Abstract
The E-26 transforming specific (ETS)-related gene TEL, also known as ETV6, is involved in a large number of chromosomal rearrangements associated with leukemia and congenital fibrosarcoma. The encoded protein contains two functional domains: a helix–loop–helix (HLH) domain (also known as pointed domain) located at the N terminus and a DNA-binding domain located at the C terminus. The HLH domain is involved in protein–protein interaction with itself and other members of the ETS family of transcription factors such as FLI1. TEL is a transcription factor, and we and others have shown that it is a repressor of gene expression. To understand further the role of TEL in the cell, we have used an in vivo interaction system to identify proteins that interact with TEL. We show that a protein, UBC9, interacts specifically with TEL in vitro and in vivo. UBC9 is a member of the family of ubiquitin-conjugating enzymes. These enzymes usually are involved in proteosome-mediated degradation; however, our data suggest that interaction of TEL with UBC9 does not lead to TEL degradation. Our studies show that UBC9 binds to TEL exclusively through the HLH domain of TEL. We also show that TEL expressed as fusion to the DNA-binding domain of Gal4 completely represses a Gal4-responsive promoter, but that the coexpression of UBC9 in the same system restores the activity of the promoter. Targeted point mutation of conserved amino acids in UBC9 essential for enzymatic ubiquitination of proteins does not affect interaction nor transcriptional activity. Based on our data, we conclude that UBC9 physically interacts with TEL through the HLH domain and that the interaction leads to modulation of the transcription activity of TEL.
TEL, also known as ETV6, is involved in a large number of chromosomal rearrangements associated with leukemia and congenital fibrosarcoma (1–7). TEL contains two domains: a helix–loop–helix (HLH) domain at the N terminus and a DNA-binding domain [E-26 transforming specific (ETS) domain] at the C terminus. Both domains are conserved in other ETS proteins. The HLH domain is involved in protein–protein interaction, leading to the formation of either a homodimer or heterodimers with other ETS proteins, such as FLI1 (8, 9). TEL is a transcription regulator, and we showed that it is a repressor of gene transcription (6). TEL is expressed in several tissues, but its precise role is not known. The gene often is deleted in patients with leukemia (10). Homozygous deletion of the gene by targeted mutation leads to embryonic death at days E10.5–11.5 because of defective yolk sac angiogenesis (11). Recent studies showed that TEL is the first transcription factor required specifically for hematopoiesis within the bone marrow (12) and support the critical importance of this protein in hematopoiesis.
The breakpoints of several chromosomal translocations involving TEL have been cloned, and it was shown that the translocations lead to gene fusion and the expression of chimeric proteins. The chromosomal breakpoints in TEL flank the two domains of the protein and are clustered 3′ of the HLH domain or 5′ of the ETS domain. The majority of the genes that fuse to TEL encode tyrosine kinases (1, 3, 7), and it was shown that the HLH domain is involved in dimerization of the fusion proteins leading to constitutive activation of the tyrosine kinase. However, in the most frequent translocation involving TEL, the t(12;21), TEL fuses upstream of the transactivator AML1. The resulting chimeric transcription factor TEL/AML1 is a repressor of promoter activated by AML1 (6), and the repression depends in part on the HLH domain of TEL (6).
To investigate the role of TEL in regulation of gene transcription, we used a yeast interacting system to identify a protein that interacts specifically with TEL. This protein is the ubiquitin-conjugating enzyme, UBC9 (13), involved in cell cycle regulation and DNA repair (13–15). Our results indicate that UBC9 is involved with TEL in the control of transcription regulation rather than in promotion of TEL degradation and are supported by the results of assays with selected mutants of UBC9 with impaired ubiquitination function. Our data indicate that UBC9 is not involved in TEL degradation, and we suggest that the interaction of UBC9 and TEL results in modulation of the transcription activity of TEL.
MATERIALS AND METHODS
Yeast Two-Hybrid Screen.
We used the Matchmaker Two-Hybrid Kit with a human bone marrow cDNA library Kit (CLONTECH). The assays were performed as recommended by the manufacturer. The full-length cDNA clone of TEL (bait) was subcloned in-frame with the Gal4 DNA-binding domain in the pGBT9 vector, resulting in pGBT9-TEL. We screened 6 × 106 clones (corresponding to two library titers) and selected the positive clones as suggested by the manufacturer. The liquid β-galactosidase assay was performed according to the manufacturer’s instruction.
Site-Specific Mutagenesis of UBC9 Protein.
Oligonucleotides that encode mutated amino acids at positions Cys-93, Asn-37, Pro-80, and Lys-101 were synthesized. By using the Quick Change PCR based site-directed mutagenesis kit (Stratagene), the following mutations were obtained: Cys-93 to Ser, Asn-37 to Asp, Pro-80 to Gly, and Lys-101 to Trp. The mutations were confirmed by sequencing with an Applied Biosystems Big-Dye automated DNA sequencer.
Glutathione S-Transferase (GST) Pull-Down and in Vitro Translation Assays.
