 | 72 | |
| 952 | 46 | 25 | 0.106 | 55 | 72 | 83 | ||
| 1,400 | 25 | 32 | 0.093 | 67 | 87 | 99 | ||
| Tyrannosaurus | 4,500 | 25 | 69 | 0.062 | 120 | 150 | 168 | |
| 5,654 | 23 | 80 | 0.057 | 134 | 167 | 187 | ||
| 6,650 | 46 | 89 | 0.054 | 145 | 180 | 201 | ||
| 10,200 | 48 | 118 | 0.046 | 177 | 218 | 242 |
Lower-end predictions of the age at RM result when a high neonate mass is assumed, and upper-end predictions of RM result when a low neonate mass is assumed. K is the instantaneous relative growth rate at RM.
Discussion and Conclusions
Our results have several important implications. First, the large body size of many dinosaurs precludes the slow growth rates and long generation times suggested by the reptilian models of growth. To grow like living reptiles scaled to equivalent size and to reach skeletochronological estimates of RM requires that individuals of these taxa (i) started growth as relatively massive neonates, (ii) reached RM at unreasonably low body masses (≪one-tenth asymptotic size), and (iii) grew potentially for 50–100 years. No evidence exists to support any of these requirements, and conservative mass estimates (25) and histological data (refs. 7, 12, 22, and 23 and Fig. 2, curves labeled 1) refute them.
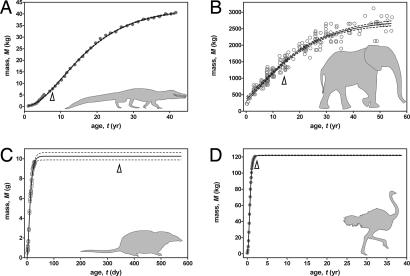
Second, despite relatively rapid growth rates (Fig. 2, curves labeled 1), the reproductive strategy of dinosaurs is surprisingly similar to that of living reptiles (Fig. 3A). In both, the onset of RM occurs while growth is still active. Asymptotic size in dinosaurs and reptiles requires growth over several to tens of years [this is not “indeterminate” growth (26) because growth trajectories do not remain infinitely plastic after reaching asymptotic size (27)], so early reproduction at one-third to one-half asymptotic size increases lifetime reproductive success. Interestingly, medium- to large-sized mammals that require similar lengths of time to reach asymptotic size also reach reproductive age at a fraction of asymptotic size (14, 28) (Fig. 3B). This strategy of RM during active growth in dinosaurs, reptiles, and medium- to large-sized mammals is strikingly different from the strategy of RM well after growth cessation in small mammals and in all living birds (Fig. 3 C and D). In these taxa, late reproduction occurs because asymptotic size is attained rapidly within the first year of life, and mechanical (e.g., flight at fledging), ecological (e.g., overwintering), or behavioral (e.g., social learning) factors preclude reproduction in that same first year. Together, these data suggest that a strategy of prolonged growth is correlated with a strategy of reproduction before reaching asymptotic size.
Fig. 3.
Growth curves and RM for Alligator (A), Loxodonta (elephant) (B), Sorex (shrew) (C), and Struthio (D). Relatively early maturity correlates with a growth strategy involving prolonged growth. Arrows indicate age at female RM. Growth and reproductive data are from published studies (34, 49–52).
Third, the reproductive strategy of dinosaurs offers insights into their survivorship and degree of neonatal development. A strong correlation in amniotes between relatively early reproduction and high adult mortality (28–30) suggests that dinosaurs also experienced high adult mortality. A recent demographic study of the theropod Albertosaurus, which showed high mortality in neonates and adults (31), supports our inference. Furthermore, the combination of early reproduction and high adult mortality suggests that the offspring of these dinosaurs were born relatively precocial.
Fourth, early reproduction would have allowed the largest dinosaurs to have ecologically reasonable generation times. For example, in the most massive sauropods [100 tons (32)], asymptotic growth likely required several decades to achieve (refs. 4 and 33 and references therein), but reproduction in actively growing individuals could have been possible in 19 [16, 27 prediction intervals (PI)] years.
