Abstract
Previous interethnic comparative studies on the susceptibility to malaria performed in West Africa showed that Fulani are more resistant to Plasmodium falciparum malaria than are sympatric ethnic groups. This lower susceptibility is not associated to classic malaria-resistance genes, and the analysis of the immune response to P. falciparum sporozoite and blood stage antigens, as well as non-malaria antigens, revealed higher immune reactivity in Fulani. In the present study we compared the expression profile of a panel of genes involved in immune response in peripheral blood mononuclear cells (PBMC) from Fulani and sympatric Mossi from Burkina Faso. An increased expression of T helper 1 (TH1)-related genes (IL-18, IFNγ, and TBX21) and TH2-related genes (IL-4 and GATA3) and a reduced expression of genes distinctive of T regulatory activity (CTLA4 and FOXP3) were observed in Fulani. Microarray analysis on RNA from CD4+CD25+ (T regulatory) cells, performed with a panel of cDNA probes specific for 96 genes involved in immune modulation, indicated obvious differences between the two ethnic groups with 23% of genes, including TGFβ, TGFβRs, CTLA4, and FOXP3, less expressed in Fulani compared with Mossi and European donors not exposed to malaria. As further indications of a low T regulatory cell activity, Fulani showed lower serum levels of TGFβ and higher concentrations of the proinflammatory chemokines CXCL10 and CCL22 compared with Mossi; moreover, the proliferative response of Fulani to malaria antigens was not affected by the depletion of CD25+ regulatory cells whereas that of Mossi was significantly increased. The results suggest that the higher resistance to malaria of the Fulani could derive from a functional deficit of T regulatory cells.
One of the possible approaches in the study of human variation in the susceptibility to malaria consists in comparing malariologic indicators between populations differing in their genetic background and living in the same epidemiological context, i.e., exposed to the same transmission level and to the same parasite strains. Actually, the possible observation of interethnic differences of susceptibility in such conditions may provide new opportunities to detect factors associated to protection. This interethnic comparative approach was applied in extensive studies performed in hyperendemic rural areas of Burkina Faso and Mali, West Africa. These studies showed obvious interethnic heterogeneities in the susceptibility to Plasmodium falciparum malaria among sympatric ethnic groups, Fulani, Mossi, Rimaibé, and Dogon (1, 2), with different genetic background (3). Despite similar exposure to malaria and comparable use of protective measures, the Fulani were less parasitized and less affected by the disease. This resistance was not associated to classic malaria resistance genes (4), and the analysis of the antibody response to P. falciparum antigens revealed higher immune reactivity in Fulani than in sympatric ethnic groups (1, 5, 6). The hypothesis of a stronger activation of the immune system in the Fulani is also suggested by the higher frequency in this group of the tropical splenomegaly syndrome (2, 7) and by the higher humoral immune response to other pathogens (Schistosoma haematobium, hepatitis B, and cytomegalovirus). (D.M., unpublished data). This higher reactivity could involve any cellular or soluble mediator of the T helper 1 (TH1) or TH2 response, as well as diverse mechanisms of immune tolerance. The generation of T regulatory cells (Treg), in particular, has been demonstrated to modulate the immune response in malaria infection and in other infectious diseases (8–10).
In this context, the analysis of the expression, in selected cellular populations of the immune system, of a large panel of genes involved in the immune response by microarray and real-time PCR techniques might be helpful in the identification of genes for genetic susceptibility studies.
In the present study we analyzed, in PBMC from Fulani and sympatric Mossi, the expression profile of a large panel of genes involved in the immune response and obtained evidence suggesting a functional deficit of the mechanisms of immune regulation in Fulani: They showed an increased expression of some genes related to TH1 or TH2 function, together with a reduced expression of CTLA4 and FOXP3, two genes involved in the immune modulation operated by T cells (11). Microarray analysis on RNA purified from peripheral blood CD4+CD25+ Treg showed great differences between the two ethnic groups, with important genes such as TGFβ, TGFβRs, CTLA4, and FOXP3 being less expressed in Fulani compared with Mossi, as well as compared with European donors not living in malaria endemic areas. The reduced expression of genes related to suppressive activity seriously affected the ability of Treg cells to suppress P. falciparum-induced cell proliferation in Fulani because depletion of these cells did not significantly increase the proliferation of PBMC to P. falciparum antigens in this group, whereas it restored an optimal response to the same antigens in the sympatric Mossi.
Overall, these results suggest that a functional deficit of Treg in Fulani could be involved in the lower susceptibility to malaria of this ethnic group.
Results
Study Population.
The 26 subjects included in the study [13 Mossi (seven females and six males), mean age ± SD = 38.24 ± 7.6 years; 13 Fulani (eight females and five males), mean age ± SD = 41.3 ± 9.7 years] were selected on the basis of their stable residence in the rural area of Barkoundouba, northeast of Ouagadougou, Burkina Faso. Previous epidemiological surveys in this area (1) demonstrated that Mossi and Fulani are homogeneously exposed to P. falciparum malaria (entomological inoculation rates > 100 per person per year), with the Mossi showing higher P. falciparum parasite rates (50–90% according to age group) than Fulani (0–70%) and higher incidence of clinical malaria. Blood collections were performed at the end of the rainy season (October 2005), when entomological inoculation rates reach one to two infective bites per person per night. At the clinical examination the subjects did not show any clinical symptom referred to malaria or other infectious diseases. P. falciparum infection was observed in one of the Mossi subjects (180 parasites per microliter) whereas all of the 13 Fulani were negative.
