Abstract
Periplasmic Cu, Zn-cofactored superoxide dismutase (SodC) protects Gram-negative bacteria from exogenous oxidative damage. The virulent Salmonella typhimurium strain ATCC 14028s has been found to contain two discrete periplasmic Cu, Zn-SOD enzymes that are only 57% identical at the amino acid level. SodCI is carried by a cryptic bacteriophage, and SodCII is closely related to the Cu, Zn-superoxide dismutase of Escherichia coli. All Salmonella serotypes appear to carry the sodCII locus, but the phage-associated sodCI gene is found only in certain strains belonging to the most highly pathogenic serotypes. Expression of either sodC locus appears to be enhanced during stationary phase, but only sodCII is regulated by the alternative sigma factor σs (RpoS). Mutants lacking both sodC genes are less lethal for mice than mutants possessing either sodC locus alone, indicating that both Cu, Zn-SOD enzymes contribute to Salmonella pathogenicity. The evolutionary acquisition of an additional sodC gene has contributed to the enhanced virulence of selected Salmonella strains.
Prokaryotic organisms generally contain one or more superoxide dismutases (SODs) cofactored by manganese (Mn), iron (Fe), or copper and zinc (Cu, Zn). Cu, Zn-SOD, encoded by the sodC gene, has been identified in a large number of Gram-negative bacterial genera including Actinobacillus, Brucella, Caulobacter, Escherichia, Francisella, Haemophilus, Legionella, Neisseria, Pasteurella, Photobacterium, and Salmonella (1–11). Periplasmic localization of Cu, Zn-SOD in bacteria has suggested a role in protection from exogenous oxidative stress, and this has been confirmed in several instances by in vitro assays (12–17).
The first Cu, Zn-SOD of Salmonella typhimurium was discovered by PCR amplification of genomic DNA using primers derived from consensus sodC sequence motifs (8). Sequence analysis of flanking regions revealed bacteriophage structural genes (13, 14), suggesting that the Salmonella sodC gene was acquired by bacteriophage-mediated horizontal transfer. Construction of a null mutant showed that Cu, Zn-SOD is required for survival in phagocytes and full virulence in mice (13, 14). Further characterization in immunodeficient mice demonstrated that Cu, Zn-SOD helps Salmonella to resist synergistic interactions of the phagocyte NADPH oxidase and nitric oxide synthase (13), possibly by diverting superoxide and limiting peroxynitrite formation.
We now report that some strains of Salmonella carry two distinct Cu, Zn-superoxide dismutases. The second Salmonella Cu, Zn-SOD was discovered when two-dimensional electrophoretic analysis of S. typhimurium was performed to identify acid stress-induced proteins regulated by the alternative sigma factor σs. Unexpectedly, the NH2-terminal sequence of one acid-induced σs-dependent protein [(acid shock protein (ASP)71] was found to be highly homologous to the SodC protein of Escherichia coli, but clearly distinguishable from the known S. typhimurium SodC sequence (8, 13, 14). The new gene has been designated sodCII, and the present study describes its sequence analysis, regulation, functional characterization, and implications for the evolution of Salmonella pathogenicity.
MATERIALS AND METHODS
Media.
Bacterial strains were routinely cultivated and stored in LB broth (10 mg/ml tryptone/5 mg/ml yeast extract/10 mg/ml NaCl)(Difco) at 37°C. Evans blue uranine medium was used to identify pseudolysogen-free transductants (18). M9 minimal medium (7 mg/ml Na2HPO4/3 mg/ml KH2PO4/0.5 mg/ml NaCl/1 mg/ml NH4Cl) or xylose-lysine-deoxycholate medium (Difco) was used for selection of recombinant Salmonella mutant strains. LB medium with the omission of NaCl and the addition of 6% sucrose was used at 30°C (19) for allelic exchange procedures. Vogel and Bonner E minimal medium (20) supplemented with 0.4% glucose was used for two-dimensional SDS/PAGE studies. Penicillin (250 μg/ml), ampicillin (60 μg/ml), kanamycin (50 μg/ml), chloramphenicol (30 μg/ml), or tetracycline (10 μg/ml in minimal and 20 μg/ml in rich media, respectively), all from Sigma, was added as indicated. Agar (1.5%) was added to solid media.
Strains and Plasmids.
