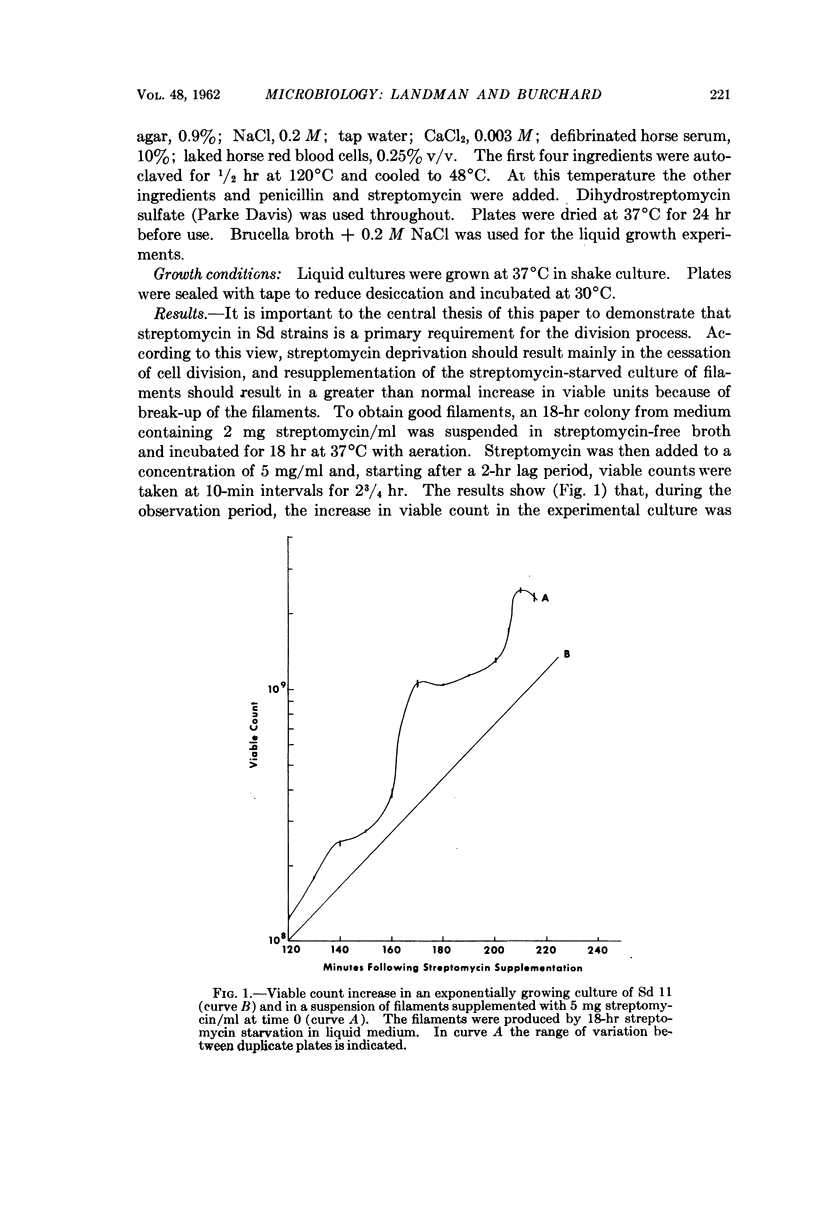
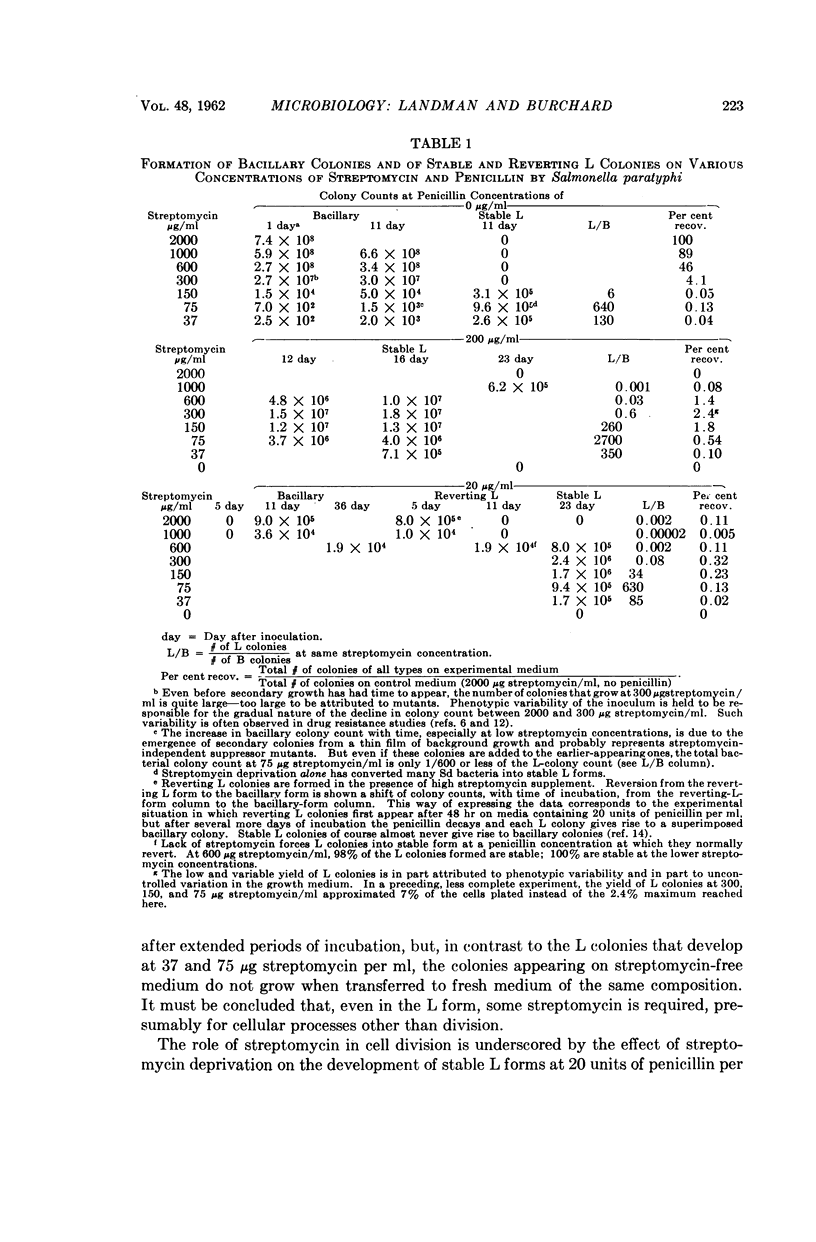
Full text
PDF


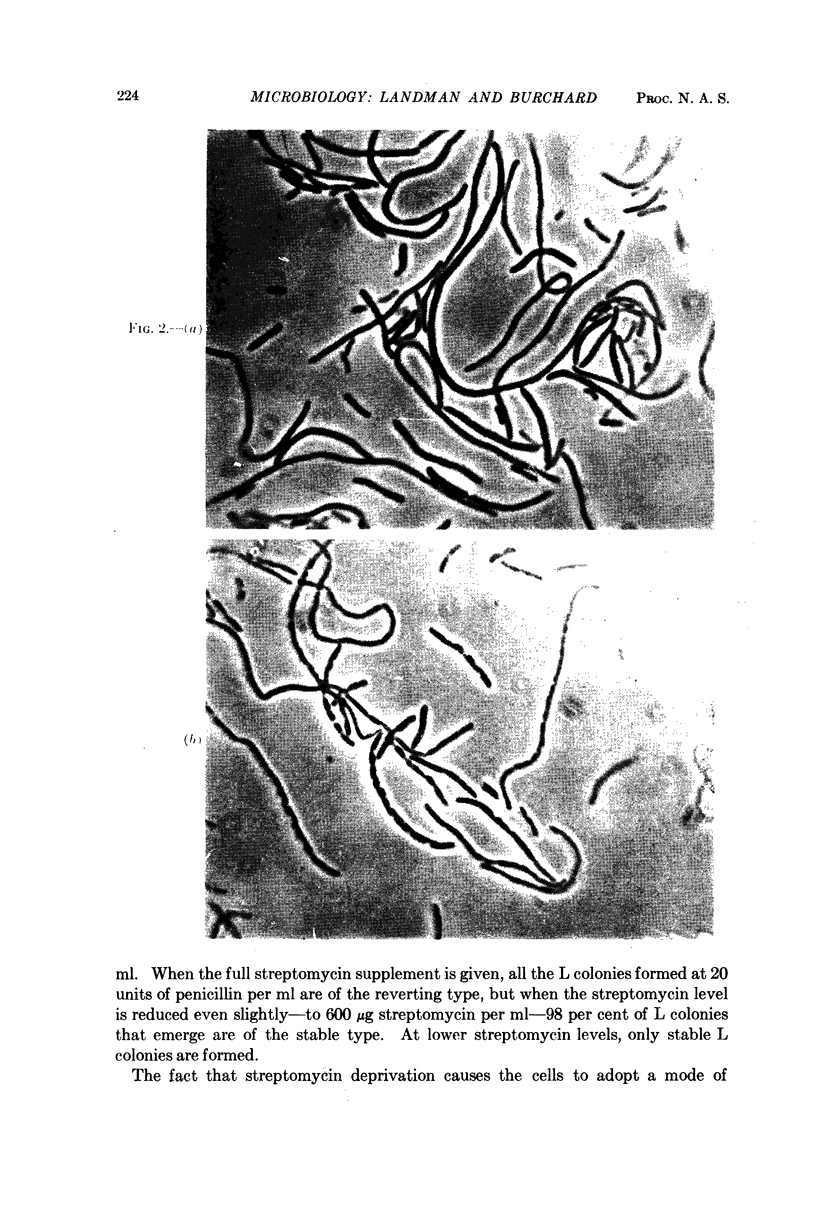
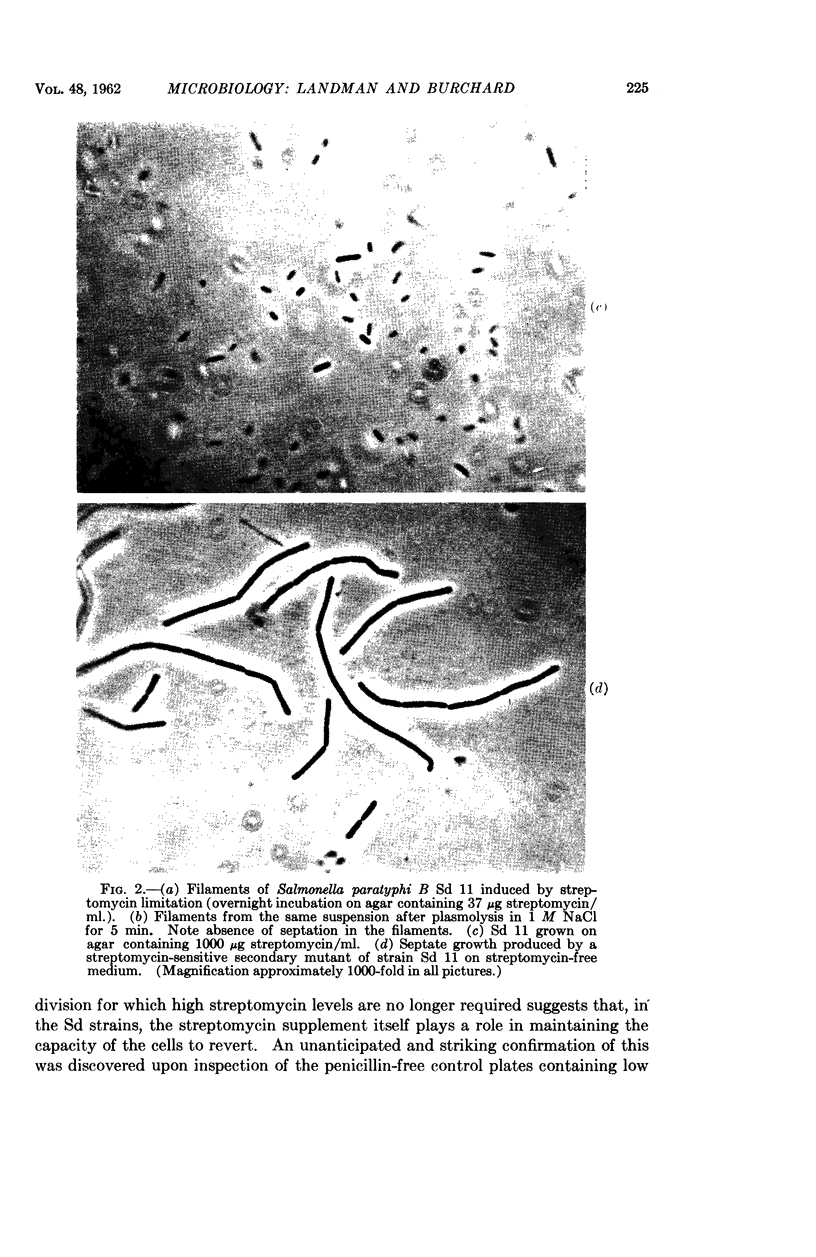



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTENBERN R. A., LANDMAN O. E. Growth of L-forms of Proteus mirabilis in liquid media. J Bacteriol. 1960 Apr;79:510–518. doi: 10.1128/jb.79.4.510-518.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ANAND N., DAVIS B. D. Damage by streptomycin to the cell membrane of Escherichia coli. Nature. 1960 Jan 2;185:22–23. doi: 10.1038/185022a0. [DOI] [PubMed] [Google Scholar]
- BROCK T. D., BROCK M. L. Effect of novobiocin on permeability of Escherichia coli. Arch Biochem Biophys. 1959 Nov;85:176–185. doi: 10.1016/0003-9861(59)90461-8. [DOI] [PubMed] [Google Scholar]
- COOPER P. D. Site of action of radiopenicillin. Bacteriol Rev. 1956 Mar;20(1):28–48. doi: 10.1128/br.20.1.28-48.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE LEY J., DOCHY R. On the localisation of oxidase systems in Acetobacter cells. Biochim Biophys Acta. 1960 May 20;40:277–289. doi: 10.1016/0006-3002(60)91352-4. [DOI] [PubMed] [Google Scholar]