The previously reported interethnic differences in the immune reactivity to P. falciparum were confirmed also in these groups of Mossi and Fulani since significant differences were found in IgG against the P. falciparum circumsporozoite protein (CSP) and the merozoite surface protein (MSP119) [supporting information (SI) Fig. 6].
Functional and Phenotypical Studies in Mossi and Fulani.
As first assessment of selected populations we performed a phenotypical analysis with a panel of mAbs in PBMC. The results did not show any significant differences in the percentage of CD3+, CD4+, CD8+, CD19+, and CD14+ cells between the two groups (data not shown).
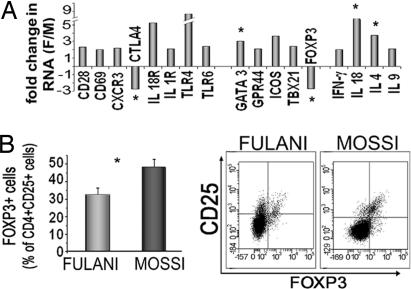
Thus, we compared the expression of genes involved in immune response in PBMC by using the quantitative TH1, TH2, TH3 PCR Array (SuperArray) and primers specific for FOXP3 as marker of Treg (11). Fig. 1A shows the results relative to PBMC from five Fulani and five Mossi randomly selected from the two groups of 13 recruited in the study. Different genes, peculiar of TH1 (IFNγ, TBX21, IL-18, and CXCR3) and TH2 (IL-4, IL-9, and GATA3) (12) response, were more expressed in Fulani than Mossi, with a fold increase between 2 and 9. Higher amounts of CD69 T cell activation marker and CD28 were also found in Fulani. On the contrary, FOXP3 and CTLA4, two genes distinctive of Treg activity, were less expressed in Fulani.
Fig. 1.
Gene expression analysis and surface phenotype of PBMC from Fulani and Mossi. (A) Quantitative PCR mRNA expression profiling of PBMC. Total RNA was extracted from PBMC of five Fulani (three females and two males) and five Mossi (two females and three males). TH1-TH2-TH3 PCR Array (SuperArray) was used to simultaneously examine the mRNA levels of 89 genes. Separate experiments were performed to analyze Foxp3 gene expression as compared with control genes. In both cases data were normalized to the mean amounts of PPI, actin, and GAPDH as HK genes. Data are expressed as fold change in RNA from Fulani compared with Mossi calculated by using the 2−ΔΔCT method. The figure shows only the genes differentially regulated between the two groups (fold increase or decrease ≥ 2). The statistical analysis, performed by using Student's t test on ΔCT values from both groups, revealed significant differences (*) for FOXP3 (P = 0.021), CTLA4 (P = 0.041), IL-18 (P = 0.042), GATA3 (P = 0.041), and IL-4 (P = 0.001). (B) Percentage of CD25+ cells expressing FOXP3. CD25+ cells coexpressing FOXP3 were determined by cytofluorimetric analysis in all of the 13 Fulani and 13 Mossi included in the study. Quadrant lines demarcate the isotype control. Analysis was performed on CD4+ gated cells. P = 0.023, Fulani vs. Mossi.
These results suggested that the higher TH1 and TH2 responses in Fulani can be related to alterations in the mechanisms of immune suppression mediated by Treg.
The proportions of circulating Treg in both ethnic groups were then assessed by quantitating, through cytofluorimetric analysis, CD4+CD25+ T cells expressing Foxp3 in PBMC from all Fulani and Mossi included in the study. Fig. 1B shows that the percentage of Treg is higher in Mossi than in Fulani.
Pathway-Focused Microarray Analysis on Treg from Mossi, Fulani, and European Donors.
To perform a general assessment of markers involved in Treg activity in the two ethnic groups, we analyzed the expression of 367 genes involved in T cell activation and differentiation using a pathway-specific microarray (Autoimmune and Inflammatory Disease; SuperArray) in the CD4+CD25+ cell populations, known to include the most of circulating Treg. Taking into account that the two groups live in a geographical area endemic for pathogens known to induce Treg activity such as schistosomes, intestinal nematodes, and P. falciparum (13), we performed the same analysis on CD4+CD25+ cells from donors living in Europe as control.
Pooled RNA isolated from five Fulani and five Mossi randomly selected from the two groups of 13 recruited in the study and from five European donors was used for the array.
Based on the phenotypical and functional features (11, 14, 15) as well as on microarray analysis of Treg reported in literature (15), a list of 96 genes, related, specifically or not, to Treg activity was selected (SI Table 1). For convenience, these genes were divided in functional clusters: (i) cytokines and cytokine receptors, (ii) chemokines (receptors and ligands), (iii) membrane or intracellular markers, (iv) transcription factors, and (v) Toll-like receptors (TLRs).