Bacterial strains are listed in Table 1. Two-dimensional protein electrophoretic analysis was performed on strains S. typhimurium UK1 (wild-type) (21) and JF3912 (UK1 sodCII∷pRR10[ΔtrfA]). Overexpression of SodCII for microsequencing was performed in JF2892 (UK1 mviA4185∷aph) (22). β-Galactosidase, chemical susceptibility, and mouse virulence assays were performed by using S. typhimurium ATCC 14028s or its isogenic mutant derivatives. Additional Salmonella strains analyzed by Southern hybridization are from a published reference collection of Salmonella enterica subspecies I strains (23) and from our laboratory collection of clinical isolates. The construction of rpoS mutant SF1005 (rpoS∷pRR10[ΔtrfA]) is described in ref. 24, and the construction of sodCI mutant MF1005 (sodCI∷pRR10[ΔtrfA]) is described in ref. 13. The construction of sodCI mutant MF1006 (sodCI∷aph), sodCII mutants MF1007 and JF3912 (sodCII∷pRR10[ΔtrfA]) and sodCI sodCII double mutant MF1008 (sodCI∷aph sodCII∷pRR10[ΔtrfA]) is described in Results. E. coli S17–1 (25) or SM10λpir (26) were used to mobilize suicide vectors pRR10(ΔtrfA) (24) or pKNG101 (27), respectively. Plasmid pRS1274 was used to construct promoter fusions with lacZ (28). Kanamycin-resistant versions of the pRS1274-derived plasmids were constructed by inserting an aph cassette (Amersham Pharmacia) into the unique PstI site in the pRS1274 bla gene. Plasmid pBluescript KS (Stratagene) was used for routine cloning.
Table 1.
Genotypes used
| Species | Strain | Relevant genotype | Ref. |
|---|---|---|---|
| S. typhimurium | 14028s | Wild-type | American Type Culture Collection |
| JF2892 | UK1 mviA 4185∷aph | 22 | |
| JF3912 | UK1 sodCII∷pRR10(ΔtrfA) | This work | |
| MF1005 | 14028s sodCI∷pRR10(ΔtrfA) | 13 | |
| MF1006 | 14028s sodCI∷aph | This work | |
| MF1007 | 14028s sodCII∷pRR10(ΔtrfA) | This work | |
| MF1008 | 14028s sodCI∷aph | This work | |
| sodCII∷pRR10(ΔtrfA) | |||
| SF1005 | 14028s rpoS∷pRR10(ΔtrfA) | 24 | |
| UK1 | Wild-type | 21 | |
| E. coli | S17–1 | Tra+recA pro thi hsdR chr∷RP4–1 | 25 |
| SM10λpir | thi leu hsd recA Kmr RP4–2-Tc∷Mu λpir | 26 |
Standard Genetic Procedures.
Routine genetic manipulations and PCR amplification were performed by using conventional methods (29). Southern hybridizations were performed by using EcoRI- or EcoRV-digested chromosomal DNA electrophoresed through 0.7% agarose and transferred onto a nylon membrane. A 790-bp EcoRI–BglII fragment from the sodCI locus was used as a probe for the presence of sodCI. Primers 5′-ATGAACCTCGTCACGTCGCAAGGGGTAGGGCAGTC-3′ and 5′-TTGATTTCATCCAGTGATTTCAGACGAGGCGC-3′ derived from the E. coli sodC sequence were used to amplify a 343-bp internal fragment to be used as a probe for the S. typhimurium sodCII gene. An internal fragment of the spvR coding region was PCR-amplified by using a previously described primer pair (30) and used to probe for the presence of the spv plasmid virulence genes. Probes were labeled with 32P or with a chemiluminescent labeling and detection kit (Boehringer Mannheim or NEN). Sequencing was performed on an ABI 1373A automated fluorescent sequencer (Applied Biosystems) or by the Sanger dideoxy method (29) using sequenase (United States Biochemical) and custom primers (BRL).
Pulsed-field gel electrophoresis of S. typhimurium 14028s genomic DNA was performed by extracting DNA, embedding it in agarose, and digesting with XbaI (New England Biolabs) according to manufacturer’s specifications. Plugs containing digested DNA were stored overnight and electrophoresed for 20 hr through 1.2% SeaKem LE agarose (FMC) on a Chef DRIII apparatus (Bio-Rad) with a field strength of 6 V/cm at an included angle of 120°. Initial switch time was 5 sec, and the final switch time was 40 sec. The electrophoresis buffer was 0.5× TBE run at 14°C. The DNA was transferred onto a nylon membrane to allow hybridization.