- EAGLE H., FLEISCHMAN R., LEVY M. Development of increased bacterial resistance to antibiotics. I. Continuous spectrum of resistance to penicillin, chloramphenicol, and streptomycin. J Bacteriol. 1952 May;63(5):623–638. doi: 10.1128/jb.63.5.623-638.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENGELBERG H., ARTMAN M. Studies on streptomycin dependent bacteria: effect of growth in limiting amounts of streptomycin on respiration and fermentation of a streptomycin dependent mutant of Escherichia coli. Biochim Biophys Acta. 1961 Mar 4;47:553–560. doi: 10.1016/0006-3002(61)90550-9. [DOI] [PubMed] [Google Scholar]
- GHOSH A., CHARALAMPOUS F., SISON Y., BORER R. Metabolic function of myo-inositol. I. Cytological and chemical alterations in yeast resulting from inositol deficiency. J Biol Chem. 1960 Sep;235:2522–2528. [PubMed] [Google Scholar]
- HANCOCK R. Reduced oxidative activities in Escherichia coli and Bacillus megaterium in relation to other changes during inhibition of growth by streptomycin. J Gen Microbiol. 1961 Jul;25:429–440. doi: 10.1099/00221287-25-3-429. [DOI] [PubMed] [Google Scholar]
- LANDMAN O. E., GINOZA H. S. Genetic nature of stable L forms of Salmonella paratyphi. J Bacteriol. 1961 Jun;81:875–886. doi: 10.1128/jb.81.6.875-886.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARK K. G. Abnormal growth induced by penicillin in a strain of Alcaligenes fecalis. Can J Microbiol. 1958 Apr;4(2):165–177. doi: 10.1139/m58-017. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., ST CLAIR J. Protoplasts and L-type growth of Escherichia coli. J Bacteriol. 1958 Feb;75(2):143–160. doi: 10.1128/jb.75.2.143-160.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., FRANCOMBE W. H., MAYALL B. H. The effect of penicillin on the structure of staphylococcal cell walls. Can J Microbiol. 1959 Dec;5:641–648. doi: 10.1139/m59-078. [DOI] [PubMed] [Google Scholar]
- McQUILLEN K. The bacterial surface. IV. Effect of streptomycin on the electrophoretic mobility of Escherichia coli and Staphylococcus aureus. Biochim Biophys Acta. 1951 May;7(1):54–60. doi: 10.1016/0006-3002(51)90005-4. [DOI] [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK J. T., STROMINGER J. L. Mode of action of penicillin. Science. 