Abstract
It is becoming increasingly apparent that heat shock proteins play an important role in the survival of Plasmodium falciparum against temperature changes associated with its passage from the cold-blooded mosquito vector to the warm-blooded human host. Interest in understanding the possible role of P. falciparum Hsp70s in the life cycle of the parasite has led to the identification of six HSP70 genes. Although most research attention has focused primarily on one of the cytosolic Hsp70s (PfHsp70-1) and its endoplasmic reticulum homolog (PfHsp70-2), further functional insights could be inferred from the structural motifs exhibited by the rest of the Hsp70 family members of P. falciparum. There is increasing evidence that suggests that PfHsp70-1 could play an important role in the life cycle of P. falciparum both as a chaperone and immunogen. In addition, P. falciparum Hsp70s and Hsp40 partners are implicated in the intracellular and extracellular trafficking of proteins. This review summarizes data emerging from studies on the chaperone role of P. falciparum Hsp70s, taking advantage of inferences gleaned from their structures and information on their cellular localization. The possible associations between P. falciparum Hsp70s with their cochaperone partners as well as other chaperones and proteins are discussed.
Keywords: PfHsp70, heat shock protein, Hsp40, molecular chaperone, malaria
Heat shock proteins are highly conserved, ubiquitous proteins that occur in most life forms, whose main role is to act as molecular chaperones. As molecular chaperones, heat shock proteins bind to nonnative proteins, facilitating their refolding to the native state (Ellis 1987). Heat shock protein 70 (Hsp70) forms one of the major heat shock protein families. Generally Hsp70 proteins are induced in response to stress, although some Hsp70 species are constitutively expressed in cells. Hsp70 binds to peptide substrate, allowing it to refold, followed by release of the substrate in ATP-expending cycles (Szabo et al. 1994). In the ADP-bound state, Hsp70 has high affinity for the peptide substrate, while its affinity for the substrate is reduced in the ATP-bound state (Suh et al. 1999). ATP binding induces a conformational change that transcends to the peptide-binding domain from the ATPase domain, resulting in the protein attaining a low substrate affinity status, leading to release of substrate (Liberek et al. 1991). Therefore, nucleotide exchange is essential for the Hsp70 functional cycle to proceed. To this end, Bcl-2-associated athanogene (Bag-1) (Höhfeld and Jentsch 1997) and Hsp70-binding protein (HspBP1) (Kabani et al. 2002) have been identified as the nucleotide exchange factors (NEFs) for mammalian Hsp70s, while GrpE facilitates nucleotide exchange by DnaK, the bacterial Hsp70 homolog (Harrison et al. 1997). Besides their role in refolding nascent proteins, Hsp70s participate in several other processes in the cell such as the assembly or disassembly of multiprotein complexes (Song et al. 2005), protein translocation (Gambill et al. 1993), protein degradation (Bercovich et al. 1997), signal transduction (Asea et al. 2002), and prion replication (Song et al. 2005). Hsp70 proteins have a molecular mass of ∼70 kDa and consist of two distinct domains; the 45-kDa N-terminal domain that binds ATP, and the 25-kDa peptide-binding domain (Flaherty et al. 1990; Wang et al. 1993). Hsp70 proteins are localized in the Escherichia coli cytosol and all compartments of eukaryotic cells such as chloroplast, endoplasmic reticulum lumen, mitochondrial matrix, and the cytosol (Yalovsky et al. 1992; Johnson and Craig 1997).
Approximately 3 billion people live in malaria endemic areas, and at least 1 million of these die from the disease (World Health Organization 2005). The following four species of Plasmodium cause malaria: Plasmodium falciparum, Plasmodium vivax, Plasmodium malaria, and Plasmodium ovale. Of these, it is P. falciparum that accounts for the highest mortality rate (Thiel 2005). Therefore, it is falciparum malaria that has received a lot of research attention. The production and localization of inducible Hsp70 proteins in P. falciparum have been well documented (Kumar et al. 1991; Joshi et al. 1992; Biswas and Sharma 1994). The overproduction of certain isoforms of Hsp70 proteins has been complemented by confirmation of the production of antibodies against these proteins in human subjects living in malaria endemic areas (Kumar et al. 1990; Behr et al. 1992).
There is growing evidence that heat shock proteins from P. falciparum could play a cytoprotective role in the life cycle of the parasite. The importance of chaperones at the host–parasite interface as a survival strategy has been reviewed (Feder and Hofmann 1999). Because the life cycle of P. falciparum transcends across two habitats (the cold-blooded mosquito vector and the warm-blooded human host), it is thought that the production of chaperones by the parasite in response to stress is a survival strategy against temperature and physiological changes experienced by the parasite (Sharma 1992). During the development of febrile malaria, body temperature rises to 41°C because of the release of pro-inflammatory cytokine tumor necrosis factor (Karnumaweera et al. 1992), and the development of this fever is known to promote the pathogenesis of malaria by enhancing the ability of the parasite-infected erythrocytes to adhere to blood vessels (Udomsangpetch et al. 2002). Another study established that P. falciparum parasites that were initially exposed to heat shock were not only able to mount better heat resistance to subsequent heat treatments but also showed better survival resilience and improved infectivity (Pavithra et al. 2004). It has also been proposed that febrile episodes that mark the development of malaria also promote the intra-erythrocytic development of the parasite (Pavithra et al. 2004). This phenomenon has been observed in other protozoan species (Soete et al. 1994; Wiesgigl and Clos 2001).
Pathogenic organisms produce heat shock proteins during the invasion of host cells (Maresca and Kobayashi 1994; Zügel and Kaufmann 1999), and as a result, heat shock proteins have become targets of vaccine research (Newport 1991). Because of their ubiquity and conservation, it has been proposed that heat shock proteins are at the interface between infection and autoimmunity through recognition of conserved epitopes or through cross-reactivity (Zügel and Kaufmann 1999). In addition, there is evidence that links heat shock proteins with drug resistance, and both heat shock protein 90 (Hsp90) and its functional partner calcineurin have been implicated (Sanglard et al. 2003; Cowen and Lindquist 2005).
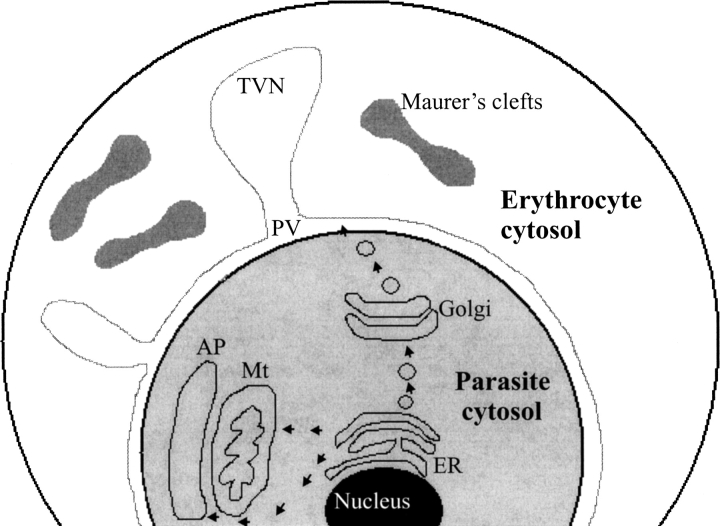
Since chaperones constitute one of the major protein families in the parasitophorous vacuole in an infected erythrocyte (▶; Nyalwidhe and Lingelbach 2006), they could play an important role in the trafficking of proteins of parasitic origin into the erythrocyte. Furthermore, the fact that chaperones of parasitic origin have been detected in other cell compartments outside the parasite cytoplasm such as the Maurer's clefts (▶), is further evidence for their possible involvement in the export of parasite proteins into the erythrocyte (Vincensini et al. 2005; Lanzer et al. 2006). However, it should be noted that it seems that the parasite exploits host cell chaperones during the assembly of multiprotein subunits at the erythrocyte surface (Banumathy et al. 2002). Host Hsp70 and Hsp90 in parasite-infected erythrocytes were membrane-associated, whereas the same chaperones were found to occur in the cytosol of uninfected cells (Banumathy et al. 2002). This suggests that in infected cells, host chaperones could play a role in the trafficking of parasitic proteins into the erythrocyte (Banumathy et al. 2002).
Figure 1.
Cellular components involved in the trafficking of proteins from P. falciparum into an infected erythrocyte. A diagrammatic representation of the structure of an erythrocyte infected by the parasite P. falciparum. Some proteins originating from the parasite enter the secretory pathway via the endoplasmic reticulum (ER), and possibly the Golgi apparatus, before being shuttled into the erythrocyte across the parasitophorous vacuole (PV). The PV extends into the interconnected tubulovesicular network (TVN). The membrane network of the parasite also gives rise to the Maurer's clefts, which are thought to play a key role in the export of proteins of parasitic origin to the erythrocyte. Several molecular chaperones (among them Hsp70s) of parasitic origin are thought to reside in the PV and Maurer's clefts, where they are implicated in the trafficking of proteins from the parasite to the erythrocyte (Vincensini et al. 2005; Lanzer et al. 2006; Nyalwidhe and Lingelbach 2006). A localized protein transport network exists inside the parasite and involves the export of proteins from the cytosol into the mitochondria (Mt) and apicoplast (AP), as represented by the arrows leading to these organelles. The apicoplast has its own genome that encodes functions mainly for the maintenance of the organelle. Parasite nuclear-encoded proteins key to the survival of the parasite are imported into the apicoplast from the cytosol of the parasite (Foth et al. 2003). The successful trafficking of these proteins across the apicoplast membrane very likely requires Hsp70 proteins of the parasite cytosol and apicoplast.