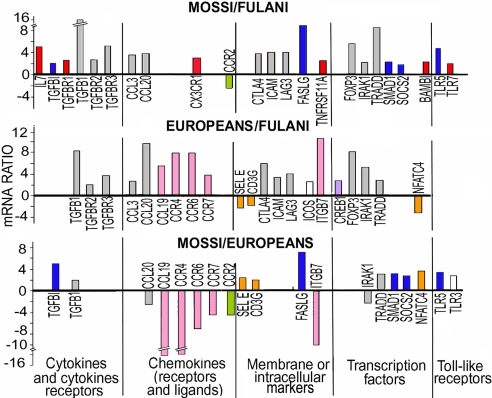
The results obtained for each group, normalized to the mean value of actin, GAPDH, and PPI as housekeeping (HK) genes, were compared by scatter plot analysis with specific software (GeneArray). Fig. 2 lists the genes where at least one difference in the level of expression among the three groups was observed. The general picture clearly shows that higher levels of expression were observed in the Mossi and Europeans compared with Fulani. Fifty percent of the recorded differences (17/34) were represented by genes more expressed in Mossi and Europeans compared with Fulani whereas only the CCR2 gene was overexpressed in Fulani compared with Mossi. Both African ethnic groups showed higher expression of SELE, NFATC4, and CD3G compared with Europeans.
Fig. 2.
Expression of genes involved in Treg function in Mossi, Fulani, and European donors. One hundred nanograms of total RNA from CD4+CD25+ cells, extracted independently from five of the 13 Mossi and five of the 13 Fulani included in the study and from five European donors were pooled and used to hybridize human gene probe arrays (Autoimmune and Inflammatory Disease; SuperArray). Image processing and intensity data extraction were performed through specific software (GeneArray). The results obtained for each group, normalized to a mean value of PPIA, actin, and GAPDH as HK genes, were compared by Scatter Plot Analysis (GeneArray Expression Analysis Suite) with 1.9 as boundary value for fold change. The results of one experiment of two performed are shown. Gray bars, genes more expressed in Mossi and Europeans compared with Fulani (11/34); red bars, genes more expressed in Mossi compared with Fulani (6/34); blue bars, genes more expressed in Mossi compared with Fulani and Europeans (5/34); pink bars, genes more expressed in Europeans compared with Mossi and Fulani (5/34); green bars, genes more expressed in Fulani and Europeans compared with Mossi (1/34); orange bars, genes more expressed in Mossi and Fulani compared with Europeans (3/34).
Cytokines and cytokine receptors.
Among the cytokines, the gene for TGFβ1, an important mediator of Treg activity (16), is more expressed in Treg from Mossi (16-fold) and European donors (8-fold) as compared with Fulani. In contrast, IL-10 gene expression showed no differences in the three populations (data not shown). Also, TGFβR2 and TGFβR3 genes were more expressed in both Mossi and European donors compared with Fulani whereas TGFβR1 was differentially modulated only compared with Mossi. With the exception of TGFβ1, these genes were not changed when Mossi were compared with European donors.
Among genes for cytokines related to Treg differentiation, survival, or proliferation, such as IL-4, IFN-γ, and IL-15, only IL-7 gene expression was 5-fold more expressed in Treg from Mossi as compared with Fulani.
Chemokines (receptors and ligands) and membrane or intracellular markers.
Fig. 2 also shows that Treg from Mossi and European donors have an increased expression of three markers reported to be highly expressed by Treg, CTLA4, LAG-3 (16), and ICAM1 (17), as compared with Fulani. In contrast, no differences for these genes were observed between Mossi and European donors.
Interestingly, FAS ligand (FASLG), which has been involved in the suppression of activated monocytes (18), was also more expressed in Treg from Mossi group as compared with Fulani (Fig. 2); however, this gene was more expressed in Mossi even when compared with European donors.
Transcription factors.
Fig. 2, consistent with the phenotypic analysis (Fig. 1), shows that Treg from Mossi and European donors have higher expression of FOXP3, the transcription factor responsible for Treg differentiation, as compared with Fulani. SOCS2, a member of the suppressor of cytokine signaling family, whose expression is confined to Treg subset and is dependent on FOXP3 expression (15), was also more expressed in Treg from Mossi as compared with Fulani. Only SOCS2 but not FOXP3 was more expressed in Mossi as compared with Europeans.
TLRs.
Among the TLR genes, Treg from Mossi, compared with Fulani, have a higher expression of TLR5 and TLR7, which have been reported as expressed at significantly higher levels in Treg than in conventional T cells (19). The expression of TLR5 was increased in Mossi also compared with European donors.
On the whole these results indicated that Fulani differ in the expression of genes determinant for Treg activity such as TGFβ, TGFβRs, CTLA4, and FOXP3 compared with both Mossi and European donors. In contrast, gene expression of Mossi Treg was superimposable to that of European donor Treg except for such genes as TRADD, SOCS2, FASLG, and TLR5, likely to be involved in Treg activation dependent on “area-specific” stimuli.
Validation of Microarray Results Through Quantitative PCR.
Based on the results of microarray analysis, for each category we selected one to three genes and assessed, through quantitative PCR, the RNA expression level in CD4+CD25+ cells isolated from 12 Mossi and 12 Fulani, on the 13 described above and from 10 European donors. The selected genes were CTLA4 and FASLG for the membrane/intracellular markers, FOXP3, SOCS2, and TRADD for the transduction factors, TLR5 for TLRs, TGFβ1 and TGFβR2 for cytokines and cytokine receptors, and CX3CR1 for chemokines. As in microarray analysis, the amount of mRNA for each gene was normalized to the mean values of three HK genes (actin, GAPDH, and PPI).