1957 Jan 18;125(3238):99–101. doi: 10.1126/science.125.3238.99. [DOI] [PubMed] [Google Scholar]
- PRESTIDGE L. S., PARDEE A. B. Induction of bacterial lysis by penicillin. J Bacteriol. 1957 Jul;74(1):48–59. doi: 10.1128/jb.74.1.48-59.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine T. F., Jr, Finland M. Observations on Bacteria Sensitive to, Resistant to, and Dependent upon Streptomycin. J Bacteriol. 1948 Aug;56(2):207–218. doi: 10.1128/jb.56.2.207-218.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFFER P. Recherches sur le métabolisme bactérien des cytochromes et des porphyrines. IV. Effets de la carence en streptomycine sur des mutants bactériens streptomycino-exigeants. Biochim Biophys Acta. 1952 Nov;9(5):563–571. doi: 10.1016/0006-3002(52)90207-2. [DOI] [PubMed] [Google Scholar]
- SMITH P. H., OGINSKY E. L., UMBREIT W. W. The action of streptomycin; the metabolic properties of resistant and dependent strains. J Bacteriol. 1949 Dec;58(6):761–767. doi: 10.1128/jb.58.6.761-767.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STORCK R., WACHSMAN J. T. Enzyme localization in Bacillus megaterium. J Bacteriol. 1957 Jun;73(6):784–790. doi: 10.1128/jb.73.6.784-790.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZYBALSKI W., BRYSON V. Genetic studies on microbial cross resistance to toxic agents. I. Cross resistance of Escherichia coli to fifteen antibiotics. J Bacteriol. 1952 Oct;64(4):489–499. doi: 10.1128/jb.64.4.489-499.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD J. R., MADOFF S., DIENES L. In vitro sensitivity of some bacteria, their L forms and pleuropneumonia-like organisms to antibiotics. Proc Soc Exp Biol Med. 1958 Jan;97(1):132–135. doi: 10.3181/00379727-97-23667. [DOI] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. I. Transfer of resistance factors by conjugation. J Bacteriol. 1961 May;81:669–678. doi: 10.1128/jb.81.5.669-678.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDEL W., FRANK H., MARTIN H. H. The rigid layer of the cell wall of Escherichia coli strain B. J Gen Microbiol. 1960 Feb;22:158–166. doi: 10.1099/00221287-22-1-158. [DOI] [PubMed] [Google Scholar]