P. falciparum HSP 70 subfamilies
At least six P. falciparum Hsp70 homologs with features spanning across the cytosolic, endoplasmic reticulum (ER), and the mitochondrial forms have been identified (▶; Peterson et al. 1988; Sargeant et al. 2006). The compartmentalization of Hsp70 protein homologs in eukaryotic cells ensures that they are able to efficiently undertake specialized cellular roles. A phylogenetic analysis of Hsp70 proteins from P. falciparum (▶) illustrates the major Hsp70-like proteins to which these proteins belong. The cytosolic form of P. falciparum Hsp70 (PfHsp70-1) clusters with Hsp70s from eukaryotes. PfHsp70-x, a closely related homolog of PfHsp70-1, shows a close phylogenetic link to PfHsp70-1. PfHsp70-2 (ER Hsp70 homolog) and PfHsp70-3 (mitochondrial Hsp70 homolog) form clusters with their homologs from other eukaryotic organisms. On the other hand, PfHsp70-z and PfHsp70-y (whose molecular mass is at least 100 kDa) form a clade with the Hsp110/Grp170 (Lhs1) subfamily of Hsp70-like proteins. The organization of Hsp70s of parasitic origin into distinct organelle specific groups with distinct expression patterns has been documented (Kappes et al. 1993; Olson et al. 1994; Šlapeta and Keithly 2004).
Table 1.
Hsp70s from Plasmodium falciparum
Figure 2.
Phylogenetic analysis of P. falciparum Hsp70s. Hsp70-like proteins from P. falciparum (bold) and other sources were analyzed. NCBI accession numbers of proteins from other organisms, besides P. falciparum, are as follows: Theileria annulata Hsp110 (TaHsp110, XP_952474); Arabidopsis thaliana Hsp110 (AtHsp110, NP_567510); yeast Lhs1 (Lhs1, P36016); Escherichia coli ClpB (ClpB, AAB49540); human Hsp100 (NP_006651); Agrobacterium tumafaciens DnaK (AgtDnaK, AAR84665); E. coli DnaK (EcDnaK, BAA01595.1); Trypanosoma cruzi mitochondrial Hsp70 (TcmtHsp70, AAA30215); human BiP (CAA61201); T. annulata Hsp70 (TaHsp70, A44985); Plasmodium berghei Hsp70 (PbHsp70, AAL34314); T. cruzi Hsp70 (TcHsp70, P05456); and human Hsc70 (AF352832). The Hsp70-like proteins were subdivided into different subgroups as shown on the right-hand side. Dendrograms were constructed using the BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html)–Protdist neighbor phylogenetic analysis tool. The TreeView (Page 1996) option housed on the BioEdit program was used to generate the dendrograms.
Pairwise sequence identity analysis for P. falciparum Hsp70s (▶) shows a wide range of percentage identities across these proteins. The highest identity (73%) is between PfHsp70-1 and PfHsp70-x, which is consistent with phylogenetic data that shows that the two proteins are closely related (▶). PfHsp70-x also shares relatively high identity (54%) with the putative ER homolog PfHsp70-2. PfHsp70-2 and PfHsp70-3 (mitochondrial homolog) share 46% identity between them. The rest of the proteins share very low identity between them, signifying that P. falciparum Hsp70s share a divergent range of identities between them that might be an indication of the wide range of activities that these chaperones could play across the different organelle locations in which they occur. It has been estimated that in vitro cultures of P. falciparum produce a minimum of 87% of total protein as new proteins in 48 h, and this figure rises to at least 90% in a 60-h growth cycle (Nirmalan et al. 2004). Given the challenges that P. falciparum encounters in its life cycle, it is not surprising that this organism has Hsp70 homologs that have a relative degree of structural diversity in order to be able to deal with the protein-folding challenges that it manages. This is extremely important in the P. falciparum life cycle since the development of malaria fever adds a further strain to its protein-folding machinery.
Table 2.
Percentage identities for Hsp70s from Plasmodium falciparum
Cytosolic and nuclear Hsp70 proteins
Of the six Hsp70s from P. falciparum, it is only PfHsp70-1 (▶) that has received widespread research attention with respect to its chaperone properties (Sharma 1992; Matambo et al. 2004; Ramya et al. 2006) and as a potential vaccine candidate (Kumar et al. 1990; Behr et al. 1992). PfHsp70-1 is a cytosolic/nuclear-localized P. falciparum Hsp70 (Kappes et al. 1993). The protein is largely confined to the cytosol, and its nuclear localization is enhanced in response to heat stress (Kappes et al. 1993). PfHsp70-1 has a molecular mass of ∼74 kDa, and possesses the C-terminal EEVD motif, characteristic of eukaryotic cytosolic Hsp70s. The EEVD motif of Hsp70 binds to cochaperones including Hop, whose role is to facilitate the partnership between Hsp70 and Hsp90 (Demand et al. 1998). PfHsp70-x, which shares close identity to PfHsp70-1, has a molecular mass of ∼76 kDa (▶). However, PfHsp70-x contains a C-terminal EEVN motif in place of the EEVD motif. PfHsp70-x could be an alternate cytosolic Hsp70 of P. falciparum (Sargeant et al. 2006). There is very little information on the role of PfHsp70-x, and perhaps it represents the constitutively expressed cytosolic Hsp70 form of P. falciparum. The fact that both PfHsp70-1 and PfHsp70-x share high sequence identity, with both possessing highly conserved bipartite nuclear localization signals (▶; Robbins et al. 1991), strongly suggests that PfHsp70-x, like PfHsp70-1, may also move into the nucleus.
Figure 3.


Amino acid sequence alignments of P. falciparum Hsp70s against E. coli DnaK and human Hsc70. (A) Alignment of the amino acid sequences for the ATPase domains of the P. falciparum Hsp70s against corresponding sequences of E. coli DnaK (BAA01595.1) and human Hsc70 (AF352832). Some of the key residues in the ATPase domain are highlighted as follows: (purple box) N-terminal ER leader sequence; (red box) the bipartite nuclear localization signal (Robbins et al. 1991); and (blue boxes with broken lines) highly conserved residues associated with heat shock proteins. Based on the DnaK sequence, some residues that are important for its function and their corresponding residues in Hsc70 and PfHsp70 are highlighted as follows: (blue boxes with continuous lines) P143 (proline allosteric switch) and R151, both of which are important for interdomain function (Vogel et al. 2006a); (green boxes) Y145, N147, D148, N170, and T173, which interact with DnaJ (Gässler et al. 1998; Suh et al.1998); and (yellow box) T199, a DnaK phosphorylation site (McCarty and Walker 1991). The different motifs that interact with subunits of the nucleotide (phosphate 1, phosphate 2, and adenosine) are shown. These motifs are linked by residues represented by the segments connect 1 and connect 2. The numbers on the left-hand side represent the positions of residues in the respective proteins. (B) An alignment of amino acid sequences of the peptide-binding domains of P. falciparum Hsp70s, E. coli DnaK, and human Hsc70. Highlighted by the different rectangular boxes are the following residues: (blue box) linker; (orange box) terminal EEV-motifs; (yellow boxes) the terminal Hsp70 ER retention signal (Pelham 1989); (arrows) residues that are in contact with the substrate (Zhu et al. 1996), of which those that constitute the hydrophobic arch and hydrophobic pocket (Mayer et al. 2000) are highlighted by red arrows and a green arrow, respectively. (Blue arrows) The rest of the residues that occur in contact with substrate; (red boxes) residues that constitute the different subdomains of the β-sheet. (Black line) Residues constituting the different lid subdomains (A–E) of DnaK; (blue lines) residues constituting the lid subdomains of Hsc70 (Chou et al. 2003). (White on black background) Identical residues; (black on gray background) similar residues. The BioEdit program ClustalW (http://www.mbio.ncsu.edu/BioEdit/bioedit.html) (Thompson et al. 1994) based alignment option was used to carry out sequence alignment. The numbers on the left-hand side represent the positions of residues in the respective proteins.
The expression of PfHsp70-1 at the blood stages of the parasite has been confirmed, and this protein has been reported to be soluble (Joshi et al. 1992; Kappes et al. 1993). Although PfHsp70-1 is primarily localized to the cytosol and nucleus (Kappes et al. 1993), it is has been reported to be present in the parasitophorous vacuole (Nyalwidhe and Lingelbach 2006). It has also been detected in the Maurer's clefts (Vincensini et al. 2005), a structure that is thought to be connected to the parasite but that extends into the erythrocyte (Langreth et al. 1978). This has raised the possibility that this protein could be exported into the erythrocyte. The detection of antibodies to PfHsp70-1 in malaria patients suggests that this protein could join the host circulatory system, thereby invoking an immune response (Kumar et al. 1990). Alternatively, PfHsp70-1’s localization in the parasitophorous vacuole and the Maurer's clefts suggests a role for this chaperone in the export of P. falciparum proteins into the erythrocyte.