TheUBC9 cDNA was cloned into the pGEX-KT vector (Amersham Pharmacia) to produce pGEX-KT-UBC9 encoding the fusion protein GST-UBC9. Expression and purification of pGEX-KT (control) or pGEX-KT-UBC9 using Glutathione Sepharose 4B (Amersham Pharmacia) were performed as suggested by the manufacturer. In vitro translation of TEL and the internal deletion mutant TELΔHLH (6) was performed with rabbit reticulocyte lysate (Promega) and 35S-Met according to the manufacturer’s instruction. The beads (30 μl) and 25 μl of in vitro-translated TEL or TELΔHLH (6) were incubated with 200 μl of bead-binding buffer [50 mM K-phosphate, pH 7.5/100 mM KCl/10% glycerol (vol/vol)/0.1% Triton X-100] at 4°C for 2 h. The glutathione Sepharose beads were washed once with complete bead-binding buffer and twice with bead-binding buffer devoid of glycerol and Triton X-100.
Mammalian Two-Hybrid Analysis.
The UBC9 cDNA was cloned in pCMV-Gal4 (16), a mammalian vector used for expression of hybrid proteins with the DNA-binding domain of Gal4, which resulted in pCMV-Gal4-UBC9. The TEL cDNA was cloned into pVP16-HA (17), resulting in pVP16-HA-TEL. NIH 3T3 cells (0.7 × 106 cells/100-mm plate) were cotransfected with pCMV-Gal4-UBC9, VP16-HA-TEL, and the Gal4 responsive reporter gene (G5E1βLUC) (18). pRLtk (Promega) was used to normalize the efficiency of transfection. The expression of luciferase was measured with the Dual Luciferase Reporter Assay System (Promega).
Cell Transfections and Chloramphenicol Acetyltransferase (CAT) Assays.
Full-length TEL cDNA was cloned into pSG424 (19), as a C-terminal fusion with the DNA-binding domain of Gal4. NIH 3T3 cells (0.7 × 106 cells/100-mm plate) were transfected with the calcium phosphate precipitation method (Invitrogen). Each precipitate included 5 μg of the internal reference pCH110, expressing the β-galactosidase enzyme (Amersham Pharmacia), 4 μg of each effector plasmid (or as noted for titration experiment), and 10 μg of reporter pGal45tkCAT (20). Forty-eight hours after transfection, cell extracts were prepared and assayed for β-galactosidase activity for normalization. The levels of CAT protein were determined by ELISA (Boehringer Mannheim). Monkey COS7 cells were transfected transiently by the calcium phosphate method.
DNA Electrophoretic Mobility-Shift Assay (EMSA).
The cell extracts prepared for CAT assays also were used for EMSA analysis. The probe was a 140-bp HindIII/XbaI fragment purified from pGal45tkCAT with the QIAquick gel extraction kit (Qiagen, Santa Clarita, CA). This fragment contains five copies of the 17-bp Gal4 binding sites. The DNA probe was labeled by incorporation of 32P-dCTP with the DNA polymerase Klenow enzyme and purified with Microspin G-25 columns (Amersham Pharmacia). The EMSAs were performed as described (21).
Metabolically Labeling of COS7 Cells and Immunoprecipitation.
COS7 cells (0.7 × 106 cells/100-mm plate) were transiently transfected with pCMV-Gal4-TEL or cotransfected with pCMV-Gal4-TEL and pCMV-Flag-UBC9 as described. The cells were metabolically labeled with 35S-Met, according to Cheng et al. (22) with the following modifications. Approximately 42 h after transfection, the cells were washed with PBS and grown for 1 h in Met-free DMEM (ICN) with 10% dialyzed FBS. The cells were maintained for 2 additional h in the same medium supplemented with 75 μCi/ml 35S-Met (Amersham Pharmacia). For immunoprecipitation, the cells were lysed with 1 ml of low-stringent NP-40 lysis buffer [10 mM Hepes, pH 7.5/250 mM NaCl/0.1% NP-40/5 mM EDTA/protease inhibitors (1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 1 mM PMSF)], and the lysate was rocked gently overnight at 4°C with 5 μg/ml of anti-Gal4 antibody (Santa Cruz Biotechnology) or with preimmune rabbit serum and 25 μl of protein A-agarose beads (Sigma). After incubation, the beads were washed three times with NP-40 buffer, boiled for 3 min in 2× SDS/PAGE sample buffer, and separated on SDS/PAGE. After drying, the gel was exposed to x-ray film overnight and analyzed.
Immunostaining of Cells.
COS7 cells were cultured on coverslips and transiently transfected with either pCMV-HA-TEL or pCMV-Flag-UBC9 or with both plasmids. About 36–48 h posttransfection, the cells were washed in PBS, fixed in 2% paraformaldehyde for 10 min at 4°C, and permeabilized either with methanol at −20°C for 2 min (for anti-Flag immunostaining), or with 0.1% Triton X-100 for 10 min at 4°C. The cells were treated with 5% normal goat serum and immunostained with anti-Flag M5 mouse mAb (Eastman Kodak), anti-hemagglutinin (HA) rat mAb (Boehringer Mannheim), or with both antisera at concentrations of 14 μg/ml and 1 μg/ml. The F(ab/)2 fragment of goat anti-rat FITC-conjugated IgG (Boehringer Mannheim), was used to visualize the anti-HA rat mAb. Goat anti-mouse FITC-conjugated IgG (Becton Dickinson Immunocytometry Systems) or Texas red-conjugated F(ab/)2 fragment of donkey anti-mouse IgG were used to visualize the anti-Flag M5 mouse mAb. Appropriate controls were performed to ensure lack of crossreactivity between the mouse primary antibody and the anti-rat secondary antibody and vice versa. Cells were mounted in 90% glycerol containing 1% p-phenylenediamine (Sigma).
RESULTS
TEL Interacts with UBC9 Through the HLH Domain.