Two-Dimensional SDS/PAGE.
Two dimensional SDS/PAGE was performed as described (31). ASPs were identified by comparing cultures at pH 7.7 to cultures shifted from pH 7.7 to pH 4.4 for 20 min. Typically, the samples were labeled for 5 min with 35S-trans label (40 μCi/ml, ICN; 1 Ci = 37 GBq). After the addition of chloramphenicol, a 1.5-ml sample of cells was pelleted and suspended in SDS lysis buffer. Approximately 5 μg of protein was analyzed for each sample. Basic and acidic proteins are situated to the left and right of the autoradiograph, respectively. The first dimension was obtained by using a pH 5–7 isoelectric-focusing gel containing 1.6% (pH 5–7) and 0.4% (pH 3–10) ampholytes (Bio-Rad), and the second dimension was obtained by using an SDS/12% PAGE gel without stacking gel.
Protein Purification from Two-Dimensional SDS/PAGE.
Isolation and purification of the σs-dependent ASPs was accomplished by scaling up the standard two-dimensional gel protocol with a few modifications and additions. Minimal E glucose medium (0.5 liters) was inoculated with a 1:100 dilution of an overnight culture of JF2892 (mviA). At 0.6 OD600, a portion of the culture (6 ml) was removed for trans 35S labeling (ICN). The labeled and unlabeled cultures were combined and centrifuged, and the pellets were resuspended in 1× sonication buffer. After sonication, the cellular debris was pelleted by low- (12,100 × g for 10 min) and high- (380,000 × g for 20 min) speed spins. The supernatant was loaded onto a Centricon-100 (Amicon) concentration column with a 100,000 MW protein cutoff. The filtrate from the centrifuged Centricon-100 was then loaded onto a Centricon-30 concentration column. Retentates from both Centricons were processed for two-dimensional gels. After electrophoresis, the polyacrylamide gels were transferred to poly(vinylidene difluoride) (Bio-Rad). The blots containing proteins from the Centricon-30 retentate were sent to The Wistar Institute (Philadelphia) for proteolytic digestion and protein sequencing of ASP71. Analysis of nucleotide and protein sequences were performed by using the Wisconsin Package, Version 8.1-Unix of the Genetics Computer Group (Madison, WI).
β-Galactosidase Assays.
Overnight cultures of S. typhimurium 14028s (wild-type) or SF1005 (rpoS) carrying PsodCI-lacZ, PsodCII-lacZ, or control plasmids were diluted 1:1000 into fresh LB broth and placed in a shaker-incubator at 275 rpm and 37°C. Samples were assayed at timed intervals for β-galactosidase activity from SDS/chloroform-permeabilized cells with o-nitrophenyl-β-d-galactopyranoside (ONPG) as a substrate (32). Each assay was performed a minimum of two times.
Mouse Virulence Assays.
C57BL/6 (Itys, genetically Salmonella-susceptible) or C3H/HeN (Ityr, genetically Salmonella-resistant) mice (The Jackson Laboratory) were administered intraperitoneal inocula of wild-type or sodC mutant S. typhimurium diluted in 200 μl of PBS. Inocula were determined by dilutional plating, and varied from 600–1,200 organisms. Each virulence assay was performed at least twice by using four to seven mice per group, with similar results.