PfHsp70-1 has relatively high basal ATPase activity and ATP-dependent in vitro chaperone activity (Matambo et al. 2004; Ramya et al. 2006). In addition, PfHsp70-1 displayed in vivo chaperone function by reversing the thermosensitivity of an Escherichia coli dnaK mutant strain (Shonhai et al. 2005). The protein also possesses a functional linker region since mutations in this segment of the protein resulted in loss of functionality in vivo (Shonhai et al. 2005). Using a chimeric protein composed of the ATPase domain of E. coli DnaK fused to the peptide-binding domain of PfHsp70-1, evidence for interdomain communication between the two domains of these proteins was established (Shonhai et al. 2005). The fact that both full-length PfHsp70-1 and its chimeric derivative composed of the ATPase domain of E. coli DnaK linked to the peptide-binding domain of PfHsp70-1 displayed in vivo function in E. coli DnaK756 cells suggests that the peptide-binding domain of PfHsp70-1 was able to interact with E. coli DnaK substrates (Shonhai et al. 2005). Furthermore, a recent study showed that the J-domains (essential for the interaction of Hsp40s with their Hsp70 counterparts) of P. falciparum Hsp40 proteins (PfJ4 and PfJ1) were able to interact with E. coli DnaK in vivo (Nicoll et al. 2007). This shows that there is a high degree of functional overlap between E. coli and P. falciparum chaperone systems.
Although information on the direct chaperone role of PfHsp70-1 in P. falciparum is scanty, there is evidence that points to its possible role in actin polymerization (Tardieux et al.1998). Actin polymerization is a phenomenon that implicates the role of actin filaments in facilitating host cell invasion by parasites (Dobrowolski et al. 1997). Furthermore, the fact that PfHsp90 is essential for the parasite's survival places PfHsp70-1 into the spotlight, since PfHsp70-1 and PfHsp90 have been observed to associate in ATP-dependent complexes, suggesting that the two proteins could form a functional partnership during the growth and development of P. falciparum (Banumathy et al. 2003).
A translation initiation factor, 2α (eIF-2α), has been identified in P. falciparum whose phosphorylation inhibits protein synthesis, leading to cell death (Surolia and Padmanaban 1991). Hsp70 has been shown to suppress the activity of a heme-regulated kinase, thus preventing the phosphorylation of eIF-2α and indirectly allowing protein synthesis to proceed (Ramya et al. 2007a). The antimalarial 15-deoxyspergulain (DSG) has been proposed to indirectly inhibit protein synthesis by titrating Hsp70 protein and promoting the phosphorylation of eIF-2α (Ramya et al. 2007a). Ramya et al. (2006) have provided evidence that DSG was able to modulate the activity of PfHsp70-1, possibly by interacting with its C-terminal EEVD motif. Therefore PfHsp70-1 has been proposed to be indirectly linked to the regulation of protein translation in P. falciparum (Ramya et al. 2007b). However, since the EEVD motif is in close proximity to the peptide-binding domain, by binding at or near the EEVD motif of PfHsp70-1, DSG may also inhibit protein folding by interfering with substrate binding by PfHsp70-1. Furthermore, it has been proposed that by sequestering PfHsp70-1, DSG inhibits the trafficking of some nuclear-encoded proteins into the apicoplast, killing the parasite (Ramya et al. 2007b). This is consistent with the proposed role for P. falciparum Hsp70s in the trafficking of proteins destined for the apicoplast (Foth et al. 2003). Proteins that are meant for uptake by the apicoplast are enriched in Hsp70-binding sites, and mutation of these sites led to mistargeting of the proteins in P. falciparum cells (Foth et al. 2003).
Heat shock protein 70 interacting protein (Hip) is a cochaperone of Hsp70 that competes with Bag-1 for a contact site in the ATPase domain of Hsp70 and acts in direct opposition to Bag-1 by stabilizing the Hsp70–peptide complex (Höhfeld et al. 1995; Höhfeld and Jentsch 1997). Hip's ability to regulate Hsp70 function in vivo has been confirmed (Nollen et al. 2001). Ramya et al. (2006) observed that P. falciparum Hip (PFE1370w) was able to modulate the chaperone function of PfHsp70-1 and PfHsp70-3 in vitro. Therefore, there is growing interest in identifying cochaperones that modulate the chaperone activity of PfHsp70-1 and their mechanism of action.
Allosteric communication is an essential feature of Hsp70 protein function, regulating both substrate release and ATP hydrolysis (Han and Christen 2003). The elucidation of the partial full-length structure of bovine Hsc70 by Jiang et al. (2005) revealed structural details that suggest that the interaction between Hsp70 and Hsp40 occurs at the linker interface, allowing Hsp40 to interact with both the ATPase and peptide-binding domain of Hsp70. Not surprisingly, some residues on Hsp70 that are important for interaction with Hsp40 are implicated in interdomain communication (Gässler et al. 1998; Jiang et al. 2005). Hsp70 residues implicated in interdomain communication and their interaction with Hsp40 are conserved in PfHsp70-1 (▶; Gässler et al. 1998; Jiang et al. 2005; Vogel et al. 2006b). The fact that it has previously been shown that PfHsp70-1 possesses a functional linker whose disruption compromised its function, suggests that the linker region of PfHsp70-1 is an important structural component of its function (Shonhai et al. 2005). Except for PfHsp70-y and PfHsp70-z, the rest of the P. falciparum Hsp70s display highly conserved linker regions (▶), suggesting that this motif might be important in the modulation of their function.
Endoplasmic reticulum Hsp70
PfHsp70-2 is a homolog of the mammalian ER 78-kDa glucose-regulated protein (Grp78), also referred to as immunoglobulin-binding protein (BiP) (Kumar and Zheng 1992; Kappes et al. 1993). The protein has a molecular mass of ∼73 kDa (▶; Kumar and Zheng 1992). PfHsp70-2 possesses the ER N-terminal leader sequence and a possible C-terminal ER retention signal (SDEL) that differs from that of Grp78/BiP, which contains a typical eukaryotic C-terminal ER retention signal (KDEL) (Pelham 1989). Indeed, PfHsp70-2 has been observed to be largely confined to ER-like structures in P. falciparum (Kumar et al. 1991). In addition, PfHsp70-2 has been detected in the Maurer's clefts (Vincensini et al. 2005). Although PfHsp70-2 was slightly induced in response to heat stress, no induction of the protein was invoked by partial glucose deprivation and other known inducers of Grp78 proteins (Lee 1987; Kumar et al. 1991).
PfHsp70-2 shows a very close phylogenetic link with human BiP (▶). Although there is no study that has been conducted to verify the direct chaperone role of PfHsp70-2 in P. falciparum, a study by LaCount et al. (2005) based on the yeast two-hybrid system (▶) showed that PfHsp70-2 potentially interacts with more P. falciparum proteins than PfHsp70-1 and PfHsp70-3 (the mitochondrial Hsp70 homolog). This study implicated PfHsp70-2 in association with the following protein families: cytoskeletal and membrane proteins, translational and transcriptional machinery, proteosome and proteolytic enzymes, enzymes involved in physiological and metabolic pathways, and DNA repair and replication enzymes. Human BiP is known to interact with proteins exported to the ER, maintaining them in states ideal either for export or degradation (Gething 1999). Assuming that PfHsp70-2 also serves the same purpose, it could explain the possible association of PfHsp70-2 with a wide spectrum of P. falciparum proteins (▶; LaCount et al. 2005). One of the proteins that potentially interacts with PfHsp70-2 is the erythrocyte membrane protein (PFI1830c) (▶), a protein that is exported into the erythrocyte (Baruch et al. 1996). Therefore, the potential localization of PfHsp70-2 to the Maurer's clefts (Vincensini et al. 2005) could be perceived as a strategy to promote the export of P. falciparum proteins into the erythrocyte.
Figure 4.
The potential P. falciparum Hsp70 interactome of proteins. The network of interactions involving Hsp70s from P. falciparum and other proteins from the parasite was constructed based on data from yeast two-hybrid studies (LaCount et al. 2005). Chaperones: (solid circle) Hsp70s; (solid square) Hsp40-like proteins; (solid arrow) PfHip; (open rectangle) disulfide isomerase. The rest of the proteins involved in the network are classified into the following subgroups: (open oval) cytoskeletal and membrane proteins; (open rectangle) translational and transcriptional machinery; (open rhombus) proteosome and proteolytic enzymes; (dotted rectangle) enzymes involved in physiological and metabolic pathways; and (open star) DNA repair and replication enzymes. The arrow points in the direction of the prey. Reciprocal association is represented by arrows facing opposite directions. A broken arrow links PfHsp70-3 and PFL1385c. Self-association of chaperones is illustrated by two adjacent shapes representing the particular protein. The interactors are represented by their locus numbers.
Mitochondrial Hsp70
PfHsp70-3 is the mitochondrial Hsp70 homolog (šlapeta and Keithly 2004; Sargeant et al. 2006). The mitochondrial and cytosolic Hsp70 homologs from the apicomplexan kingdom and other closely related species display distinct phylogenetic features, suggesting that these proteins have distinct roles (šlapeta and Keithly 2004). Despite the fact that this protein possesses a weakly conserved mitochondrial pre-sequence, it is phylogenetically related to its mitochondrial Hsp70 from other eukaryotic organisms (šlapeta and Keithly 2004). PfHsp70–3 forms a monophyletic clade with its Trypanosoma cruzi mitochondrial Hsp70 homolog (TcmtHsp70), which clusters closely with the prokaryotic cytosolic Hsp70s from Escherichia coli (EcDnaK) and Agrobacterium tumafaciens DnaK (AgtDnaK) (▶).
There is very little experimental information on the possible role of PfHsp70-3. In eukaryotic cells, mitochondrial Hsp70 proteins play an important role in the import of pre-proteins into the mitochondria (Bauer et al. 2000). However, it is yet to be established whether PfHsp70-3 is involved in the import of P. falciparum pre-proteins from the parasite cytosol to the mitochondrion. However, the high homology between PfHsp70-3 and human HspA9B has been presented as evidence that PfHsp70-3, like HspA9B, could be involved in the export of proteins since HspA9B is implicated in antigen processing and protein export (Wadhwa et al. 2002; Botha et al. 2007).