The yeast two-hybrid system was used to identify proteins that interact with TEL. Yeast strain Y190 was cotransformed with pGBT9-TEL and a human bone marrow cDNA library. We screened approximately 6 × 106 transformants and identified 15 clones that were positive for the expression of the selection markers (β-galactosidase and histidine). Seven of the 15 clones interacted specifically with TEL. Subsequent sequence analysis showed that the majority of the clones had an identical 1.1-kb insert containing a single ORF encoding 158 aa. GenBank search revealed 100% homology with the human ubiquitin-conjugating enzyme UBC9 (13).
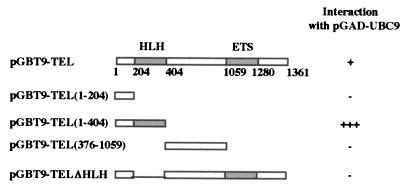
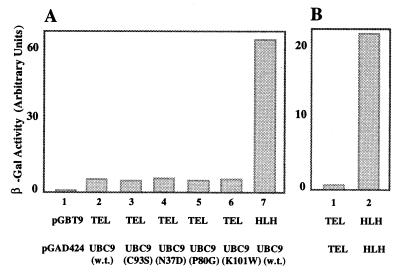
To determine the domains of TEL necessary for the interaction with UBC9, specific TEL deletion mutants were cloned in pGBT9 (Fig. 1), and the strength of the interaction was determined by liquid β-galactosidase assay, which is a very sensitive assay easily quantitated by colorimetric analysis. The results (Fig. 1) indicate that the HLH domain of TEL is required for the interaction with UBC9. To determine whether UBC9 has binding affinity for the HLH domain of other ETS repressors, we used the HLH domain of Yan, which is highly homologous to the HLH domain of TEL (8), and UBC9 by the yeast two-hybrid assay. The results (data not shown) indicate that UBC9 does not interact with the HLH domain of Yan, suggesting that the binding between TEL and UBC9 is specific. By comparing the strength of the association between UBC9 and either the wild type (w. t.) TEL (Fig. 2A, bar 2) or the deletion mutant of TEL consisting of only the HLH domain (Fig. 2A, bar 7), it would appear that the w. t. TEL has about 10-fold lower affinity for UBC9, as determined by liquid β-galactosidase expression. Similar results also were observed for interaction of TEL with itself (Fig. 2B), and we found that the association between two TEL clones consisting of only the HLH domain was about 40-fold stronger (Fig. 2B, bar 3) than the interaction between two w. t. TEL clones (Fig. 2B, bar 2). Although the liquid β-galactosidase assay measures the final result of a complex process, and differences in β-galactosidase activity could be the result of differences in efficiency of synthesis, folding, and stability of the proteins, or their transport to the nucleus, we must keep in mind that the results of these assays also depend on the transcriptional activation of the β-galactosidase enzyme. It is therefore possible that TEL could, in fact, interact strongly with itself in this assay, but because TEL is a powerful transcriptional repressor the interaction could result in reduction of β-galactosidase expression.
Figure 1.
Diagram of the TEL deletion mutants used in this study. (Left) The ORF of TEL is indicated as a box (first line). The functional domains of TEL (HLH at the amino terminus and ETS domain at the carboxyl terminus) are shaded. The numbers below TEL represent the nucleotide positions used for the construction of the mutants. The type and the extent of each deletion is indicated by the numbers in the parenthesis, in the remaining lines. (Right) Yeast cells were cotransformed with pGAD-UBC9 and one of the various pGBT9-TEL plasmids. Liquid β-galactosidase assay were used as described in Materials and Methods. The intensity of color is scored as: 0.001 (−), 7 (+), and 53.6 (+++).
Figure 2.
Quantitation of UBC9-TEL association as a function of β-galactosidase expression. Yeast cells were cotransformed with two separate plasmids, one of which encoded either TEL or the HLH domain of TEL fused to the Gal4 DNA-binding domain (pGBT9), whereas the second plasmid encoded UBC9 or a mutant of UBC9 fused to the activation domain of Gal4 (pGAD424). Liquid β-galactosidase assays were performed as described in Materials and Methods. (A) The interaction between the HLH domain of TEL and UBC9 (bar 7) results in 10-fold higher expression of β-galactosidase than the interaction between the w. t. TEL and UBC9 (bar 2). UBC9 mutants do not affect β-galactosidase expression (bars 3–6). (B) The interaction between two plasmids encoding the HLH domain results in about 40-fold higher expression of β-galactosidase than when using the w. t. TEL.
To determine whether the amino acids essential for the ubiquitination properties of the conjugating enzymes are important in the interaction between UBC9 and TEL, we used four different mutants in which specific amino acids had been mutated. These amino acids are conserved among the conjugating enzymes across species, and they were selected because, based on the crystal structure of UBC9 (23), they might be essential for the function of conjugating enzymes. Cys-93 is responsible for binding the conjugating enzyme to ubiquitin (24); Asn-37 is close to the N-terminus helix; Pro-80 is close to the C-terminus helix; and Lys-101 is likely to mediate the catalytic action of the enzyme because the side chain is oriented toward the ubiquitin-accepting sulfhydryl group (23) and it forms a small protruding loop near the active Cys site. Other mutants are not yet characterized. Unpublished results suggest that they have not lost their ability to bind RAD51 (Z.S., unpublished results). Mutation of these amino acids did not affect the association of UBC9 and TEL (Fig. 2A, bars 3–6), which indicates that the structural domain of UBC9 essential for TEL binding was not perturbed by these mutations. Based on the crystal structure, these amino acids of UBC9 might be responsible for the conjugation of modifier to the target protein.