RESULTS
S. typhimurium Has Two sodC Genes.
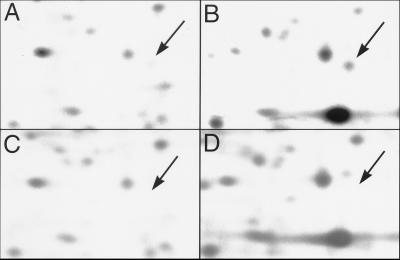
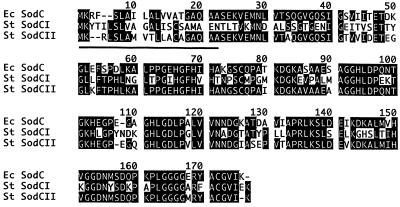
Previous studies have identified the protein designated ASP71 to be a σs-dependent ASP (33). The mviA mutant JF2892 overproduces many σs-dependent proteins, apparently because σs degradation depends on the mviA regulatory locus (22). Consequently, strain JF2892 was subjected to a modified two-dimensional SDS/PAGE protocol to obtain quantities of ASP71 sufficient for microsequencing. Two-dimensional protein gel electrophoresis of total cell lysates from S. typhimurium exposed to acid stress had revealed a σs-dependent protein of approximately 16 kDa (Fig. 1B). NH2-terminal sequencing of this protein revealed a peptide identical to the mature E. coli SodC protein at 20 of 23 residues, suggesting that this protein was in fact a Cu, Zn-SOD. However, the sequence differed from the known corresponding S. typhimurium sodC sequence at 16 of 23 residues (Fig. 2), raising the possibility that S. typhimurium has two sodC genes. Primers derived from the E. coli sodC sequence (described in Materials and Methods) were subsequently used to amplify a 343-bp internal fragment of the newly identified S. typhimurium gene, designated sodCII, from genomic DNA of ATCC S. typhimurium strain 14028s (34).
Figure 1.
Two-dimensional SDS/PAGE analysis of SodCII (ASP71) in wild-type Salmonella and a sodCII mutant. Wild-type UK1 (A and B) and sodCII mutant JF3912 (C and D) were grown to logarithmic phase at pH 7.7 in minimal EG medium. Cells were either processed directly for two-dimensional SDS/PAGE (A and C) or acid shocked for 1 hr at pH 4.4 before processing (B and D). The arrow indicates the position of SodCII. The panels show equivalent small areas of larger gels.
Figure 2.
Sequence Comparison of E. coli and S. typhimurium SodC proteins. The two S. typhimurium SodC proteins share 57% identity. S. typhimurium SodCII is 82% identical to E. coli SodC, but SodCI is only 54% identical to the E. coli protein. Each SodC possesses a typical hydrophobic NH2 terminus signal sequence (underlined), consistent with secretion of Cu, Zn-SOD into the periplasmic space.
The 343-bp sodCII fragment was labeled with 32P and used to probe S. typhimurium 14028s genomic DNA completely digested with BglII and BamHI endonucleases. A hybridizing 3.8-kbp chromosomal fragment containing the entire sodCII gene was cloned and sequenced (GenBank accession no. AF056931). The nucleotide sequence of sodCII revealed a 522-bp gene encoding a predicted 174-aa protein (Fig. 2). The predicted protein sequence is 82% identical to the E. coli sodC gene and 54% identical to S. typhimurium sodCI (Fig. 2). Like other prokaryotic Cu, Zn-SODs, the SodCII protein possesses a hydrophobic NH2-terminal sequence consistent with its secretion into the periplasmic space.
sodCI Is Associated with Highly Virulent Salmonella Serotypes.
Chemiluminescence-labeled sodCI and sodCII fragments were used to probe an XbaI digest of S. typhimurium 14028s DNA, a reference collection of chromosomal DNA from 75 strains representing 37 serotypes of S. enterica subspecies I (23) and DNA from additional clinical isolates in our laboratory collection (Table 2). The presence of the Salmonella spvR plasmid virulence gene was determined for comparison, because the spv genes, like sodCI, are associated with mobile DNA elements (35). The sodCI and sodCII loci were found to reside on distinct XbaI fragments separated by pulsed-field gel electrophoresis (data not shown); sodCI is found on an XbaI fragment of ≈750 kbp, whereas sodCII is located on an ≈250-kbp fragment.
Table 2.