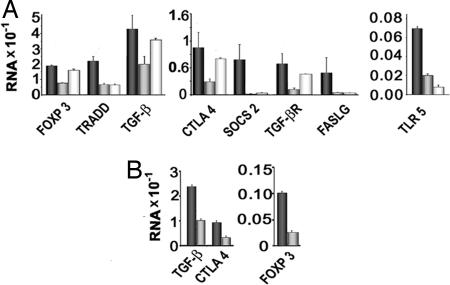
Fig. 3A shows that, in agreement with the microarray analysis, eight of nine genes tested (CTLA4, FOXP3, SOCS2, TRADD, TGFβ, TGFβR, TLR5, and FASLG) were less expressed in Fulani compared with Mossi, and, with the exception of FASLG, the differences between the two groups were statistically significant. FOXP3, CTLA4, TGFβ, and TGFβR were significantly reduced in Fulani even when compared with European donors.
Fig. 3.
Quantitative PCR analysis of selected genes related to Treg function in CD4+CD25+ cells from Mossi, Fulani, and European donors. (A) CD4+CD25+ cells were isolated from 12 of 13 Mossi (black bars) and 12 of 13 Fulani (gray bars) included in the study and 10 European donors (white bars) and lysed to obtain total RNA. Equal amounts of RNA (50 ng) from each donor were reverse-transcribed and amplified in duplicate in RT2 Custom PCR Array to simultaneously examine the mRNA levels of nine selected genes related to Treg activity and PPIA, GAPDH, and actin as HK genes. Data were normalized to the mean values of HK genes, and a relative amount of RNA was calculated by using the 2−ΔCT method. Statistically significant differences between Fulani and Mossi were as follows: FOXP3, P = 0.011; CTLA4, P = 0.041; TGFβ, P = 0.045; SOCS2, P = 0.049; TLR5, P = 0.002; FASLG, P = 0.22; TRAD, P = 0.0002; TGFβR, P = 0.048. Statistically significant differences between Fulani and European donors were as follows: FOXP3, P = 0.001; CTLA, P = 0.042; TGFβ, P = 0.011; TGFβR, P = 0.012. (B) RNA was extracted from CD4+CD25+high cells isolated from three Fulani (gray bars) and three Mossi (black bars) of the 13 included in the study, and the expression of selected genes was investigated as reported in A.
The comparison with Mossi and European donors confirmed that Treg from Mossi express significantly more TLR5 and SOCS2. Higher expression of TRADD and FASLG was also found, but the differences were not significant.
The whole results of microarray and PCR analysis and in particular the reduced expression of the CTLA4, TGFβ, and TGFβR genes suggested that an early block in the differentiation process driven by TGFβ (TGFβ/CTLA4/FOXP3/CTLA4 positive loop) (20) affects the generation of Treg in Fulani. To support this hypothesis and to rule out that differences in gene expression simply reflect the differences in the percentage of FOXP3+ cells (see Fig. 1), in selected cases CD4+CD25+high cells, which include all FOXP3+ Treg (12, 21), were sorted by FACS and analyzed for the expression of TGFβ, FOXP3, and CTLA4 genes. Fig. 3B shows that the expression of these genes is lower in Fulani CD4+CD25+high cells. Furthermore, in two Fulani that showed percentages of CD4+CD25+FOXP3+ cells comparable to that of Mossi, the expression of CTLA4, TGFB, and TGFβR was lower than Mossi (data not shown).
Effect of CD25+ Cell Depletion on P. falciparum-Induced Cell Proliferation.
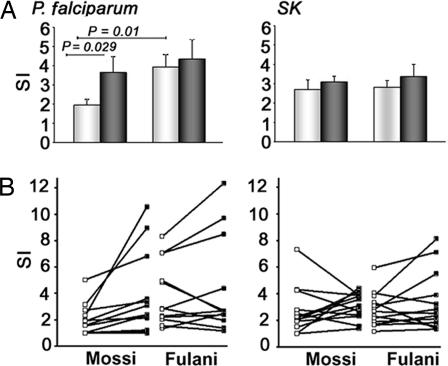
CD4+CD25+ cells have been reported to inhibit immune responses induced by P. falciparum antigens in both human and murine models of malaria infection (8, 9, 22). The reduced expression of key regulatory genes, revealed by microarray and PCR analysis in CD4+CD25+ cells from Fulani, suggests that these cells could be less efficient in suppressing T cell proliferation induced by P. falciparum antigens. To investigate this issue we compared the in vitro proliferative response to P. falciparum soluble extracts of both unfractionated and CD25+-depleted PBMC from all of the Mossi and Fulani included in the study. As control, the proliferative response toward the recall antigen streptokinase (SK) (23) was also evaluated. Fig. 4A shows that the proliferative response to P. falciparum in Fulani was higher than in Mossi and that the depletion of CD25+ cells significantly increased the stimulation index only in the Mossi group. In contrast, the depletion of CD25+ cells did not influence the SK-induced proliferation of PBMC in both groups, thus suggesting that few if any SK-specific Treg are present. Fig. 4B shows the changes in the stimulation index obtained after CD25+ cell depletion in each individual donor. SI Table 2 shows that a significant increase (stimulation index ≥ 50%) after CD25+ cell depletion was observed in six of 13 Mossi and in two of 13 Fulani. Moreover, the mean of this increase was 151% among the six Mossi and 69.4% in the two Fulani.
Fig. 4.