A study based on the yeast two-hybrid system (▶) shows that some of the proteins associated with PfHsp70-3 are a malaria antigen (MAL13P.304) and two asparagine-rich antigen proteins (PF08_0060 and PF11_0111). This suggests a possible role of PfHsp70-3 during protein trafficking into the erythrocyte since both malaria antigen and asparagine-rich proteins are exported into the erythrocyte (Weber et al. 1988; Barale et al. 1997). Perhaps the fact that PfHsp70-3 has been detected in the Maurer's clefts (Vincensini et al. 2005; Lanzer et al. 2006) suggests that this chaperone might facilitate the export of proteins of parasitic origin into the erythrocyte. Assuming that PfHsp70-3 occurs in several cellular organelles as is true for HspA9B, perhaps it is not surprising that PfHsp70-3 could be found in the parasite mitochondrion and Maurer's clefts in the infected erythrocytes. However, it is confounding to reconcile how the same protein could act as a protein import system (in the mitochondrion) and as a possible protein export system (in the Maurer's clefts). Therefore, the proposed localization of PfHsp70-3 as observed by Vincensini et al. (2005) may need to be verified by independent localization experiments.
Hsp110/Grp170 homologs
PfHsp70-y and PfHsp70-z have approximate molecular masses of 100 kDa and 108 kDa, respectively. Although the two Hsp70-like proteins have relatively conserved ATPase domains, they display very low conservation in the peptide-binding domains (▶). Of the six P. falciparum Hsp70s, it is only these two that do not have a conserved nuclear localization signal (Robbins et al. 1991). PfHsp70-z and PfHsp70-y also do not have the threonine residue corresponding to the T199 of DnaK, which is a crucial phosphorylation site of DnaK (McCarty and Walker 1991). Generally, the linker is conserved in all the Hsp70s from P. falciparum, except in PfHsp70-y and PfHsp70-z. In addition, the fact that both PfHsp70-y and PfHsp70-z lack a distinct linker structure that is crucial for allosteric control of Hsp70s (Han and Christen 2001; Vogel et al. 2006b) suggests that these Hsp70s are regulated differently or have a distinct role that is divergent from the typical Hsp70 chaperone function. The close phylogenetic association of PfHsp70-y and PfHsp70-z with Hsp70-like proteins of higher molecular masses such as TaHsp110 from another apicomplexan species, Theileria annulata, and AtHsp110 from a plant species (Arabidopsis thaliana) hints at the fact that the two Hsp70-like proteins are Grp110/Grp170 homologs. The Grp110/Grp170 family of proteins has structural–functional features distinct from Hsp70s, although they also have some degree of similarity to Hsp70s (Easton et al. 2000). The Hsp110/Grp170 family of proteins has a longer helical lid compared to Hsp70 proteins, which enables them to bind larger amounts of substrate (Easton et al. 2000). The fact that PfHsp70-y contains a putative terminal ER-retention signal and shows close phylogenetic connection to the yeast Hsp70-like Hsp110/Grp170 homolog Lhs1 (Steel et al. 2004) hints at the possibility that PfHsp70-y could be a NEF for PfHsp70-2. Besides Lhs1 (Steel et al. 2004), another yeast Hsp110, Sse1p has been identified as a NEF (Dragovic et al. 2006). Therefore, PfHsp70-y possibly acts as the ER-based NEF of the Hsp110/Grp170 family, while PfHsp70-z is the cytosolic Hsp110/Grp170 protein of P. falciparum.
Characterization of the chaperone features of P. falciparum Hsp70s
Although relatively divergent in structure, P. falciparum Hsp70s must display conserved features that are important for their chaperone role. These include key residues for interaction with their Hsp40 partners, NEFs, and peptide substrates. Since PfHsp70-1 is the most extensively studied P. falciparum Hsp70 protein, its key functional residues were analyzed relative to the structures of the well-studied E. coli DnaK and human Hsc70. Since there is evidence that PfHsp70-1 could interact with a subpopulation of E. coli substrates in vivo (Shonhai et al. 2005), we analyzed its structural features important for chaperone activity compared to E. coli DnaK and human Hsc70, with particular reference to residues that interact with cochaperones and substrate. The structural comparison between PfHsp70-1 and Hsc70 was used in order to identify important structural motifs that are conserved between the two Hsp70s of eukaryotic origin. Furthermore, a comparative analysis of the chaperone properties of PfHsp70-1 and human Hsc70 could be used to establish whether the two proteins could be regulated by the same cochaperones since it has been hypothesized that human Hsp70s could be regulated by P. falciparum Hsp40s that are exported into the erythrocyte (Sargeant et al. 2006; Botha et al. 2007).
P. falciparum Hsp70 residues important for interaction with substrate
Only PfHsp70-1 and PfHsp70-x possess the typical eukaryotic Hsp70 arch residues that are inverted compared to those of E. coli DnaK (the DnaK arch is made up of M404 and A429, and most eukaryotic Hsp70s have A and Y at corresponding positions) (▶; Zhu et al. 1996). PfHsp70-2 has residues V/Y, while PfHsp70-3 has residues L/A at the arch position. However, both PfHsp70-2 and PfHsp70-3 have the conserved valine residue in the hydrophobic pocket. PfHsp70-z and PfHsp70-y have arch residues different from those of PfHsp70-1 and PfHsp70-x, and neither of the two possess the conserved valine residue associated with the hydrophobic pocket position. Instead, PfHsp70-z and PfHsp70-y have leucine and isoleucine residues, respectively, in the position corresponding to the Hsp70 hydrophobic pocket. The lack of consensus in arch residues observed between cytosolic P. falciparum Hsp70s and the rest of their homologs is consistent with the fact that arch residues are the least-conserved substrate-binding residues, thus perfectly placing them as determinants of Hsp70 substrate specificity (Rüdiger et al. 1997; Mayer et al. 2000). Based on sequence alignment data, PfHsp70-1, like other Hsp70s (▶; Zhu et al. 1996), possesses a substrate-binding cavity that is rich in hydrophobic residues.
Phosphorylation of Hsp70 proteins in P. falciparum
Phosphorylation of Hsp70 proteins is an important functional regulatory aspect of their chaperone role (McCarty and Walker 1991). The concomitant expression and phosphorylation of major chaperones from Plasmodium has been observed (Wiser and Plitt 1987; Kappes et al. 1993). PfHsp70-1 and PfHsp70-2 display growth-phase-dependent phosphorylation in vivo through their threonine and serine residues (Kumar et al. 1991; Kappes et al. 1993). The phosphorylation of Hsp70 proteins is important in the regulation of their activities (McCarty and Walker 1991; Cvoro et al. 1999). The concomitant expression and phosphorylation of Hsp70 proteins ensures that their chaperone activity is enhanced during stressful conditions (McCarty and Walker 1991). Therefore, the fact that at least four of the P. falciparum Hsp70s possess a threonine residue that corresponds to the DnaK phosphorylation site (T199) (▶; McCarty and Walker 1991) suggests that most of them may have phosphorylation-regulated activities.
Using the KinasePhos phosphorylation prediction tool (Huang et al. 2005a,b), potential phosphorylation sites in the six Hsp70s from P. falciparum were identified (▶). Based on this phosphorylation prediction tool, P. falciparum Hsp70s are predicted to be phosphorylated mostly through serine and threonine residues, in agreement with a previous observation based on studies on the phosphorylation of PfHsp70-1 and PfHsp70-2 (Kappes et al. 1993). PfHsp70-1 has been observed to be phosphorylated more through its serine residues than through threonine sites, while PfHsp70-2 was phosphorylated more through its threonine than through serine residues (Kappes et al. 1993). Based on the predicted number of phosphorylation sites, PfHsp70-1 and PfHsp70-2 each have approximately the same number of threonine and serine phosphorylation sites (▶). Therefore it is not clear why phosphorylation through the threonine and serine sites occurs preferentially through one of the two residues in each protein (Kappes et al. 1993). Perhaps this represents a unique regulatory mechanism for the two proteins. However, the fact that no phosphorylation was observed to occur through tyrosine residues for both PfHsp70-1 and PfHsp70-2 could be because of the low number of possible tyrosine phosphorylation sites in these two chaperones (▶; Kappes et al. 1993). Compared to their P. falciparum homologs, PfHsp70-y and PfHsp70-z exhibit high numbers of potential tyrosine phosphorylation sites. This is further evidence that the chaperone properties of these two proteins could be distinct from the rest of the P. falciparum Hsp70s. This is particularly the case for PfHsp70-z, which has only one predicted threonine phosphorylation site.
Figure 5.
Comparative potential phosphorylation status of P. falciparum Hsp70s. A bar graph representation for the potential phosphorylation status of the Hsp70 proteins from P. falciparum. The KinasePhos phosphorylation prediction tool (Huang et al. 2005a,b) was used to predict potential serine, threonine, and tyrosine residues that could be phosphorylated by kinases. The bar graphs are for (1) PfHsp70-1, (x) PfHsp70-x, (2) PfHsp70-2, (3) PfHsp70-3, (y) PfHsp70-y, and (z) PfHsp70-z.