Virulence genes and serovars carrying them
| Virulence gene
| |||
|---|---|---|---|
| sodCI | sodCII | spvR | Serovars |
| + | + | + | S. choleraesuis, S. dublin, S. enteritidis, S. gallinarum, S. heidelberg, S. pullorum, S. typhimurium, S. typhisuis |
| + | + | S. enteritidis, S. gallinarum, S. haifa, S. heidelberg, S. newport, S. paratyphiB, S. saintpaul | |
| + | + | S. arizona, S. paratyphiC | |
| + | S. agona, S. anatum, S. barta, S. benta, S. brandenburg, S. cerro, S. choleraesuis, S. decatur, S. derby, S. dublin, S. duisburg, S. emek, S. enteritidis, S. hadar, S. indiana, S. infantis, S. mbandaka, S. miami, S. montevideo, S. muenchen, S. newport, S. panama, S. paratyphiB, S. paratyphiC, S. reading, S. rubislaw, S. saintpaul, S. schwarzengrund, S. senftenberg, S. stanley, S. stanleyville, S. typhi, S. typhimurium, S. typhisuis, S. wien, S. worthington | ||
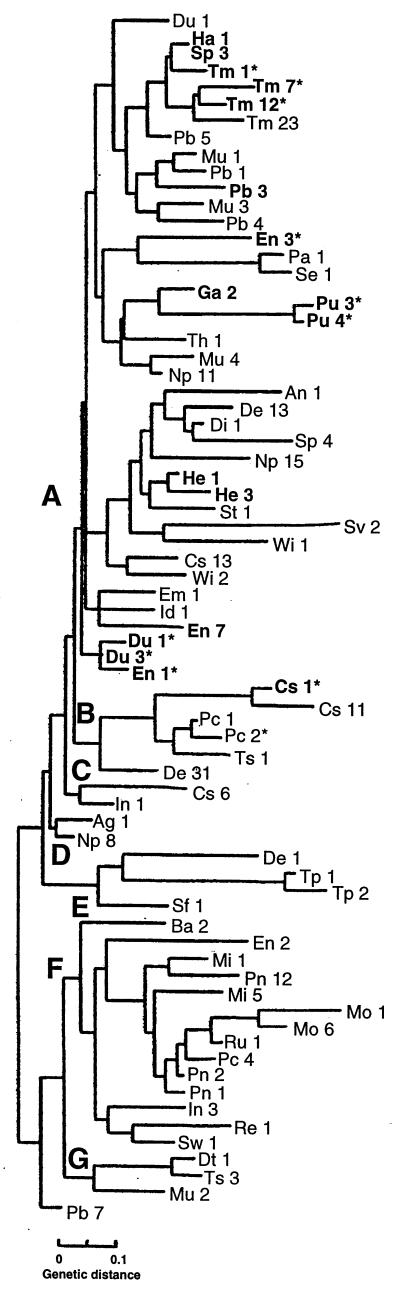
Although there are more than 2,000 recognized serotypes of Salmonella, specific serotypes are overrepresented among patients with invasive extraintestinal infection. Salmonella enteritidis, S. typhimurium, Salmonella heidelburg, and Salmonella dublin are responsible for nearly 70% of all episodes of Salmonella bacteremia in the United States (36). Representative strains from these highly virulent Salmonella serotypes were found to have a full complement of the sodCI, sodCII, and spv virulence-associated genes, whereas serotypes associated with intermediate virulence phenotypes (e.g., Salmonella newport, Salmonella saintpaul, Salmonella arizona) have some but not all of these factors, and the majority of serotypes possess only sodCII. Serotype was found to correlate imperfectly with the presence of bacteriophage- or plasmid-associated virulence loci sodCI and spvR, as the presence of these loci was found to vary among isolates of Salmonella choleraesuis, S. dublin, S. enteritidis, Salmonella gallinarum, S. newport, Salmonella paratyphi B, S. paratyphi C, S. saintpaul, Salmonella typhisuis, and S. typhimurium (Table 2, Fig. 3). Discrepancies were noted between our results with the spvR probe (Fig. 3) and those recently reported by Boyd and Hartl (37) for strains Tm23, De13, Du1, Cs11, Pc1, and De31 of the SARB reference collection (23). These differences might be attributable to the smaller probe used in our studies, or less likely to plasmid loss during laboratory passage of these strains.
Figure 3.
Presence of sodCI and spvR in Salmonella Reference Collection B Strains. Strains and evolutionary tree are modified from ref. 23, with permission. Strains carrying sodCI are indicated in bold type. Strains carrying spvR are indicated by ∗. Abbreviations of Salmonella serotypes: Ag, S. agona; An, S. anatum; Ba, S. brandenburg; Cs, S. cholerasuis; De, S. derby; Di, S. duisburg; Dt, S. decatur; Du, S. dublin; Em, S. emek; En, S. enteritidis; Ga, S. gallinarum; Ha, S. haifa; He, S. heidelberg; Id, S. indiana; In, S. infantis; Mi, S. miami; Mo, S. montevideo; Mu, S. muenchen; Np, S. newport; Pa, S. paratyphi A; Pb, S. paratyphi B; Pc, S. paratyphi C; Pn, S. panama; Pu, S. pullorum; Re, S. reading; Ru, S. rubislaw; Se, S. sendai; Sf, S. senftenberg; Sp, S. saintpaul; St, S. stanley; Sv, S. stanleyville; Sw, S. schwarzengrund; Th, S. thompson; Tm, S. typhimurium; Tp, S. typhi; Ts, S. typhisuis; Wi, S. wien. [Reproduced with permission from ref. 23 (Copyright 1993, Society for General Microbiology)].
sodCI and sodCII Expression Are Differentially Regulated.