Proliferative response to P. falciparum and SK antigens by total and CD25-depleted Fulani- and Mossi-derived PBMC. Unfractionated PBMC (white bars) and CD25-depleted PBMC (black bars) obtained from the 13 Mossi and the 13 Fulani included in the study were stimulated with P. falciparum extracts or SK antigens as control. Cell proliferation was evaluated by thymidine incorporation after a 5-day culture. (A) Data are presented as mean stimulation index (cpm of stimulated cultures/cpm of unstimulated cultures) ± SE of Mossi and Fulani. (B) Results are expressed as stimulation index of PBMC before (□) and after (■) CD25+ cell depletion for each individual donor.
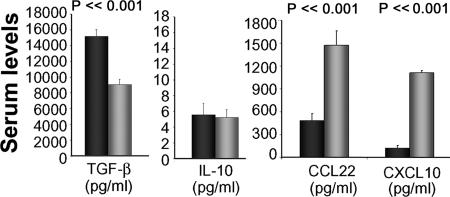
Serum Levels of the Regulatory Cytokine TGFβ Are Higher in Mossi than in Fulani.
To provide support to the in vitro data showing higher Treg activity in Mossi than in Fulani, sera from 58 Fulani (21 males and 37 females, mean age 26.9 ± 15.2 years) and 82 Mossi (35 males and 47 females, mean age 30.5 ± 8.1 years) collected in a previous survey (August 1994) in the same Barkoundouba area were assessed for the presence of the Treg-related cytokines TGFβ1 and IL-10. The proinflammatory chemokines CXCL10 and CCL22, considered as markers of TH1 and TH2 activity, respectively (24), were also measured. Fig. 5 shows that the levels of the immunoregulatory TGFβ1 were significantly higher in Mossi than in Fulani whereas IL-10 concentrations were comparable. In contrast, serum levels of CXCL10 and CCL22 were markedly higher in Fulani, supporting the hypothesis that an imbalance between the regulatory and the effector functions of the immune system could be responsible for the high response to P. falciparum antigens.
Fig. 5.
Serum levels of Treg-related and proinflammatory cytokines in Mossi and Fulani. Levels of TGF-β, IL-10, CXCL10, and CCL22 were evaluated in the sera of 82 Mossi (black bars) and 58 Fulani (gray bars). Columns represent the mean values (± SE) of the indicated cytokine/chemokine concentration. Statistical analysis was performed by using Student's t test.
Discussion
In this work we tried to dissect the mechanisms determining the strong immune reactivity toward P. falciparum of the Fulani, a West African ethnic group with low susceptibility to malaria. We reported that PBMC from Fulani express higher amounts of RNA for several TH1- and TH2-related genes compared with sympatric Mossi. In contrast, gene expression of FOXP3 and CTLA4, two important genes related to the mechanisms of immune tolerance mediated by Treg, was markedly lower in Fulani than in Mossi. These results are in agreement with previous reports showing a more efficient TH1 and TH2 response toward P. falciparum antigens in this group (25). The reduced expression of FOXP3 and CTLA4 found in PBMC from Fulani, compared with sympatric Mossi, directly involves the activity of Treg, key agents of immune regulation (11, 26). Treg, either naturally produced or induced in response to a specific stimulus (Tr1 and TH3) (10, 16), exert their function through a number of potential mediators such as CTLA4, FOXP3, and the antiinflammatory cytokines TGF-β and IL-10 (12, 16, 26). In parasitic infections, natural and adaptive Treg preserve host homeostasis by controlling excessive immune response, but they can favor pathogen survival and pathogen persistence (13). In malaria infection, in particular, Hisaeda et al. (8) demonstrated that depletion of CD4+CD25+ Treg protects mice from death upon infection with a lethal strain of Plasmodium yoelii. Recently, Walther et al. (9) demonstrated, in a longitudinal study of malaria sporozoite infection performed in malaria naïve vaccinated and control subjects, that both the production of TGFβ and the presence of CD4+CD25+FOXP3+ Treg are associated with higher rates of parasite growth in vivo.
In our study, cytofluorimetric analysis showed that the percentage of circulating CD4+CD25+ cells expressing FOXP3 was lower in Fulani. More important, a detailed assessment of gene expression in circulating CD4+CD25+ cells, which include most of the circulating Treg, revealed, together with reduced amounts of FOXP3 mRNA, a lower expression of several genes essential for Treg activity such as CTLA4, TGFβ, and TGFβ receptors in Fulani compared with sympatric Mossi. The fold decrease of differentially regulated genes in Fulani ranged between 2 and 16, suggesting that it does not merely reflect the differences in Treg percentage found with phenotypical analysis (see Fig. 2). Furthermore, the differences in TGFβ, CTLA4, and FOXP3 expression between Mossi and Fulani were evident also in CD4+CD25+high cells, known to include all of the FOXP3+ cells (21), suggesting that alterations in the maturation process of Treg in Fulani account also for the generation of a lower number of Treg.
The comparison of gene expression profiles of CD4+CD25+ between West African ethnic groups and European donors was deemed necessary to identify differences in those genes activated by infectious agents endemic in the study area and known to induce Treg-mediated immunosuppression, such P. falciparum, intestinal nematodes, and schistosomes (13). The results obtained clearly showed that, among the genes less expressed in Fulani, there are TGFβ1, TGFβR2, TGFβR3, FOXP3, and CTLA4, which normally are expressed on Treg, irrespective of their activation state (15). On the other side, SOCS2, FASLG, TGFβ2, and TRADD were overexpressed in Mossi, even when compared with European donors, a finding that suggests that their activation might depend on stimuli present in the study area.