Finally, the delayed reproductive strategy in all extant birds likely evolved through decoupled changes in growth rate and duration. Although early Mesozoic birds show decreases in bone vascularity and numbers of LAGs (suggesting that initial miniaturization was achieved by decreasing growth rates and durations), asymptotic size still required several years to achieve (6, 8, 26). These early birds probably still retained the primitive reproductive strategy of early RM. Later, ornithuran and neoavian birds show further increases in bone vascularity and an extreme reduction and elimination of LAGs, which suggests that miniaturization was achieved by truncating the duration of rapid growth (6, 8, 26). Most likely, this extreme reduction in growth duration is correlated with the origin of the modern avian reproductive strategy and occurred within Ornithurae or at the base of Neoaves at the latest.
Methods
Reproductive Scaling Across Organisms.
A previous study (24) reported a strong positive correlation between the maturation time (i.e., age of RM) and adult mass across 61 taxa of a broad range in size (10−14 to 107 g). The published equation, however, contains detransformation bias and provides no means to estimate the uncertainty of prediction. To address both problems, we digitized the data from that study and added data from the blue whale (34). These data were loge-transformed, and a linear regression analysis was performed. The regression equation in loge scale is:
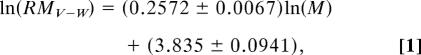 |
where RMV-W is the predicted age at RM in days based on data from viruses to whales, M is adult mass in g, and error terms are standard errors. To convert Eq. 1 back into the original scale, the intercept was detransformed and multiplied with the bias correction factor. In the original scale, Eq. 1 becomes:
Predictions using Eq. 2 are not without uncertainty. To approximate that uncertainty, nominal 95% PI about each prediction were calculated by using Cox's method (35, 36). Cox's method was preferred over others [e.g., nonparametric bootstrap or Angus' conservative method (35)] because it is computationally simple, produces PIs with the smallest coverage error given moderate sample sizes, and has an acceptably small relative bias when the residual mean squares is <1. Lower and upper PIs were calculated by raising e to the following:
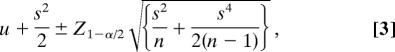 |
where for the current study u is loge(RMV-M), s2 is the residual mean squares [= 0.7361], Z1-α/2 is the appropriate value from the standard normal distribution [= 1.96], and n is the sample size [= 62].
Calculating Maximum Growth Rates.
No living reptile reaches dinosaur-sized proportions. To determine how rapidly a living reptile of typical size (102 to 105 g) scaled to dinosaur-sized proportions (105 to 108 g) might grow, we gathered maximum mass growth rates and corresponding adult masses from a published study (37). The data in that study comprise 1 sphenodontid, 1 alligatorid, 10 chelonians, and 33 squamates. Because the published data lack representation of large-bodied nonserpentian squamates, we added the maximum growth rate of Komodo dragons (Varanus komodoensis) raised in zoos in the United States (38). It should be noted that Komodo dragons growing in temperate climates reach smaller adult sizes and grow for a longer period than those growing in tropical climates (38). However, the growth in tropical dragons was not recorded over sufficiently long duration to calculate the maximum growth rate and adult size. Instead, it is possible to calculate those life-history parameters for temperate dragons, so we cautiously use a maximum growth rate of 16 g per day and an adult size of 40 kg (38).
Originally, a log10 transformation was used to improve the variance (i.e., homoscedascity) of the data (1). Once the linear regression analysis was performed, the resulting regression equation was detransformed back into the original scale. Although data values can be transformed and detransformed without bias, the same is not true for regression equations (39), so the direct application of the original regression equation would produce biased growth rate estimates. To address this issue, we ln-transformed the original data (including the data from V. komodoensis) and performed a linear regression by using Prism (Graphpad). The regression equation in ln scale is:
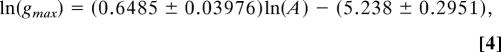 |
where gmax is the maximum growth rate in g per day, A is the adult mass in g, and error terms are standard errors. Upon detransformation, the intercept was multiplied by a correction factor of exp(s2/2), where s2 is the residual mean squares (39). In the original scale, the bias-corrected regression equation is:
Modeling Reptilian Growth and RM in Dinosaurs.