Regulation of P. falciparum Hsp70s by Hsp40 cochaperones
Several Hsp40-binding sites corresponding to those identified to be important for this process in E. coli DnaK (Gässler et al. 1998; Suh et al. 1999) are generally conserved in the ATPase domains of P. falciparum Hsp70s (▶). Watanabe (1997) established that certain P. falciparum Hsp40s may be induced by heat stress. At least some of the P. falciparum Hsp70s would be expected to have chaperone activities that are modulated by Hsp40s (Botha et al. 2007). At least 43 Hsp40-like proteins are encoded on the P. falciparum genome (Sargeant et al. 2006; Botha et al. 2007). Of these, 19 are predicted to be exported based on export signal motifs that they carry (Sargeant et al. 2006; Botha et al. 2007). Therefore, there are 24 Hsp40 proteins that could be restricted to the cytoplasm of P. falciparum. Ironically, all the P. falciparum Hsp70s do not possess export signal motifs (Marti et al. 2004; Sargeant et al. 2006) and are therefore predicted not to be exported into the erythrocyte (Botha et al. 2007). It would be expected that part of this large entourage of Hsp40s that P. falciparum possibly exports into the erythrocyte could serve as cochaperone partners of host Hsp70s (Sargeant et al. 2006; Botha et al. 2007). A study showed that host chaperones (including human Hsp70 and Hsp90) interact with P. falciparum proteins exported into the erythrocyte (Banumathy et al. 2002). Therefore, these exported P. falciparum Hsp40s possibly scavenge for P. falciparum proteins, directing them to the host Hsp70 machinery.
It has been proposed that of the 24 Hsp40-like proteins confined to the P. falciparum cytoplasm, two type I Hsp40s (PfJ1 and PF14_0359) (Watanabe 1997; Sargeant et al. 2006) or a type II Hsp40 (PFB059w) possibly act as cochaperones of PfHsp70-1 (Botha et al. 2007). It is thought that protein disulfide isomerase (PDI) related P. falciparum Hsp40s (PfJ2 and PF13_0102) could serve as the ER-based cochaperones of PfHsp70-2 (Botha et al. 2007). Yeast two-hybrid data for PfHsp70-3 suggested it has possible direct and indirect interactions with a ring-infected erythrocyte surface antigen (RESA)-like protein, and mature parasite-infected erythrocyte surface antigen (MESA) Hsp40-like protein (▶). These associations could be partner protein interactions; however, this is difficult to reconcile with the erythrocyte membrane localization of the RESA-like and MESA proteins. Therefore, one cannot exclude the possibility that the RESA-like and MESA proteins are substrates of PfHsp70-3 during their export to the host.
The possible regulation of P. falciparum Hsp70s by nucleotide exchange factors
There is evidence that Hsp70s differ with respect to their intrinsic rate of ADP release, justifying the need for them to have specialized nucleotide exchange systems (Russell et al. 1998; Silberg and Vickery 2000). Variations within structural features of the NEF-binding site of Hsp70s are responsible for providing unique Hsp70–nucleotide exchange partnerships (Brehmer et al. 2001). For this reason, Brehmer et al. (2001) subdivided nucleotide exchange systems in Hsp70s into three prototypes: DnaK proteins, HscA (Hsc66), and Hsc70 proteins, as determined by their distinct interaction with GrpE and Bag-1.
Although structurally unrelated to GrpE, Bag-1 is considered the functional equivalent of GrpE (Höhfeld and Jentsch 1997). This is because structures of GrpE–DnaK and Bag-1–Hsc70 complexes displayed identical conformational changes in the ATPase domains of the respective Hsp70 protein (Harrison et al. 1997; Sondermann et al. 2001). On the other hand, the mechanism by which nucleotide exchange occurs on eukaryotic Hsp70 through the action of HspBP1 is distinct from the GrpE/Bag-1-driven one (Shomura et al. 2005). However, despite the fact that GrpE and Bag-1 manifest similar conformational changes in Hsp70 proteins, the specific mechanistic details in which these two NEFs interact with their Hsp70 partners are not the same. GrpE functions as a dimer (Harrison et al. 1997), as opposed to Bag-1, which uses a single domain (Sondermann et al. 2001). GrpE has more contact points with DnaK than exist between Bag-1 and Hsc70 (Sondermann et al. 2001). It is becoming clear that there are variations in the way GrpE and Bag-1 operate to effect nucleotide exchange. For example, whereas GrpE triggers the release of both ADP and ATP from DnaK, Bag-1 induces only ADP release (Brehmer et al. 2001). HspBP1, though a eukaryotic NEF, binds and induces nucleotide exchange conformation in Hsp70 proteins in a different mechanism from Bag-1 (Shomura et al. 2005).
The possible role of NEFs in the regulation of P. falciparum Hsp70s was investigated by exploring the conservation of residues crucial for nucleotide exchange in PfHsp70-1. Amino acid sequence alignments were conducted in order to identify residues known to be crucial for the interaction of Hsp70s with GrpE, Bag-1, and HspBP1. A conserved loop region that occurs in subdomain IB of DnaK is thought to ensure the stable interaction of DnaK with GrpE (▶; Buchberger et al. 1994). This loop is also implicated in the regulation of nucleotide binding by DnaK and its role is essential for DnaK function (Buchberger et al. 1994). In addition, a salt bridge that is formed by R34 and E369 in DnaK that is conserved in other Hsp70s regulates nucleotide exchange by preventing nucleotide release in the absence of GrpE, and opens up to release nucleotide when GrpE binds to the neighboring loop (▶; Buchberger et al. 1994). Salt bridges that are equivalent to the R34–E369 salt bridge of DnaK (Buchberger et al. 1994) are represented in both Hsc70 and PfHsp70-1 (▶). In addition, DnaK has two extra salt bridges that link subdomains IB and IIB that further regulate nucleotide exchange (Brehmer et al. 2001). On the other hand, both PfHsp70-1 and Hsc70 only have one such salt bridge each (▶).
Figure 6.
Identification of residues of PfHsp70-1 predicted to be important in nucleotide exchange. (A) Conservation of residues constituting interdomain loops and salt bridges in DnaK, Hsc70, and PfHsp70 and (B) three-dimensional images for the ATPase domains of DnaK, Hsc70, and PfHsp70 showing the loops and salt bridges crucial in nucleotide exchange. Residues in subdomain IB (red) form a highly conserved loop and salt bridge (green) that link subdomains IA and IB. The loop and salt bridge are both important for nucleotide exchange in DnaK (Buchberger et al. 1994). DnaK has two additional salt bridges that link subdomains IB and IIB (green). Hsc70 and PfHsp70 each have only one of these. An additional feature that confers specificity to the interaction of NEF with a particular Hsp70 is the GrpE signature loop, whose (yellow) tip (motif) is made up of highly divergent residues in Hsp70s (Brehmer et al. 2001). The predicted structures for the ATPase domains of human Hsc70 and PfHsp70 were generated by homology modeling (SWISS-MODEL, first approach mode) (Schwede et al. 2003) using the structure of bovine Hsc70 as a template (1YUW.pdb) (Jiang et al. 2005). The images of E. coli DnaK (1DKG.pdb), human Hsc70, and PfHsp70 were rendered using PyMOL (DeLano 2002). The figure was based on a previous analysis by Brehmer et al. (2001). (C) Residues of Hsc70 that interact with HspBP1 and Bag-1 (Sondermann et al. 2001; Shomura et al. 2005) together with their corresponding residues in PfHsp70 and DnaK are shown. Bag-1 contact sites are red; HspBP1 contact sites (blue) are highlighted; residues that are implicated as both Bag-1 and HspBP1 contact sites are shown in yellow background. (D) A top view of the three-dimensional structure of PfHsp70 ATPase domain showing the potential solvent exposure of residues that may have contact with nucleotide exchange factors: Bag-1 (red); HspBP1 (blue); and contact residues common to both NEFs (yellow). The predicted structure for the ATPase domain PfHsp70 was generated by homology modeling (SWISS-MODEL, first approach mode; Schwede et al. 2003) using the structure of bovine Hsc70 as a template (1YUW.pdb; Jiang et al. 2005). The image was rendered using PyMOL (DeLano 2002).
Hsp70s are classified as DnaK, HscA, or Hsc70 protein families according to their mechanism of interaction with NEFs (Brehmer et al. 2001). Key to this classification system are the salt bridges between subdomains IB and IIB and a segment that is referred to as the GrpE signature loop (▶; Brehmer et al. 2001). Within the GrpE signature motif is a substructure, the GrpE motif (▶; Brehmer et al. 2001). The GrpE motif in DnaK forms a loop that is essential for its interaction with GrpE (▶; Brehmer et al. 2001). Both PfHsp70-1 and Hsc70 have a GrpE motif that is structurally divergent from that of DnaK (▶). The fact that PfHsp70-1 has high sequence similarity to human Hsc70 in the critical GrpE motif section (▶; Brehmer et al. 2001) suggests that NEFs that interact with Hsc70 could also recognize PfHsp70-1. Based on this decisive feature, there is evidence that PfHsp70-1 falls into the category of cytosolic Hsp70s (▶; Brehmer et al. 2001; Sondermann et al. 2001). Structurally, PfHsp70-1 has salt bridges and motifs implicated in nucleotide exchange that are more identical to human Hsc70 than E. coli DnaK (▶).
Of the five Hsc70 residues that interact with both Bag-1 and HspBP1, PfHsp70-1 has four of these residues identical to corresponding residues in Hsc70 (▶). It should be noted that most of the residues implicated in Bag-1 and HspBP1 contact (Sondermann et al. 2001; Shomura et al. 2005) are not only conserved in PfHsp70-1, but they are also predicted to be solvent-exposed (▶), suggesting their accessibility for protein–protein interaction (Rajamani et al. 2004). It is therefore conceivable that nucleotide exchange in PfHsp70-1 could occur through the same mechanism as that of Hsc70, since most of the residues of PfHsp70-1 implicated in its interaction with NEF are similar to those of Hsc70. P. falciparum contains a GrpE homolog (PF11_0258). However, GrpE homologs of eukaryotic origin such as yeast are located in the mitochondria (Westermann et al. 1995). Therefore, since PfHsp70-1 is cytosolic, PfGrpE may not be its NEF.