TEL Interacts with UBC9 in Vitro.
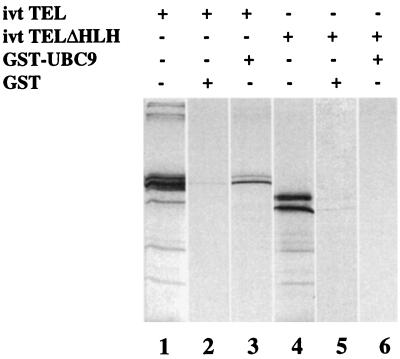
To confirm that UBC9 and TEL interact physically, we performed GST-fusion pull-down assays. Positive results of this assay correlate well with protein–protein interactions detected in the yeast two-hybrid system (25). The fusion protein GST-UBC9 was expressed, purified, and incubated with in vitro translated 35S-Met-labeled TEL as described in Materials and Methods. After extensive washing, the proteins bound to glutathione Sepharose beads were separated by SDS/PAGE and detected by autoradiography. Fig. 3 shows the results of the assay. As reported previously (26), translated TEL shows two bands, the result of two different translation start sites (Fig. 3, lane 1). Similarly, an internal deletion mutant of TEL that removes only the HLH domain also shows two bands, although of smaller size (Fig. 3, lane 4). Incubation of GST-UBC9 with w. t. TEL (Fig. 3, lane 3) and with the deletion mutant lacking the HLH domain (Fig. 3, lane 6) indicates that only full-length TEL was retained efficiently by GST-UBC9. In contrast, GST alone is unable to interact with TEL and the mutant TEL (Fig. 3, lanes 2 and 5).
Figure 3.
The in vitro interaction of TEL and UBC9 requires the HLH domain. In vitro-translated (ivt) TEL (lane 1) or the deletion mutant TELΔHLH (lane 4) were incubated with GST-UBC9, washed, and separated by SDS/PAGE as described in Materials and Methods. Only the w. t. TEL (lane 3), but not the HLH deletion mutant (lane 6), was retained by UBC9.
TEL Interacts with UBC9 in Mammalian Cells.
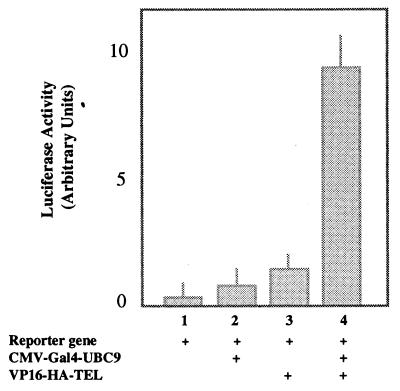
We used the mammalian two-hybrid system to establish the specificity of interaction in mammalian cells. The assay is based on functional reconstitution of Gal4-VP16, an artificial transcription factor containing the DNA-binding domain of yeast Gal4 protein (27) fused to an acidic transactivation domain of the herpes simplex virus VP16 protein (28). In mammalian cells, the Gal4-VP16 hybrid protein readily stimulates transcription of reporter plasmids bearing the UASG sequence, a recognition site for the DNA-binding domain of Gal4. We used pCMV-Gal4-UBC9 and pVP16-HA-TEL in cotransfection assays of NIH 3T3 cells, and we measured the activation of the reporter enzyme luciferase expressed by a Gal4-responsive promoter (Fig. 4). Expression of Gal4-UBC9 alone or VP16-TEL alone failed to activate significantly the transcription of the reporter gene (Fig. 4, bars 2 and 3). However, coexpression of Gal4-UBC9 and VP16-TEL generated an 8-fold increase in luciferase activity (Fig. 4, bar 4), suggesting that the interaction of UBC9 and TEL also occurs in mammalian cells.
Figure 4.
TEL and UBC9 interact in mammalian cells. NIH 3T3 cells were transiently transfected with pCMV-Gal4-UBC9 and pVP16-TEL separately or together as indicated. The luciferase activity was normalized for transfection efficiency and plotted as shown. Bar 1, cells transfected with only the reporter plasmid. Addition of UBC9 alone (bar 2) or TEL alone (bar 3) does not result in significant change. However cotransfection of both plasmids (bar 4) results in about 8-fold increase of luciferase expression.
Subcellular Localization of TEL and UBC9.
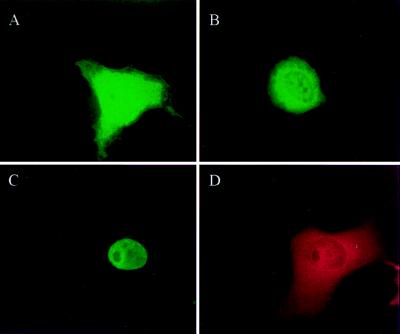
We analyzed the distribution of HA-TEL and Flag-UBC9 in cytoplasmic and nuclear fractions of transfected cells by Western blot analysis. When nuclear and cytoplasmic extracts from equivalent numbers of cells were separated by SDS/PAGE, the anti-Flag mAbs detected a polypeptide of approximately 18 kDa corresponding to Flag-UBC9 in both subcellular fractions, whereas HA-TEL was detected predominantly in the nucleus (data not shown). These expected results confirm those previously reported by other investigators (14, 29–31). Immunocytochemistry analysis showed a predominant nuclear localization of HA-TEL (data not shown) in agreement with results of other investigators (26). The distribution of Flag-UBC9 is shown in Fig. 5. When the expression of Flag-UBC9 was high, its overwhelming presence in the cytoplasm made it difficult to visualize its nuclear distribution by using Nomarski optics (Fig. 5A). Cells expressing lower levels of Flag-UBC9, however, showed a distinct nuclear fluorescence (Fig. 5B). When cells were cotransfected with both plasmids, immunocytochemical staining showed a similar distribution of HA-TEL (Fig. 5C) and Flag-UBC9 (Fig. 5D) in the nucleus of cells with lower levels of Flag-UBC9, and both proteins were excluded from subnuclear regions, probably corresponding to nucleoli.