The estimation of the age at RM for dinosaurs growing like scaled-up living reptiles is complicated by uncertainty in (i) the model of growth, (ii) neonate mass, and (iii) asymptotic mass. In light of these complications, we used different models and masses to assess their effects on our estimates of RM.
(i) We applied two traditional models of mass accumulation in reptiles (2, 40, 41). The first is described by the von Bertalanffy equation:
and the second is described by the logistic equation:
where for both, A is the asymptotic mass, a is the neonate mass, t is the age in years, and K is the instantaneous relative growth rate (14). The calculation of the instantaneous relative growth rate, K, from the maximum growth rate depended on whether von Bertalanffy or logistic growth was modeled. For the former:
For the latter:
Each equation describes a curve with a single inflection point that corresponds to female RM in many mammals and reptiles (14, 15). Of the two, the von Bertalanffy equation (Eq. 6) models an earlier growth inflection at one-third adult mass when the age at inflection, ti, is:
where K is the instantaneous relative growth rate and a is the neonate mass.
The logistic equation (Eq. 7) models a later inflection at one-half adult mass when the age is:
where K is the instantaneous relative growth rate, A is the adult mass, and a is the neonate mass.
(ii) Because no neonate skeletal material is known for Tenontosaurus, Allosaurus, or Tyrannosaurus, we used two estimates of neonate mass based on volumetric reconstructions of small and large nonavian dinosaur eggs (1). The larger estimate of neonate mass always generated a younger estimate of RM, whereas the smaller one always generated an older estimate of RM.
(iii) To address the problem of estimating adult mass in the three dinosaurs examined in this study, we gathered three to four estimates of asymptotic mass from the literature (see Table 1). The effect of asymptotic mass on RM is the same as with neonate mass. Ages of inflection and RM modeled on scaled reptilian growth rates were compared with those ages estimated by skeletochronology.
Calculating Longevity.
Here, longevity refers to the length of time required to reach 95% of asymptotic mass. We calculated this longevity, L, by:
where RM is the age corresponding to the inflection point in the growth curve, or the age at RM (42). As with modeled ages at RM, we compared the modeled longevities with those estimated by skeletochronology.
Supplementary Material
ACKNOWLEDGMENTS.
We thank R. Burkhalter, P. J. Bybee, K. Davies, E.-T. Lamm, R. Lupia, and J. Person for technical assistance; A. Chinsamy for input on avian pathologies; P. Holroyd, P. O'Connor, K. Padian, N. Stevens, D. Wake, J. Brown, and two anonymous reviewers for comments; Utah Museum of Natural History and R. Cifelli at OMNH for samples; R. Heaney and Creighton Medical Center (Omaha, NE) for access to the Foote Histological Collection; and the OMNH, University of California Museum of Paleontology, and University of Oklahoma Department of Geology for equipment. This work was supported by grants from the Department of Integrative Biology and Jurassic Foundation (to A.H.L.) and grants from the Geological Society of America, Paleontological Society, and University of Oklahoma Graduate Student Senate (to S.W.). This is University of California Museum of Paleontology contribution no. 1939.
Note.
We are delighted that the independent results of Erickson et al. (43) complement our results. Note that our results are based on the skeletochronological age when MB formed, whereas theirs are based on the age when brooding occurred. Whereas our results show reproduction began relatively early, theirs show that reproduction was still possible in individuals approaching asymptotic size. We conclude that our independent studies generally characterize the reproductive strategies of dinosaurs.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0708903105/DC1.