Conclusion and future perspectives
P. falciparum displays a functionally and structurally diverse group of Hsp70 proteins. Although we have very little information on the role of these proteins, studies that have been conducted so far strongly suggest a pivotal role for Hsp70 proteins in the life cycle and pathogenicity of P. falciparum. It is clear that Hsp70 proteins play an important role in the translation and export of proteins into the apicoplast (Foth et al. 2003; Ramya et al. 2007b). Hsp70 proteins could be important for sustaining reactions in the apicoplast of the parasite. This finding begs for further research attention to establish the direct input of P. falciparum Hsp70s in this process. This is important since the apicoplast hosts metabolic pathways that are distinct from those of the host cell, making it a target for drug design (Ralph et al. 2001). Furthermore, cochaperones that are responsible for the modulation of the chaperone activities of the different Hsp70s in P. falciparum need to be identified. For example, the identification of cochaperones of PfHsp70-1 is long overdue since the role of this protein in the life cycle of the parasite has been highlighted. Perhaps most interesting is the possible development of drugs that target Hsp70 function in P. falciparum. However, because of the high conservation between human Hsp70s and P. falciparum Hsp70s, the design of potential antimalarials targeting P. falciparum Hsp70s is a real challenge. A more feasible approach might be the design of drugs that interfere with the Hsp70 interactome in P. falciparum. An example of this will be to identify inhibitors that may selectively interfere with the possible partnership between PfHsp70-1 and PfHsp90, without interfering with the functional partnership between host cell Hsp70 and Hsp90 proteins.
Acknowledgments
This work was funded in part by a Wellcome Trust Grant (066705; United Kingdom), a National Research Foundation Grant (NRF; South Africa), and a Medical Research Council Grant (MRC; South Africa), all awarded to G.L.B. A.S. was awarded an NRF Ph.D. Grant-Holder Bursary and a Ph.D. Scholarship from the Cannon Collins Educational Trust of Southern Africa.
Footnotes
Reprint request to: Gregory L. Blatch, Department of Biochemistry, Microbiology and Biotechnology, Rhodes University, Grahamstown 6140, South Africa; e-mail: g.blatch@ru.ac.za; fax: 27-46-622-3984.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.072918107.
References
- Asea A., Rehli, M., Kabingu, E., Boch, J.A., Bare, O., Auron, P.E., Stevenson, M.A., and Calderwood, S.K. 2002. Novel signal transduction pathway utilized by extracellular HSP70: Role of toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277 15028–15034. [DOI] [PubMed] [Google Scholar]
- Banumathy G., Singh, V., Pavithra, S.R., and Tatu, U. 2002. Host chaperones are recruited in membrane bound complexes by Plasmodium falciparum . J. Biol. Chem. 277 3902–3912. [DOI] [PubMed] [Google Scholar]
- Banumathy G., Singh, V., Pavithra, S.R., and Tatu, U. 2003. Heat shock protein 90 is essential for Plasmodium falciparum growth in human erythrocytes. J. Biol. Chem. 278 18336–18345. [DOI] [PubMed] [Google Scholar]
- Barale J.C., Candelle, D., Attal-Bonnefoy, G., Dehoux, P., Bonnefoy, S., Ridley, R., Pereira da Silva, L., and Langsley, G. 1997. Plasmodium falciparum AARP1, a giant protein containing repeated motifs rich in asparagine and aspartate residues, is associated with the infected erythrocyte membrane. Infect. Immun. 65 3003–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch D.I., Gormley, J.A., Ma, C., Howard, R.J., and Pasloske, B.L. 1996. Plasmodium falciparum erythrocyte membrane protein 1 is a parasitized erythrocyte receptor for adherence to CD36, thrombospondin, and intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. 93 3497–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M.F., Hofmann, S., Neupert, W., and Brunner, M. 2000. Protein translocation into mitochondria: The role of TIM complexes. Trends Cell Biol. 10 25–31. [DOI] [PubMed] [Google Scholar]
- Behr C., Sarthou, J.L., Rogier, C., Trape, J.F., Dat, M.H., Michel, J.C., Aribot, G., Dieye, A., Claverie, J.M., and Druihle, P. 1992. Antibodies and reactive T cells against the malaria heat-shock protein Pf72/hsp70-1 and derived peptides in individuals continuously exposed to Plasmodium falciparum . J. Immunol. 149 3321–3330. [PubMed] [Google Scholar]
- Bercovich B., Stancovski, I., Mayer, A., Blumenfeld, N., Laszlo, A., Schwartz, A.L., and Ciechanover, A. 1997. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J. Biol. Chem. 272 9002–9010. [DOI] [PubMed] [Google Scholar]
- Biswas S. and Sharma, Y.D. 1994. Enhanced expression of Plasmodium falciparum heat shock protein PFHSP70-1 at higher temperatures and parasite survival. FEMS Microbiol. Lett. 124 425–430. [DOI] [PubMed] [Google Scholar]
- Botha M., Pesce, E.-R., and Blatch, G.L. 2007. The Hsp40 proteins of Plasmodium falciparum and other apicomplexa: Regulating chaperone power in the parasite and host. Int. J. Biochem. Cell Biol. doi: 1016/j.biocel.2007.02.011. [DOI] [PubMed]
- Brehmer D., Rüdiger, S., Gässler, C.S., Klostermeier, D., Packschies, L., Reinstein, J., Mayer, M.P., and Bukau, B. 2001. Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat. Struct. Biol. 8 427–432. [DOI] [PubMed] [Google Scholar]
- Buchberger A., Schröder, H., Buttner, M., Valencia, A., and Bukau, B. 1994. A conserved loop in the ATPase domain of the DnaK chaperone is essential for stable binding of GrpE. Nat. Struct. Biol. 1 95–101. [DOI] [PubMed] [Google Scholar]
- Chou C.-C., Forouhar, F., Yeh, Y.-H., Shr, H.-L., Wang, C., and Hsiao, C.-D. 2003. Crystal structure of the C-terminal 10-kDa subdomain of Hsc70. J. Biol. Chem. 278 30311–30316. [DOI] [PubMed] [Google Scholar]
- Cowen L.E. and Lindquist, S. 2005. Hsp90 potentiates the rapid evolution of new traits: Drug resistance in diverse fungi. Science 309 2185–2189. [DOI] [PubMed] [Google Scholar]
- Cvoro A., Dundjerski, J., Trajkovic, D., and Matic, G. 1999. The level and phosphorylation of Hsp70 in the rat liver cytosol after adrenalectomy and hyperthermia. Cell Biol. Int. 23 313–320. [DOI] [PubMed] [Google Scholar]
- DeLano W.L. 2002. The PyMOL molecular graphics system. DeLano Scientific, San Carlos, CA.