Figure 5.
Subcellular localization of TEL and UBC9. COS7 cells transfected with pCMV-Flag-UBC9 showing a high (A) or a relatively low (B) expression of Flag-UBC9. Cells were immunostained with anti-Flag M5 mouse mAb and anti-mouse FITC-conjugated secondary antibody. UBC9 is present both in the cytoplasm and nucleus of cells. Cells cotransfected with pCMV-Flag-UBC9 and pCMV-HA-TEL were immunostained with anti-HA rat/anti-rat FITC antibodies for HA-TEL detection (C) or with anti-Flag mouse/anti-mouse Texas red antibodies for Flag-UBC9 detection (D). Both TEL and UBC9 show a diffuse nuclear staining and are excluded from subnuclear regions that may correspond to nucleoli.
UBC9 Relieves Transcription Repression by TEL.
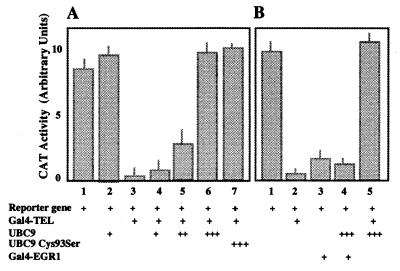
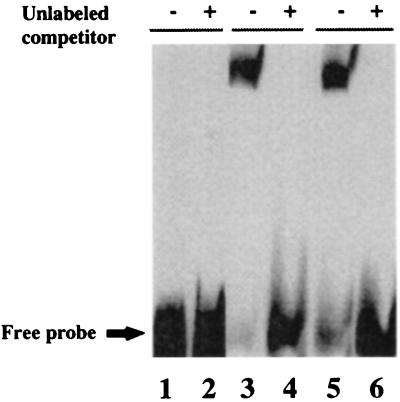
The Gal4-TEL fusion protein was tested for its ability to regulate transcription of a Gal4-dependent promoter in the reporter plasmid pGal45tkCAT (20). Addition of Gal4-TEL repressed CAT expression about 20-fold (Fig. 6A, bars 1 and 3). UBC9 by itself had no significant effect (Fig. 6A, bar 2). Cotransfection of increasing amounts of UBC9 inhibited the repression of the reporter gene (Fig. 6A, bars 4 and 5), and equimolar amount of UBC9 completely restored promoter activity (Fig. 6A, bar 6). These results would indicate that UBC9 has a direct effect on the transcription activity of TEL. To rule out that overexpression of UBC9 restores promoter activity by increasing TEL degradation, we repeated the same transfection assay with a UBC9 mutant in which Cys-93 had been mutated to Ser. It was shown that the Cys-93 residue is essential for ubiquitin transfer to the target protein (23, 24), and therefore for protein degradation. The result (Fig. 6A, bar 7) indicates that mutant UBC9 has the same effect as the w. t. UBC9 in restoring promoter activity. To confirm that the effect of UBC9 is specific for TEL, we used the gene EGR-1, which encodes a strong transcription repressor (21) in similar assay. As shown in Fig. 6B, Gal4-EGR-1 strongly repressed the expression of CAT, although not as much as TEL (Fig. 6B, bars 2 and 3). However, in contrast to the results obtained with TEL, coexpression of UBC9 did not restore promoter activity (Fig. 6B, bar 4), suggesting that the mechanism by which UBC9 inhibits TEL repression is specific for TEL. To rule out that addition of UBC9 inhibits binding of Gal4-TEL to the promoter region of the reporter gene, we used the promoter region and cell extracts containing either Gal4-TEL alone, or Gal4-TEL and UBC9 in electrophoretic mobility-shift assays. The results indicated that Gal4-TEL binds to the promoter independently of expression of UBC9 (Fig. 7, lanes 3 and 5). We did not detect any supershift of the Gal4-TEL/DNA complex in the presence of UBC9, suggesting that the interaction between TEL and UBC9 is transient. Transient association between UBC9 and the target protein has been reported before (14).
Figure 6.
UBC9 restores activation of a promoter repressed by TEL. NIH 3T3 cells were cotransfected with plasmids as indicated, and normalized CAT activities are shown. (A) TEL (bar 3) represses the promoter, whereas UBC9 (bar 2) has no effect. Cotransfection of NIH 3T3 cells with TEL and with increasing amounts of UBC9 restores promoter activity (bars 4–6). The Cys-93–Ser mutant of UBC9 is as effective as the w. t. UBC9 in restoring promoter activity (bar 7). (B) The effect of UBC9 is specific for TEL (bar 5), and another strong repressor, such as EGR1, is not affected (bar 4).
Figure 7.