References
- 1.Case TJ. Paleobiology. 1978;4:320–328. [Google Scholar]
- 2.Lehman TM. In: Horns and Beaks. Carpenter K, editor. Indianapolis: Indiana Univ Press; 2006. pp. 259–318. [Google Scholar]
- 3.Chinsamy A. Mod Geol. 1993;18:319–329. [Google Scholar]
- 4.Sander PM. Paleobiology. 2000;26:466–488. [Google Scholar]
- 5.Erickson GM, Curry-Rogers K, Yerby SA. Nature. 2001;412:429–433. doi: 10.1038/35086558. [DOI] [PubMed] [Google Scholar]
- 6.Padian K, de Ricqlès AJ, Horner JR. Nature. 2001;412:405–408. doi: 10.1038/35086500. [DOI] [PubMed] [Google Scholar]
- 7.Horner JR, Padian K. Proc R Soc London Ser B. 2004;271:1875–1880. doi: 10.1098/rspb.2004.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Padian K, Horner JR, de Ricqlès AJ. J Vertebr Paleontol. 2004;24:555–571. [Google Scholar]
- 9.Stearns SC, Koella JC. Evolution (Lawrence, Kans) 1986;40:893–913. doi: 10.1111/j.1558-5646.1986.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 10.Congdon JD, van Loben Sels RC. J Evol Biol. 1993;6:547–557. [Google Scholar]
- 11.Castanet J, Francillon-Vieillot H, Meunier FJ, de Ricqlès A. In: Bone: Bone Growth. Hall BK, editor. Boca Raton, FL: CRC; 1993. pp. 245–283. [Google Scholar]
- 12.Bybee PJ, Lee AH, Lamm E-T. J Morphol. 2006;267:347–359. doi: 10.1002/jmor.10406. [DOI] [PubMed] [Google Scholar]
- 13.Zullinger EM, Ricklefs RE, Redford KH, Mace GM. J Mammal. 1984;65:607–636. [Google Scholar]
- 14.Brody S. Bioenergetics and Growth: With Special Reference to the Efficiency Complex in Domestic Animals. 2nd Ed. New York: Hafner; 1964. [Google Scholar]
- 15.Reiss MJ. The Allometry of Growth and Reproduction. New York: Cambridge Univ Press; 1989. [Google Scholar]
- 16.Schweitzer MH, Wittmeyer JL, Horner JR. Science. 2005;308:1456–1460. doi: 10.1126/science.1112158. [DOI] [PubMed] [Google Scholar]
- 17.Pahl R, Winkler DW, Graveland J, Batterman BW. Proc R Soc London Ser B. 1997;264:239–244. [Google Scholar]
- 18.Dacke CG, Arkle S, Cook DJ, Wormstone IM, Jones S, Zaidi M, Bascal ZA. J Exp Biol. 1993;184:63–88. [Google Scholar]
- 19.Hirsch KF, Stadtman KL, Miller WE, Madsen JH. Science. 1989;243:1711–1713. doi: 10.1126/science.243.4899.1711. [DOI] [PubMed] [Google Scholar]
- 20.Schweitzer MH, Elsey RM, Dacke CG, Horner JR, Lamm E-T. Bone. 2007;40:1152–1158. doi: 10.1016/j.bone.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 21.Rogers RR, Swisher CC, III, Sereno PC, Monetta AM, Forster CA, Martínez RN. Science. 1993;260:794–797. doi: 10.1126/science.260.5109.794. [DOI] [PubMed] [Google Scholar]
- 22.Werning S. Norman: University of Oklahoma; 2005. Thesis. [Google Scholar]
- 23.Erickson GM, Makovicky PJ, Currie PJ, Norell MA, Yerby SA, Brochu CA. Nature. 2004;430:772–775. doi: 10.1038/nature02699. [DOI] [PubMed] [Google Scholar]
- 24.Blueweiss L, Fox H, Kudzma V, Nakashima D, Peters R, Sams S. Oecologia. 1978;37:257–272. doi: 10.1007/BF00344996. [DOI] [PubMed] [Google Scholar]
- 25.Anderson AF, Hall-Martin A, Russell DA. J Zool. 1985;207:53–61. [Google Scholar]