- Demand J., Lüders, J., and Höhfeld, J. 1998. The carboxy-terminal domain of Hsc70 provides binding sites for a distinct set of chaperone cofactors. Mol. Cell. Biol. 18 2023–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski J.M., Carruthers, V.B., and Sibley, L.D. 1997. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii . Mol. Microbiol. 26 163–173. [DOI] [PubMed] [Google Scholar]
- Dragovic Z., Broadle, S.A., Shomura, Y., Bracher, A., and Hartl, F.U. 2006. Molecular chaperones of the Hsp110 family act as nucleotide exchange factors of Hsp70s. EMBO J. 25 2519–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton D.P., Kaneko, Y., and Subjeck, J.R. 2000. The Hsp110 and Grp1 70 stress proteins: Newly recognized relatives of the Hsp70s. Cell Stress Chaperones 5 276–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. 1987. Proteins as molecular chaperones. Nature 328 378–379. [DOI] [PubMed] [Google Scholar]
- Feder M.E. and Hofmann, G.E. 1999. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 61 243–282. [DOI] [PubMed] [Google Scholar]
- Flaherty K.M., DeLuca-Flaherty, C., and McKay, D.B. 1990. Three-dimensional structure of the ATPase fragment of a 70-K heat shock cognate protein. Nature 346 623–628. [DOI] [PubMed] [Google Scholar]
- Foth B.J., Ralph, S.A., Tonkin, C.J., Struck, N.S., Fraunholz, M., Roos, D.S., Cowman, A.F., and McFadden, G.I. 2003. Dissecting apicoplast targeting in the malaria parasite Plasmodium falciparum . Science 299 705–708. [DOI] [PubMed] [Google Scholar]
- Gambill B.D., Voos, W., Kang, P.J., Miao, B., Langer, T., Craig, E.A., and Pfanner, N. 1993. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J. Cell Biol. 123 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gässler S.C., Buchberger, A., Laufen, T., Mayer, M.P., Schröder, H., Valencia, A., and Bukau, B. 1998. Mutations in the DnaK chaperone affecting interaction with the DnaJ co-chaperone. Biochemistry 95 15229–15234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M.J. 1999. Role and regulation of the ER chaperone BiP. Semin. Cell Dev. Biol. 10 465–472. [DOI] [PubMed] [Google Scholar]
- Han W. and Christen, P. 2001. Mutations in the interdomain linker region of DnaK abolish the chaperone action of the DnaK/DnaJ/GrpE system. FEBS Lett. 497 55–58. [DOI] [PubMed] [Google Scholar]
- Han W. and Christen, P. 2003. Mechanism of the targeting action of DnaJ in the DnaK molecular chaperone system. J. Biol. Chem. 278 19038–19043. [DOI] [PubMed] [Google Scholar]
- Harrison C.J., Hayer-Hartle, M., Di Liberto, M., Hartl, F.U., and Kuriyan, J. 1997. Crystal structure of the nucleotide exchange factor GrpE bound to the ATPase domain of the molecular chaperone DnaK. Science 276 431–435. [DOI] [PubMed] [Google Scholar]
- Höhfeld J. and Jentsch, S. 1997. GrpE-like regulation of the Hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 16 6209–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhfeld J., Minami, Y., and Hartl, F.-U. 1995. Hip, a novel cochaperone involved in the eukaryotic Hsc70/Hsp40 reaction cycle. Cell 83 589–598. [DOI] [PubMed] [Google Scholar]
- Huang H.D., Lee, T.Y., Tseng, S.W., and Horng, J.T. 2005a. KinasePhos: A Web tool for identifying protein kinase-specific phosphorylation sites. Nucleic Acids Res. 33 W226–W229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.D., Lee, T.Y., Tseng, S.W., Wu, L.C., Horng, J.T., and Tsou, A.P. 2005b. Incorporating hidden Markov model for identifying protein kinase-specific phosphorylation sites. J. Comput. Chem. 26 1032–1041. [DOI] [PubMed] [Google Scholar]
- Jiang J., Prasad, K., Lafer, E.M., and Sousa, R. 2005. Structural basis of interdomain communication in the hsc70 chaperone. Mol. Cell 20 513–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.L. and Craig, E.A. 1997. Protein folding in vivo: Unraveling complex pathways. Cell 90 201–220. [DOI] [PubMed] [Google Scholar]
- Joshi B., Biswas, S., and Sharma, Y.D. 1992. Effect of heat-shock on Plasmodium falciparum viability, growth and expression of the heat-shock protein “PFHSP70-1” gene. FEBS Lett. 312 91–94. [DOI] [PubMed] [Google Scholar]
- Kabani M., McLellan, C., Raynes, D.A., Guerriero, V., and Brodsky, J.L. 2002. HspBP1, a homologue of the yeast Fes1 and Sls1 proteins, is an Hsc-70 nucleotide exchange factor. FEBS Lett. 531 339–342. [DOI] [PubMed] [Google Scholar]
- Kappes B., Suetterlin, B.W., Hofer-Warbinek, R., Humar, R., and Franklin, R.M. 1993. Two major phosphoproteins of Plasmodium falciparum are heat shock proteins. Mol. Biochem. Parasitol. 59 83–94. [DOI] [PubMed] [Google Scholar]
- Karnumaweera N.D., Grau, G.E., Gamage, P., Carter, R., and Mendis, K.N. 1992. Dynamics of fever and serum levels of tumor necrosis factor are closely associated during clinical paroxysms in Plasmodium vivax malaria. Proc. Natl. Acad. Sci. 89 3200–3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. and Zheng, H. 1992. Nucleotide sequence of a Plasmodium falciparum stress protein with similarity to mammalian 78-kDa glucose-regulated protein. Mol. Biochem. Parasitol. 56 353–356. [DOI] [PubMed] [Google Scholar]
- Kumar N., Zhao, Y., Graves, P., Perez Folgar, J., Maloy, L., and Zheng, H. 1990. Human immune response directed against Plasmodium falciparum heat shock-related proteins. Infect. Immun. 58 1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Koski, G., Harada, M., Aikawa, M., and Zheng, H. 1991. Induction and localization of Plasmodium falciparum stress proteins related to the heat shock protein 70 family. Mol. Biochem. Parasitol. 48 47–58. [DOI] [PubMed] [Google Scholar]
- LaCount D.J., Vignali, M., Chettier, R., Phansalkar, A., Bell, R., Hesselberth, J.R., Schoenfeld, L.W., Ota, I., Sahasrabudhe, S., Kurschner, C., et al. 2005. A protein interaction network of the malaria parasite Plasmodium falciparum . Nature 438 103–107. [DOI] [PubMed] [Google Scholar]
- Langreth S.J., Jensen, J.B., Reese, R.T., and Trager, W. 1978. Fine structure of human malaria in vitro. J. Protozool. 25 443–452. [DOI] [PubMed] [Google Scholar]
- Lanzer M., Wickert, H., Krohne, G., Vincensini, L., and Braun Breton, C. 2006. Maurer's clefts: A novel multi-functional organelle in the cytoplasm of Plasmodium falciparum-infected erythrocytes. Int. J. Parasitol. 36 23–36. [DOI] [PubMed] [Google Scholar]
- Lee A.S. 1987. Coordinated regulation of a set of genes by glucose and calcium ionophores in mammalian cells. Trends Biochem. Sci. 12 20–23. [Google Scholar]
- Liberek K., Skowyra, D., Zylicz, M., Johnson, C., and Georgopoulos, C. 1991. The Escherichia coli DnaK chaperone, the 70-kDa heat shock protein eukaryotic equivalent, changes conformation upon ATP hydrolysis, thus triggering its dissociation from a bound target protein. J. Biol. Chem. 266 14491–14496. [PubMed] [Google Scholar]
- Maresca B. and Kobayashi, G.S. 1994. Hsp70 in parasites: As an inducible protective protein and as an antigen. Experientia 50 1067–1074. [DOI] [PubMed] [Google Scholar]
- Marti M., Good, R.T., Rug, M., Knuepfer, E., and Cowman, A.F. 2004. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306 1930–1933. [DOI] [PubMed] [Google Scholar]
- Matambo T.S., Odununga, O.O., Boshoff, A., and Blatch, G.L. 2004. Overproduction, purification, and characterization of the Plasmodium falciparum heat shock protein 70. Prot. Expr. Purif. 33 214–222. [DOI] [PubMed] [Google Scholar]
- Mayer M.P., Schröder, H., Rüdiger, S., Paal, K., Laufen, T., and Bukau, B. 2000. Multistep mechanism of substrate binding determines chaperone activity of Hsp70. Nat. Struct. Biol. 7 586–593. [DOI] [PubMed] [Google Scholar]
- McCarty J.S. and Walker, G.C. 1991. DnaK as a thermometer: Threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc. Natl. Acad. Sci. 88 9513–9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport G.R. 1991. Heat shock proteins as vaccine candidates. Semin. Immunol. 3 17–24. [PubMed] [Google Scholar]
- Nicoll W.S., Botha, M., McNamara, C., Schlange, M., Pesce, E.R., Boshoff, A., Ludewig, M.H., Zimmermann, R., Cheetham, M.E., Chapple, J.P., et al. 2007. Cytosolic and ER J-domains of mammalian and parasitic origin can functionally interact with DnaK. Int. J. Biochem. Cell Biol. 39 736–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmalan N., Sims, P.F.G., and Hyde, J.E. 2004. Quantitative proteomics of the human malaria parasite Plasmodium falciparum and its application to studies of development and inhibition. Mol. Microbiol. 52 1187–1199. [DOI] [PubMed] [Google Scholar]
- Nollen E.A., Kabakov, A.E., Brunsting, J.F., Kanon, B., Höhfeld, J., and Kampinga, H.H. 2001. Modulation of in vivo HSP70 chaperone activity by Hip and Bag-1. J. Biol. Chem. 276 4677–4682. [DOI] [PubMed] [Google Scholar]
- Nyalwidhe J. and Lingelbach, K. 2006. Proteases and chaperones are the most abundant proteins in the parasitophorous vacuole of Plasmodium falciparum-infected erythrocytes. Proteomics 6 1563–1573. [DOI] [PubMed] [Google Scholar]
- Olson C.L., Nadeau, K.C., Sullivan, M.A., Winquist, A.G., Donelson, J.E., Walsh, C.T., and Engman, D.M. 1994. Molecular and biochemical comparison of the 70-kDa heat shock proteins of Trypanosoma cruzi . J. Biol. Chem. 269 3868–3874. [PubMed] [Google Scholar]
- Page R.D. 1996. TreeView: An application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12 357–358. [DOI] [PubMed] [Google Scholar]
- Pavithra S.R., Banumathy, G., Joy, O., Singh, V., and Tatu, U. 2004. Recurrent fever promotes Plasmodium falciparum development in human erythrocytes. J. Biol. Chem. 279 46692–46699. [DOI] [PubMed] [Google Scholar]