Gal4-TEL binds to a Gal4 probe. The cell extracts used for CAT assays were tested by gel shift analysis with a 140-bp probe containing five Gal4-binding sites. Protein-DNA complexes were detected in the absence (−) but not in the presence (+) of 200-fold molar excess of cold competitor DNA, indicating the specificity of the binding. Lanes 1 and 2: Cell extracts from untransfected cell. Lanes 3 and 4: Cell extracts from Gal4-TEL-transfected cells. Lanes 5 and 6: Cell extracts from Gal4-TEL and UBC9 cotransfected cells.
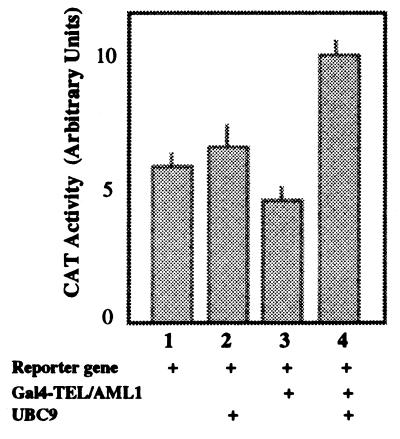
To determine whether UBC9 also can modify the transcription properties of TEL fusion proteins, we used as effector the fusion protein TEL/AML1 cloned as fusion with the Gal4 DNA-binding domain. By itself, Gal4-TEL/AML1 is a very weak repressor compared with TEL (Fig. 8, bars 2 and 3), and coexpression of UBC9 completely relieves this repression (Fig. 8, bar 4), suggesting that the HLH region of TEL, maintained in TEL/AML1, responds to the effect of UBC9, also in the fusion protein.
Figure 8.
UBC9 relieves transcription repression by TEL/AML1. Conditions are as described in the legend of Fig. 6. TEL/AML1 is a weak repressor (bar 3), and expression of UBC9 changes TEL/AML1 from a weak repressor to a weak activator (bar 4).
Overexpression of UBC9 Does Not Increase TEL Degradation.
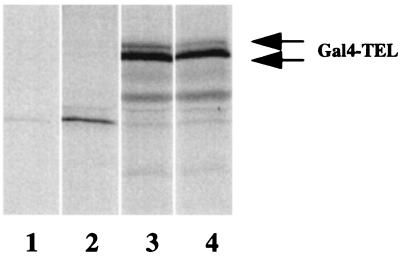
To confirm that UBC9 does not play a significant role in TEL degradation, we immunoprecipitated TEL from metabolically labeled cells transfected with TEL alone (Fig. 9, lane 4) or with TEL and UBC9 (Fig. 9, lane 3). Analysis of the autoradiogram shows a doublet corresponding to the TEL protein as reported by others (26), in both lanes (Fig. 9, lanes 3 and 4). The doublet was not detected with preimmune serum (Fig. 9, lane 1) or with extracts of mock-transfected cells (Fig. 9, lane 2). Fig. 9 shows no significant difference in the levels of TEL immunoprecipitated from extracts of cell transfected with or without UBC9, suggesting that UBC9 probably has no major role in TEL degradation.
Figure 9.
UBC9 is not involved in TEL degradation. COS7 cells were transfected either with pCMV-Gal4-TEL alone or with pCMV-Flag-UBC9 and were labeled with 35S-methionine. The labeled cells were lysed under low stringency and immunoprecipitated with anti-Gal4 polyclonal antibody or preimmune serum. After washing, the immunoprecipitates were fractionated by 12% SDS/PAGE and analyzed by autoradiography. The Gal4-TEL doublet is indicated by the double arrow. Lane 1, preimmune control from cell lysates of cells transfected with Gal4-TEL and Flag-UBC9. Lane 2, immunoprecipitation from the cells transfected with the empty vector. Lane 3, immunoprecipitation from Gal4-TEL and Flag-UBC9 cotransfected cells. Lane 4, immunoprecipitation from Gal4-TEL-transfected cells.
DISCUSSION
We have used the yeast two-hybrid system to identify candidate proteins that interact with TEL. Of the several positive clones that were initially detected, one was independently isolated several times. The clone encoded UBC9, a member of the family of ubiquitin-conjugating enzymes. These enzymes usually play a role in proteosome-mediated degradation of many cytoplasmic and nuclear proteins. The function of UBC9, however, is not known, and there are contradictory results about its involvement in protein degradation. Although some investigators suggest that UBC9 mediates protein degradation by conjugation of ubiquitin (14, 31, 32), other investigators show that UBC9 is involved in directing proteins to specific subcellular compartments by conjugation of the small modifier SUMO1 (15, 33–35).
By using various techniques, we have determined that the interaction between TEL and UBC9 requires the HLH domain of TEL and is unaffected by specific amino acid mutations that are probably necessary for the enzymatic activities of ubiquitin-conjugating enzymes. The level of TEL is unaltered in the presence or absence of UBC9, indicating that the interaction between the two proteins does not result in ubiquitin-mediated degradation of TEL. We also showed that w. t. UBC9 and a mutant form of UBC9 that presumably has lost the capability of conjugating a modifier have a similar effect on the response of a promoter to TEL and inhibit the ability of TEL to repress reporter gene expression. UBC9 also interacts with ETS1 (36), which is also a member of the ETS family of transcription factors. Although ETS1, which is a transcription activator, interacts with UBC9 through both the HLH and the DNA-binding domain, the w. t. UBC9 and the mutant form of UBC9 also have similar effect on the response of a promoter to ETS1 and increase the transcription activity of ETS1. Therefore, it would seem that UBC9 has the ability to activate promoters through a mechanism that does not involve conjugation of modifiers to target proteins. In the case of ETS1, it was speculated that the binding of UBC9 to ETS1 changes the intramolecular structure by opening the compact, closed structure of ETS1, leading to the increase in efficiency as a transcriptional activator (36).