A possible interpretation of the overall results is that the autocrine/paracrine circuits maintaining the regulatory phenotype, in particular those triggered by TGFβ, are interrupted or less efficient in Fulani. In fact, it has been shown that TGFβ, beside the role of suppressor factor, induces FOXP3 gene expression and that, in turn, FOXP3 renders T cells highly susceptible to the regulatory effects of TGFβ signaling by down-regulating inhibitory Smad proteins (27, 28). Furthermore, the interaction between CTLA4 and the CD80/CD86 determinants on the surface of target T cells (16) is needed for the induction of FOXP3 in the presence of TGFβ (20).
The reduced expression of the CTLA4 gene in Fulani, together with the lower expression of TGFβ and TGFβRs, suggest that, in this ethnic group, an early block in the differentiation process driven by TGFβ (TGFβ/CTLA4/FOXP3/CTLA4 positive loop) affects the generation of Treg and might be responsible for the inability of reaching a mature Treg phenotype, consistent with a lower expression of LAG3, ICAM, and FASLG molecules. For these reasons TGFβ, TGFβRs, FOXP3, and CTLA4 genes can be proposed as candidate genes for genetic susceptibility studies.
The phenotypic alterations of Treg in Fulani are consistent with the lower concentrations of TGFβ and the high levels of proinflammatory chemokines found in sera from this group compared with Mossi. These data suggest the hypothesis that an imbalance between the specific effector and the regulatory response to P. falciparum antigens can account for the differences in susceptibility to malaria infection. The functional correlate, however, was evident in proliferation experiments: The response to P. falciparum antigens in PBMC from Fulani was definitely higher than in Mossi, and the depletion of CD25+ cells did not affect the proliferation of PBMC from Fulani. In contrast, CD25+ depletion induced an increase in proliferation in the P. falciparum-stimulated cultures from Mossi, thus confirming that fully functional, activated Treg were modulating this immune response. Although the specificity of Treg to P. falciparum antigens needs to be further investigated, the lack of interferences of CD25+ cell depletion in the proliferative response to the control antigen SK suggests that few if any SK-specific Treg are present in both of the populations studied and that Treg in Mossi are rather derived from the expansion of cells specific for more frequently encountered antigens, such as P. falciparum.
Conclusions
In line with previous evidence from murine and human models (8, 9, 22), these results suggest that Treg activity could be central in the control of malaria infection also in populations exposed to naturally high P. falciparum transmission. Furthermore, this study highlights the existence of clear-cut differences in strategic pathways of the immunoregulatory network between sympatric populations differing in their genetic background and degree of susceptibility to malaria. The functional deficit of Treg here reported in Fulani is consistent with their higher susceptibility to diseases with autoimmune pathogenesis such as diabetes mellitus (29), pemphigus (30), and onchocercal skin disease (31). A higher resistance against infectious diseases like P. falciparum malaria could have been the driving selective force of this disorder. The definition of the genes involved could have important implications in the understanding of host–parasite relationships and in the development of anti-malaria vaccines.
Materials and Methods
Populations.
Subjects were recruited after oral informed consent for immunological studies was obtained. The study protocol was approved by the Technical Committee of the Centre National de Recherche et de Formation sur le Paludisme of the Ministry of Health of Burkina Faso. Ten- to 15-ml blood samples were collected at the end of the rainy season (October 2005). Buffy coats from 10 healthy blood donors (mean age ± SD = 39.9 ± 7.0) were supplied by Transfusional Center of Azienda Ospedaliera Careggi (Firenze, Italy).
Reagents.
Phycoerythrin-, FITC-, and allophycocyanin-conjugated anti-CD3, anti-CD4, anti-CD25, anti-CD8, anti-CD19, anti-CD14, and isotype-matched control mAbs were purchased from Becton Dickinson. Anti-FOXP3 antibody was purchased from eBiosciences. POD anti-human IgG was purchased from Behring. FOXP3 primer 20× Mix was purchased from Applied Biosystems. TH1-TH2-TH3 PCR array, custom PCR array for CTLA4, FOXP3, FASLG, TLR5, CX3CR1, TGFB, TGFBR, TRADD, SOCS2, PPI, and GAPDH, and the Human Autoimmune and Inflammatory Response Gene Array were purchased from SuperArray. yPfMSP1-19 (E-TSR)/VK1, yeast-secreted 19-kDa recombinant protein containing the carboxyl terminus of MSP1 from P. falciparum (3D7 strain) and yPfCSP-RPEU3/VK1, yeast-secreted recombinant CSP protein from P. falciparum WELCH, were supplied by MR4. SK was purchased by Behring.
Parasite Extract Preparation.
A laboratory strain (3D7) of P. falciparum was cultured in group O+ human RBC suspended in RPMI medium 1640 (GIBCO) containing 10% heat-inactivated human serum. Schizont-stage parasites were purified by sedimentation through 60% Percoll. Mature schizont-infected erythrocytes were lysed with 0.05% saponin/0.06 M NaCl, washed with PBS, and subjected to three freeze–thaw cycles (−156°C/37°C). The schizont-soluble fraction was obtained by repeated centrifugations of the lysate, and protein concentration was determined by Bio-Rad assay in 0.2-μ Millex filtered supernatants.
Flow Cytometric Analysis.