- Pelham H.R.B. 1989. Heat shock and the sorting of luminal ER proteins. EMBO J. 8 1445–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson M.G., Crewther, P.E., Thompson, J.K., Corcoran, L.M., Coppel, R.L., Brown, G.V., Anders, R.F., and Kemp, D.J. 1988. A second antigenic heat shock protein of Plasmodium falciparum. DNA 7 71–78. [DOI] [PubMed] [Google Scholar]
- Rajamani D., Thiel, S., Vajda, S., and Camacho, C.J. 2004. Anchor residues in protein–protein interactions. Proc. Natl. Acad. Sci. 101 11287–11292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph S.A., D'Ombrain, M.C., and McFadden, G.I. 2001. The apicoplast as an antimalarial drug target. Resist. Updat. 4 145–151. [DOI] [PubMed] [Google Scholar]
- Ramya T.N.C., Surolia, N.N., and Surolia, A. 2006. 15-Deoxyspergualin modulates Plasmodium falciparum heat shock protein function. Biochem. Biophys. Res. Commun. 348 585–592. [DOI] [PubMed] [Google Scholar]
- Ramya T.N., Surolia, N., and Surolia, A. 2007a. 15-Deoxyspergualin inhibits eukaryotic protein synthesis through eIF2a phosphorylation. Biochem. J. 401 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramya T.N.C., Karmodiya, K., Surolia, A., and Surolia, N. 2007b. 15-Deoxyspergualin primarily targets the trafficking of apicoplast proteins in Plasmodium falciparum . J. Biol. Chem. 282 6388–6397. [DOI] [PubMed] [Google Scholar]
- Robbins J., Dilworth, S.M., Laskey, R.A., and Dingwall, C. 1991. Two interdependent basic domains in nucleoplasmin nuclear targeting sequence: Identification of a class of bipartite nuclear targeting sequence. Cell 64 615–623. [DOI] [PubMed] [Google Scholar]
- Rüdiger S., Germeroth, L., Schneider-Mergener, J., and Bukau, B. 1997. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16 1501–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell R., Jordan, R., and McMacken, R. 1998. Kinetic characterization of the ATPase cycle of the DnaK molecular chaperone. Biochemistry 37 596–607. [DOI] [PubMed] [Google Scholar]
- Sanglard D., Ischer, F., Marchetti, O., Entenza, J., and Bille, J. 2003. Calcineurin A of Candida albicans: Involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48 959–976. [DOI] [PubMed] [Google Scholar]
- Sargeant T.J., Marti, M., Caler, E., Carlton, J.M., Simpson, K., Speed, T.P., and Cowman, A.F. 2006. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 7 R12. doi: 10.1186/gb-2006-7-2-r12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede T., Kopp, J., Guex, N., and Peitsch, M.C. 2003. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 31 3381–3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma Y.D. 1992. Structure and possible function of heat-shock proteins in Plasmodium falciparum . Comp. Biochem. Physiol. B 102 437–444. [DOI] [PubMed] [Google Scholar]
- Shomura Y., Dragovic, Z., Chang, H.C., Tzvetkov, N., Young, J.C., Brodsky, J.L., Guerriero, V., Hartl, F.U., and Bracher, A. 2005. Regulation of Hsp70 function by HspBP1: Structural analysis reveals an alternate mechanism for Hsp70 nucleotide exchange. Mol. Cell 17 367–379. [DOI] [PubMed] [Google Scholar]
- Shonhai A., Boshoff, A., and Blatch, G.L. 2005. Plasmodium falciparum heat shock protein 70 is able to suppress the thermosensitivity of an Escherichia coli DnaK mutant strain. Mol. Genet. Genomics 4 70–78. [DOI] [PubMed] [Google Scholar]
- Silberg J.J. and Vickery, L.E. 2000. Kinetic characterization of the ATPase cycle of the molecular chaperone Hsc66 from Escherichia coli . J. Biol. Chem. 275 7779–7786. [DOI] [PubMed] [Google Scholar]
- Šlapeta J. and Keithly, J.S. 2004. Cryptosporidium parvum mitochondrial-type Hsp70 targets homologous and heterologous mitochondria. Eukaryot. Cell 3 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soete M., Camus, D., and Dubremetz, J.F. 1994. Experimental induction of bradyzoite-specific antigen expression and cyst formation by the RH strain of Toxoplasma gondii in vitro . Exp. Parasitol. 78 361–370. [DOI] [PubMed] [Google Scholar]
- Sondermann H., Scheufler, C., Schneider, C., Höhfeld, J., Hartl, F.U., and Moarefi, I. 2001. Structure of a Bag/Hsc70 complex: Convergent functional evolution of Hsp70 nucleotide exchange factors. Science 291 1553–1557. [DOI] [PubMed] [Google Scholar]
- Song Y., Wu, Y.X., Jung, G., Tutar, Y., Eisenberg, E., Greene, L.E., and Masison, D.C. 2005. Role for Hsp70 chaperone in Saccharomyces cerevisiae prion seed replication. Eukaryot. Cell 4 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh W.C., Burkholder, W.F., Lu, C.Z., Zhao, X., Gottesman, M.E., and Gross, C.A. 1998. Interaction of the Hsp70 molecular chaperone, DnaK, with cochaperone DnaJ. Proc. Natl. Acad. Sci. 95 15223–15228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh W.C., Lu, C.Z., and Gross, C.A. 1999. Structural features required for the interaction of the Hsp70 molecular chaperone DnaK with its cochaperone DnaJ. J. Biol. Chem. 274 30534–30539. [DOI] [PubMed] [Google Scholar]
- Surolia N. and Padmanaban, G. 1991. Chloroquine inhibits heme-dependent protein synthesis in Plasmodium falciparum . Proc Natl. Acad. Sci. 88 4786–4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steel G.J., Fullerton, D.M., Tyson, J.R., and Stirling, C.J. 2004. Coordinated activation of Hsp70 chaperones. Science 303 98–101. [DOI] [PubMed] [Google Scholar]
- Szabo A., Langer, T., Schröder, H., Flanagan, J., Bukau, B., and Hartl, F.U. 1994. The ATP hydrolysis-dependent reaction cycle of the Escherichia coli Hsp70 system—DnaK, DnaJ and GrpE. Proc. Natl. Acad. Sci. 91 10345–10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieux I., Baines, I., Mossakowska, M., and Ward, G.E. 1998. Actin-binding proteins of invasive malaria parasites and the regulation of actin polymerization by a complex of 32/34-kDa proteins associated with heat shock protein 70kDa. Mol. Biochem. Parasitol. 93 295–308. [DOI] [PubMed] [Google Scholar]
- Thiel L. 2005. Malaria Initiative for Africa (SAMI) Report. African Centre for Gene Technologies, University of Pretoria, Pretoria, South Africa.
- Thompson J., Higgins, D., and Gibson, T. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udomsangpetch R., Pipitaporn, B., Silamut, K., Pinches, R., Kyes, S., Looareesuwan, S., Newbold, C., and While, N.J. 2002. Febrile temperatures induce cytoadherence of ring-stage Plasmodium falciparum-infected erythrocytes. Proc. Natl. Acad. Sci. 99 11825–11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincensini L., Richert, S., Blisnick, T., Van Dorsselaer, A., Leize-Wagner, E., Rabilloud, T., and Braun Breton, C. 2005. Proteomic analysis identifies novel proteins of the Maurer's clefts, a secretory compartment delivering Plasmodium falciparum proteins to the surface of its host cell. Mol. Cell. Proteomics 4 582–593. [DOI] [PubMed] [Google Scholar]
- Vogel M., Bukau, B., and Mayer, M.P. 2006a. Allosteric regulation of Hsp70 chaperones by a proline switch. Mol. Cell 21 359–367. [DOI] [PubMed] [Google Scholar]
- Vogel M., Mayer, M.P., and Bukau, B. 2006b. Allosteric regulation of Hsp70 chaperones involves a conserved interdomain linker. J. Biol. Chem. 281 38705–38711. [DOI] [PubMed] [Google Scholar]
- Wadhwa R., Taira, K., and Kaul, S.C. 2002. An Hsp70 family chaperone, mortalin/mtHsp70/PBP74/Grp75: What, when, and where? Cell Stress Chaperones 7 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.-F., Chang, J., and Wang, C. 1993. Identification of the peptide binding domain of hsc70. 18-Kilodalton fragment located immediately after ATPase domain is sufficient for high affinity binding. J. Biol. Chem. 268 26049–26051. [PubMed] [Google Scholar]
- Watanabe J. 1997. Cloning and chacterization of heat shock protein DnaJ homologues from Plasmodium falciparum and comparison with ring infected erythrocyte surface antigen. Mol. Biochem. Parasitol. 88 253–258. [DOI] [PubMed] [Google Scholar]
- Weber J.L., Lyon, J.A., Wolff, R.H., Hall, T., Lowell, G.H., and Chulay, J.D. 1988. Primary structure of a Plasmodium falciparum malaria antigen located at the merozoite surface and within the parasitophorous vacuole. J. Biol. Chem. 263 11421–11425. [PubMed] [Google Scholar]
- Westermann B., Prip-Buus, C., Neupert, W., and Schwarz, E. 1995. The role of the GrpE homologue, Mge1p, in mediating protein import and protein folding in mitochondria. EMBO J. 14 3452–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesgigl M. and Clos, J. 2001. Heat shock protein 90 homeostasis controls stage differentiation in Leishmania donovani . Mol. Biol. Cell 12 3307–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser M.F. and Plitt, B. 1987. Plasmodium berghei, P. chabaudi, P. falciparum: Similarities in phosphoproteins and protein kinase activities and their stage specific expression. Exp. Parasitol. 64 328–335. [DOI] [PubMed] [Google Scholar]
- 2005. World malaria report. http://www.who.int/malaria/me_reports.html.
- Yalovsky S., Paulsen, H., Michaeli, D., Chitnis, P.R., and Nechushtai, R. 1992. Involvement of a chloroplast HSP70 heat shock protein in the integration of a protein (light-harvesting complex protein precursor) into the thylakoid membrane. Proc. Natl. Acad. Sci. 89 5616–5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Zhao, X., Burkholder, W.F., Gragerov, A., Ogata, C.M., Gottesman, M.E., and Hendrickson, W.A. 1996. Structural analysis of substrate binding by the molecular chaperone DnaK. Science 272 1606–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zügel U. and Kaufmann, S.H.E. 1999. Role of heat shock proteins in protection and pathogenesis of infectious diseases. Clin. Microbiol. Rev. 12 19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]