Recently, it was shown that UBC9 is involved in the conjugation of SUMO1 to target proteins, and there are indications that this could be one of the major roles of UBC9 (15, 33–35, 37). There have been several speculations on the function of SUMO1 conjugation. It was reported that protein modification by SUMO1 could represent a mechanism of directing a specific protein to a subcellular compartment different from the one that is targeted by the unmodified protein. This effect was clearly shown for RanGAP1, which can be detected in the cytosol or in the nuclear pore complex depending on SUMO1 conjugation (34). We did not observe any striking difference in the localization of TEL in the presence or absence of SUMO1 by immunofluorescence analysis of transiently transfected cells, and in both instances TEL maintained a diffused nuclear staining pattern, whereas SUMO1 showed punctuate staining over a diffused nuclear distribution (data not shown). We also analyzed protein extracts of COS7 cells transiently transfected with TEL, SUMO1, and UBC9 by using the method of Desterro et al. (37) (data not shown). However, the results of immunoprecipitation analysis of TEL in the presence and absence of SUMO1 are at this time inconclusive. One of the reasons it is difficult to unambiguously show the presence of SUMO1-conjugated TEL could be that the modification of a target protein with SUMO1 is inefficiently maintained during in vitro manipulations because of the presence of hydrolases that cleave the bond between the C terminus of SUMO1 and the lysine to which it is bound (35). Another explanation could be that the modification requires the presence of other factors or proteins that may be present in limiting amounts. Two other proteins related to but distinct from SUMO1 have been reported recently (38, 39), and it is speculated that they also could be involved in protein modification. Therefore, at this time, we cannot definitively and clearly dismiss the possibility that SUMO1 or molecules of the SUMO1 family might be conjugated to and direct TEL to specific subnuclear regions, and we believe that much work will be required before the role of UBC9 is clearly understood.
Acknowledgments
We gratefully acknowledge Dr. Richard Baer for the mammalian two-hybrid system. We also thank Dr. Vincent Shankey for his advice and help with fluorescence microscopy. We are also grateful to Dr. Nancy Zeleznik-le for providing the Gal4-EGR1 plasmid. This work was supported by National Institutes of Health Grants CA67189 (G.N.), CA72675 (G.N.), and ES08353 (Z.S.). G.N. is a Scholar of the Leukemia Society of America.
ABBREVIATIONS
- ETS
E-26 transforming specific
- HLH
helix–loop–helix
- GST
glutathione S-transferase
- CAT
chloramphenicol acetyltransferase
- HA
hemagglutinin
- w. t.
wild type
References
- 1.Golub T R, Barker G F, Lovett M, Gilliland D G. Cell. 1994;77:307–316. doi: 10.1016/0092-8674(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 2.Golub T R, Barker G F, Bohlander S K, Hiebert S W, Ward D C, Bray-Ward P, Morgan E, Raimondi S C, Rowley J D, Gilliland D G. Proc Natl Acad Sci USA. 1995;92:4917–4921. doi: 10.1073/pnas.92.11.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papadopoulos P, Ridge S A, Boucher C A, Stocking C, Wiedemann L M. Cancer Res. 1995;55:34–38. [PubMed] [Google Scholar]
- 4.Romana S P, Mauchauffe M, Le Coniat M, Chumakov I, Le Paslier D, Berger R, Bernard O A. Blood. 1995;85:3662–3670. [PubMed] [Google Scholar]
- 5.Buijs A, Sherr S, van Baal S, van Bezouw S, van der Plas D, Geurts van Kessel A, Riegman P, Lekanne Deprez R, Zwarthoff E, Hagemeijer A, et al. Oncogene. 1995;10:1511–1519. [PubMed] [Google Scholar]
- 6.Fears S, Gavin M, Zhang D E, Hetherington C, Ben-David Y, Rowley J D, Nucifora G. Proc Natl Acad Sci USA. 1997;94:1949–1954. doi: 10.1073/pnas.94.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knezevich S R, McFadden D E, Tao W, Lim J F, Sorensen P H. Nat Genet. 1998;18:184–187. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 8.Jousset C, Carron C, Boureux A, Quang C T, Oury C, Dusanter-Fourt I, Charon M, Levin J, Bernard O, Ghysdael J. EMBO J. 1997;16:69–82. doi: 10.1093/emboj/16.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwiatkowski B A, Bastian L S, Bauer T R, Jr, Tsai S, Zielinska-Kwiatkowska A G, Hickstein D D. J Biol Chem. 1998;273:17525–17530. doi: 10.1074/jbc.273.28.17525. [DOI] [PubMed] [Google Scholar]