Flow cytometry analysis was performed as detailed elsewhere (32) on PBMC of the 13 Mossi and 13 Fulani described above. Briefly, cells were analyzed on a BDLSRII cytofluorimeter using Diva software (BD Biosciences). The area of positivity was determined by using an isotype-matched control mAb. Ten thousand events for each sample were acquired. Statistical analysis was performed by using Student's t test. P < 0.05 was considered significant.
Cellular Populations.
PBMC were isolated by Ficoll density gradient and frozen in liquid nitrogen until they were used. Depletion of CD25+ cells was obtained by using anti-CD25 mAb conjugated microbeads (Miltenyi Biotec) in PBMC. CD4+CD25+ cells were affinity-purified by using anti-CD25 mAb conjugated microbeads in a CD4+ enriched population as described (32). In selected experiments CD4+CD25high cells were isolated by FACS sorting (FACSVantage) using a phycoerythrin-coupled anti-CD25 mAb.
Antigen-Specific Proliferative Response.
Total PBMC or PBMC depleted of CD25+ cells (106 per milliliter), suspended in medium supplemented with 10% FCS, were cultured in triplicate on 96 U-bottomed plates (Nunc) for 5 days in the presence or absence of schizont antigen (10 μg/ml) or SK (5,000 units/ml). On day 5 cells were pulsed for a 16-h pulse with 0.5 μCi of [3H]thymidine per well (Amersham), cultures were harvested, and radioactivity was measured with a scintillation counter.
Quantitation of Cytokines and Antibodies.
The quantitative determination of CXCL10, CCL22, TGF-β, and IL-10 in the sera of patients was performed by ELISA (Quantikine; R & D Systems) according to the manufacturer's instructions. IgG against P. falciparum CSP and MSP-119 were measured by ELISA as described (6). Data were compared by using the Kruskal–Wallis test. P < 0.05 was considered significant.
Quantitative Real-Time PCR Analysis.
Total RNA was extracted with TRIzol reagent (Invitrogen) from PBMC of five Mossi (two males and three females, mean age = 42.0 ± 11.4 years) and five Fulani (three females and two males, mean age = 40.6 ± 9.8 years) and from CD4+CD25+ cells of 12 Mossi (seven females and five males, mean age = 42.4 ± 11.1 years) and 12 Fulani (seven females and five males, mean age = 39.3 ± 8.7 years). All of the Mossi and Fulani were included in the groups described above. RNA (50 ng) was reverse-transcribed by using SuperScript reverse transcriptase (Invitrogen). Specific or custom PCR array (SuperArray) was used to simultaneously examine the mRNA levels of several genes according to the manufacturer's protocol. Quantitative real-time PCR analysis was performed with the ABI PRISM 7700 sequence detector (Applied Biosystems). To examine the expression of the FOXP3 gene, FOXP3 primer control reagent Mix and TaqMan PCR Master mix (Applied Biosystems) were also used with the following protocol: 50°C/3 min, 95°C/10 min, 95°C/15 s, and 60°C/1 min for 40 cycles. Normalization was performed based on the mean of three HK genes, actin, PPIA, and GAPDH, in both cases. Relative amounts of RNA for each gene and fold increase were calculated by using the 2−ΔCT method or the 2−ΔΔCT method (33). Statistical analysis was performed by using Student's t test. P < 0.05 was considered significant.
Microarray Analysis of CD4+CD25+ Cell Populations.
RNA was extracted independently from five Mossi (three females and two males, mean age = 43.2 ± 3.1 years) and five Fulani (three females and two males, mean age = 40.8 ± 13.4 years) all included in the groups described above and five European donors (three females and two males, mean age = 38.0 ± 4.7 years). A total of 100 ng for each of the donor groups (Mossi, Fulani, and European donors) was pooled. Sample labeling and processing were performed with GEArray Ampolabeling LPR kit (SuperArray) by using [α-32P]dCTP (Amersham) according to the supplier's instructions. Radioactivity was quantified with a Packard Cyclone phosphorimager. Image processing and intensity data extraction were performed with the GEArray Expression Analysis Suite (SuperArray). The results obtained for each group, normalized to the mean value of actin, GAPDH, and PPI as HK genes, were compared by scatter plot analysis using GeneArray Expression Analysis Suite software with 1.9 as boundary value for fold change. This procedure was replicated with another set of pooled RNA from the same donors and a second set of microarrays to assess the variability associated with the preparation of targets and processing of microarrays.
Supplementary Material
ACKNOWLEDGMENTS.
This work was supported by grants from Italian Ministry of Education (FIRB N.RBAU01PXLN, N.RBNE01NWCH; COFIN, MIUR-PRIN 2006062857), from the University of Firenze, and from the European Union Sixth Framework Program BioMalPar Network of Excellence through Grant LSHP-CT-2004-503578.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0709969105/DC1.