- 26.Chinsamy-Turan A. The Microstructure of Dinosaur Bone: Deciphering Biology with Fine-Scale Techniques. Baltimore: Johns Hopkins Univ Press; 2005. [Google Scholar]
- 27.Sebens KP. Annu Rev Ecol Syst. 1987;18:371–407. [Google Scholar]
- 28.Promislow DEL, Harvey PH. J Zool. 1990;220:417–437. [Google Scholar]
- 29.Tinkle DW, Wilbur HM, Tilley SG. Evolution (Lawrence, Kans) 1970;24:55–74. doi: 10.1111/j.1558-5646.1970.tb01740.x. [DOI] [PubMed] [Google Scholar]
- 30.Promislow DEL, Harvey PH. Acta Ecologica. 1991;12:119–137. [Google Scholar]
- 31.Erickson GM, Currie PJ, Inouye BD, Winn AA. Science. 2006;313:213–217. doi: 10.1126/science.1125721. [DOI] [PubMed] [Google Scholar]
- 32.Mazzetta GV, Christiansen P, Fariña RA. Hist Biol. 2004;16:71–83. [Google Scholar]
- 33.Sander PM, Klein N, Buffetaut E, Cuny G, Suteethorn V, Le Loeuff J. Organisms Diversity Evol. 2004;4:165–173. [Google Scholar]
- 34.Nowak RM. Walker's Mammals of the World. 6th Ed. Baltimore: Johns Hopkins Univ Press; 1999. [Google Scholar]
- 35.Zhou X-H, Gao S. Stat Med. 1997;16:783–790. doi: 10.1002/(sici)1097-0258(19970415)16:7<783::aid-sim488>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Hayes JP, Shonkwiler JS. Physiol Biochem Zool. 2006;79:665–674. doi: 10.1086/502814. [DOI] [PubMed] [Google Scholar]
- 37.Case TJ. Q Rev Biol. 1978;53:243–282. doi: 10.1086/410622. [DOI] [PubMed] [Google Scholar]
- 38.Walsh T, Chiszar D, Birchard GF, Tirtodiningrat KA. In: Komodo Dragons: Biology and Conservation. Murphy JB, Ciofi C, de La Panouse C, Walsh T, editors. Washington, DC: Smithsonian Institution; 2002. pp. 178–195. [Google Scholar]
- 39.Smith RJ. Am J Phys Anthropol. 1993;90:215–228. [Google Scholar]
- 40.Shine R, Charnov EL. Am Nat. 1992;139:1257–1269. [Google Scholar]
- 41.Adolph SC, Porter WP. Oikos. 1996;77:267–278. [Google Scholar]
- 42.Brown D, Rothery P. Models in Biology: Mathematics, Statistics, and Computing. New York: Wiley; 1993. [Google Scholar]
- 43.Erickson GM, Curry Rogers K, Varricchio DJ, Norell MA, Xu X. Biol Lett. 2007;3:558–561. doi: 10.1098/rsbl.2007.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wink CS, Elsey RM. J Morphol. 1986;189:183–188. doi: 10.1002/jmor.1051890208. [DOI] [PubMed] [Google Scholar]
- 45.Bloom W, Bloom MA, McLean FC. Anat Rec. 1941;81:443–475. [Google Scholar]
- 46.Seebacher F. J Vertebr Paleontol. 2001;21:51–60. [Google Scholar]
- 47.Spotila JR, O'Connor MP, Dodson P, Paladino FV. Mod Geol. 1991;16:203–227. [Google Scholar]
- 48.Therrien F, Henderson DM. J Vertebr Paleontol. 2007;27:108–115. [Google Scholar]
- 49.Rootes WL, Chabreck RH, Wright VL, Brown BW, Hess TJ. Estuaries. 1991;14:489–494. [Google Scholar]
- 50.Hanks J. E Afr Wildl J. 1969;7:7–10. [Google Scholar]
- 51.Nesterenko V, Ohdachi SD. Mammal Study. 2001;26:145–148. [Google Scholar]
- 52.Cilliers SC, de Preez JJ, Maritz JS, Hayes JP. Anim Sci. 1995;61:161–164. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.