- 10.Fears S, Vignon C, Bohlander S K, Smith S, Rowley J D, Nucifora G. Genes Choromosomes Cancer. 1996;17:127–135. doi: 10.1002/(SICI)1098-2264(199610)17:2<127::AID-GCC8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Wang L C, Kuo F, Fujiwara Y, Gilliland D G, Golub T R, Orkin S H. EMBO J. 1997;16:4374–4383. doi: 10.1093/emboj/16.14.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L C, Swat W, Fujiwara Y, Davidson L, Visvader J, Kuo F, Alt F W, Gilliland D G, Golub T R, Orkin S H. Genes Dev. 1998;12:2392–2402. doi: 10.1101/gad.12.15.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen Z, Pardington-Purtymun P E, Comeaux J C, Moyzis R K, Chen D. Genomics. 1996;37:183–186. doi: 10.1006/geno.1996.0540. [DOI] [PubMed] [Google Scholar]
- 14.Tashiro K, Pando M P, Kanegae Y, Wamsley P M, Inoue S, Verma I M. Proc Natl Acad Sci USA. 1997;94:7862–7867. doi: 10.1073/pnas.94.15.7862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller S, Matunis M J, Dejean A. EMBO J. 1998;17:61–70. doi: 10.1093/emboj/17.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu L C, Wang Z W, Tsan J T, Spillman M A, Phung A, Xu X L, Yang M C, Hwang L Y, Bowcock A M, Baer R. Nat Genet. 1996;14:430–440. doi: 10.1038/ng1296-430. [DOI] [PubMed] [Google Scholar]
- 17.Tsan J T, Wang Z, Jin Y, Hwang L Y, Bash R O, Baer R. In: The Yeast Two-Hybrid System. Bartel P L, Fields S, editors. London: Oxford Univ. Press; 1997. [Google Scholar]
- 18.Hsu H L, Wadman I, Baer R. Proc Natl Acad Sci USA. 1994;91:3181–3185. doi: 10.1073/pnas.91.8.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sadowski I, Ptashne M. Nucleic Acids Res. 1989;17:7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Seto E, Chang L S, Shenk T. Cell. 1991;67:377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- 21.Gashler A L, Swaminathan S, Sukhatme V P. Mol Cell Biol. 1993;13:4556–4571. doi: 10.1128/mcb.13.8.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng J T, Hsu H L, Hwang L Y, Baer R. Oncogene. 1993;8:677–683. [PubMed] [Google Scholar]
- 23.Tong H, Hateboer G, Perrakis A, Bernards R, Sixma T K. J Biol Chem. 1997;272:21381–21387. doi: 10.1074/jbc.272.34.21381. [DOI] [PubMed] [Google Scholar]
- 24.Jentsch S. Annu Rev Genet. 1992;26:179–207. doi: 10.1146/annurev.ge.26.120192.001143. [DOI] [PubMed] [Google Scholar]
- 25.Saltzman A, Searfoss G, Marcireau C, Stone M, Ressner R, Munro R, Franks C, D’Alonzo J, Tocque B, Jaye M, Ivashchenko Y. FEBS Lett. 1998;425:431–435. doi: 10.1016/s0014-5793(98)00287-7. [DOI] [PubMed] [Google Scholar]
- 26.Poirel H, Oury C, Carron C, Duprez E, Laabi Y, Tsapis A, Romana S P, Mauchauffe M, LeConiat M, Berger R, et al. Oncogene. 1997;14:349–357. doi: 10.1038/sj.onc.1200829. [DOI] [PubMed] [Google Scholar]
- 27.Ma J, Ptashne M. Cell. 1987;48:847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- 28.Triezenberg S J, LaMarco K L, McKnight S L. Genes Dev. 1988;2:730–742. doi: 10.1101/gad.2.6.730. [DOI] [PubMed] [Google Scholar]
- 29.Becker K, Schneider P, Hofmann K, Mattmann C, Tschopp J. FEBS Lett. 1997;41:102–106. doi: 10.1016/s0014-5793(97)00758-8. [DOI] [PubMed] [Google Scholar]
- 30.Lee G W, Melchior F, Matunis M J, Mahajan R, Tian Q, Anderson P. J Biol Chem. 1998;273:6503–6507. doi: 10.1074/jbc.273.11.6503. [DOI] [PubMed] [Google Scholar]
- 31.Firestein R, Feuerstein N. J Biol Chem. 1998;273:5892–5902. doi: 10.1074/jbc.273.10.5892. [DOI] [PubMed] [Google Scholar]
- 32.Kho C J, Huggins G S, Endege W O, Hsieh C M, Lee M E, Haber E. J Biol Chem. 1997;272:3845–3851. doi: 10.1074/jbc.272.6.3845. [DOI] [PubMed] [Google Scholar]
- 33.Saitoh H, Sparrow D B, Shiomi T, Pu R T, Nishimoto T, Mohun T J, Dasso M. Curr Biol. 1998;8:121–124. doi: 10.1016/s0960-9822(98)70044-2. [DOI] [PubMed] [Google Scholar]
- 34.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 35.Desterro J M, Rodriguez M S, Hay R T. Mol Cell. 1998;2:233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- 36.Hahn S L, Wasylyk B, Criqui-Filipe P. Oncogene. 1997;15:1489–1495. doi: 10.1038/sj.onc.1201301. [DOI] [PubMed] [Google Scholar]
- 37.Desterro J M, Rodriguez M S, Hay R T. FEBS Lett. 1997;417:297–300. doi: 10.1016/s0014-5793(97)01305-7. [DOI] [PubMed] [Google Scholar]
- 38.Saitoh H, Pu R T, Dasso M. Trends Biochem Sci. 1997;22:374–376. doi: 10.1016/s0968-0004(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 39.Lapenta V, Chiurazzi P, van der Spek P, Pizzuti A, Hanaoka F, Brahe C. Genomics. 1997;40:362–366. doi: 10.1006/geno.1996.4556. [DOI] [PubMed] [Google Scholar]