References
- 1.Modiano D, Petrarca V, Sirima BS, Nebie I, Diallo D, Esposito F, Coluzzi M. Proc Natl Acad Sci USA. 1996;93:13206–13211. doi: 10.1073/pnas.93.23.13206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dolo A, Modiano D, Maiga B, Daou M, Dolo G, Guindo H, Ba M, Maiga H, Coulibaly D, Perlman H, et al. Am J Trop Med Hyg. 2005;72:243–248. [PubMed] [Google Scholar]
- 3.Modiano D, Luoni G, Petrarca V, Sodiomon SB, De Luca M, Simpore J, Coluzzi M, Bodmer JG, Modiano G. Tissue Antigens. 2001;57:128–137. doi: 10.1034/j.1399-0039.2001.057002128.x. [DOI] [PubMed] [Google Scholar]
- 4.Modiano D, Luoni G, Sirima BS, Lanfrancotti A, Petrarca V, Cruciani F, Simpore J, Ciminelli BM, Foglietta E, Grisanti P, et al. Trans R Soc Trop Med Hyg. 2001;95:149–152. doi: 10.1016/s0035-9203(01)90141-5. [DOI] [PubMed] [Google Scholar]
- 5.Modiano D, Chiucchiuini A, Petrarca V, Sirima BS, Luoni G, Perlmann H, Esposito F, Coluzzi M. Am J Trop Med Hyg. 1998;58:220–224. doi: 10.4269/ajtmh.1998.58.220. [DOI] [PubMed] [Google Scholar]
- 6.Modiano D, Chiucchiuini A, Petrarca V, Sirima BS, Luoni G, Roggero MA, Corradin G, Coluzzi M, Esposito F. Am J Trop Med Hyg. 1999;61:663–667. doi: 10.4269/ajtmh.1999.61.663. [DOI] [PubMed] [Google Scholar]
- 7.Greenwood BM, Groenendaal F, Bradley AK, Greenwood AM, Shenton F, Tulloch S, Hayes R. Ann Trop Med Parasitol. 1987;81:345–354. doi: 10.1080/00034983.1987.11812130. [DOI] [PubMed] [Google Scholar]
- 8.Hisaeda H, Maekawa Y, Iwakawa D, Okada H, Himeno K, Kishihara K, Tsukumo S, Yasutomo K. Nat Med. 2004;10:29–30. doi: 10.1038/nm975. [DOI] [PubMed] [Google Scholar]
- 9.Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, Andersen RF, Bejon P, Thompson F, Dunachie SJ, et al. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Belkaid Y, Rouse BT. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 11.Becker C, Stoll S, Bopp T, Schmitt E, Jonuleit H. Med Microbiol Immunol (Berlin) 2006;195:113–124. doi: 10.1007/s00430-006-0017-y. [DOI] [PubMed] [Google Scholar]
- 12.Romagnani S. Clin Exp Allergy. 2006;36:1357–1366. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 13.Belkaid Y, Blank RB, Suffia I. Immunol Rev. 2006;212:287–300. doi: 10.1111/j.0105-2896.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 14.Yi H, Zhen Y, Jiang L, Zheng J, Zhao Y. Cell Mol Immunol. 2006;3:189–195. [PubMed] [Google Scholar]
- 15.Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, Sakaguchi S. Int Immunol. 2006;18:1197–1209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 16.Beissert S, Schwarz A, Schwarz T. J Invest Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- 17.Kohm AP, Miller SD. J Autoimmun. 2003;21:261–271. doi: 10.1016/s0896-8411(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 18.Venet F, Pachot A, Debard AL, Bohe J, Bienvenu J, Lepape A, Powell WS, Monneret G. J Immunol. 2006;177:6540–6547. doi: 10.4049/jimmunol.177.9.6540. [DOI] [PubMed] [Google Scholar]
- 19.Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Trends Immunol. 2006;27:387–393. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Zheng SG, Wang JH, Stohl W, Kim KS, Gray JD, Horwitz DA. J Immunol. 2006;176:3321–3329. doi: 10.4049/jimmunol.176.6.3321. [DOI] [PubMed] [Google Scholar]
- 21.Roncador G, Brown PJ, Maestre L, Hue S, Martinez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, et al. Eur J Immunol. 2005;35:1681–1691. doi: 10.1002/eji.200526189. [DOI] [PubMed] [Google Scholar]
- 22.Nie CQ, Bernard NJ, Schofield L, Hansen DS. Infect Immun. 2007;75:2275–2282. doi: 10.1128/IAI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Youkeles LH, Soliman MY, Rosenstreich DL. J Allergy Clin Immunol. 1991;88:166–171. doi: 10.1016/0091-6749(91)90324-h. [DOI] [PubMed] [Google Scholar]
- 24.Hung CH, Suen JL, Hua YM, Chiang W, Chang HC, Chen CN, Jong YJ. Pediatr Allergy Immunol. 2007;18:378–384. doi: 10.1111/j.1399-3038.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 25.Farouk SE, Dolo A, Bereczky S, Kouriba B, Maiga B, Farnert A, Perlmann H, Hayano M, Montgomery SM, Doumbo OK, et al. Microbes Infect. 2005;7:110–117. doi: 10.1016/j.micinf.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Holm TL, Nielsen J, Claesson MH. Acta Pathol Microbiol Immunol Scand. 2004;112:629–641. doi: 10.1111/j.1600-0463.2004.apm1121001.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 29.Fisch A, Pichard E, Prazuck T, Leblanc H, Sidibe Y, Brucker G. Diabetologia. 1987;30:859–862. doi: 10.1007/BF00274794. [DOI] [PubMed] [Google Scholar]
- 30.Mahe A, Flageul B, Cisse I, Keita S, Bobin P. Br J Dermatol. 1996;134:114–119. [PubMed] [Google Scholar]
- 31.Brieger WR, Ososanya OO, Kale OO, Oshiname FO, Oke GA. Trop Med Int Health. 1997;2:529–534. doi: 10.1046/j.1365-3156.1997.d01-317.x. [DOI] [PubMed] [Google Scholar]
- 32.Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. J Exp Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.