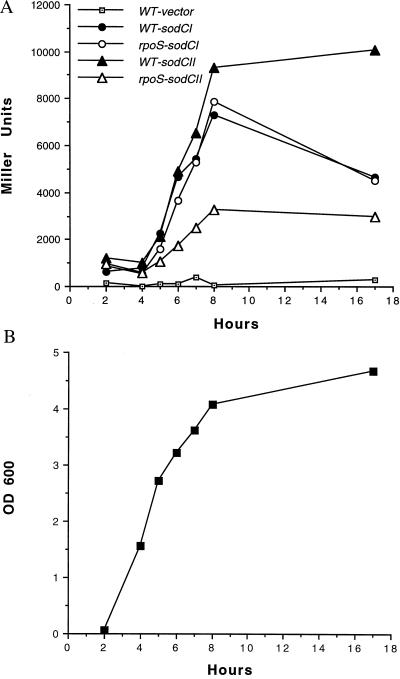
A transcriptional β-galactosidase fusion with the sodCI promoter was constructed by ligating a 277-bp fragment encompassing 81 upstream residues and the initial 196 bp of the sodCI coding region into the BamHI and EcoRI restriction sites of reporter plasmid pRS1274 (28), and an aph cassette (Amersham Pharmacia) was inserted into the unique PstI site of this plasmid to create a kanamycin-resistant derivative. An analogous plasmid carrying the sodCII promoter was constructed by using a 584-bp fragment containing 432 upstream residues and the initial 152 bp of sodCII coding sequence. The transcriptional fusions were electroporated into S. typhimurium 14028s (wild-type) and S. typhimurium SF1005 (rpoS), and β-galactosidase activity was assayed at various phases of growth in rich medium (Fig. 4). sodCII expression is maximal at stationary phase and σs-dependent. β-Galactosidase expression from a sodCI-lacZ reporter construct also appears to rise during the transition from exponential to stationary phase, but this induction is σs-independent and does not appear to be as sustained during stationary phase as that of sodCII (Fig. 4A).
Figure 4.
Effects of growth phase and σS on sodCI and sodCII expression. Expression of both PsodC1-lacZ and PsodCII-lacZ fusions increases during the transition from exponential growth to stationary phase. However, expression of PsodC1-lacZ is σS-independent and subsequently declines, whereas σS -dependent expression of PsodCII-lacZ is sustained. β-Galactosidase activity in Miller units (32) from S. typhimurium 14028s (wild-type) or SF1005 (rpoS) carrying PsodC1-lacZ or PsodCII-lacZ fusions is shown in A. The OD600 of S. typhimurium 14028s carrying PsodC1-lacZ is shown in B; growth curves were highly similar for all strains tested.
Both sodC Loci Contribute to Salmonella Virulence in Mice.
A null mutation was created in sodCI by insertion of a kanamycin-resistance cassette between the EcoRI restriction site at nucleotide 191 of the sodCI coding sequence and the ClaI site immediately downstream of the sodCI ORF. The interrupted sodCI allele was introduced into the chromosome of S. typhimurium 14028s by allelic exchange using suicide vector pKNG101 (27), resulting in strain MF1006. A null mutation was created in sodCII by cloning the 343-bp internal sodCII fragment into the EcoRI and HindIII restriction sites of suicide vector pRR10(ΔtrfA) (24) and allowing this construct to homologously recombine into the S. typhimurium 14028s chromosome, creating strain MF1007. JF3912 was constructed by bacteriophage P22-mediated transduction of the sodCII mutation into S. typhimurium UK1, and construction of the sodCI sodCII double-mutant strain MF1008 was achieved by transduction of the sodCI mutation into strain MF1007. Interruption of the sodCI and sodCII genes was confirmed by using Southern blotting (data not shown). Confirmation that sodCII encodes ASP71 was obtained by using two-dimensional SDS/PAGE analysis to compare JF3912 with the parent strain UK1 (Fig. 1).
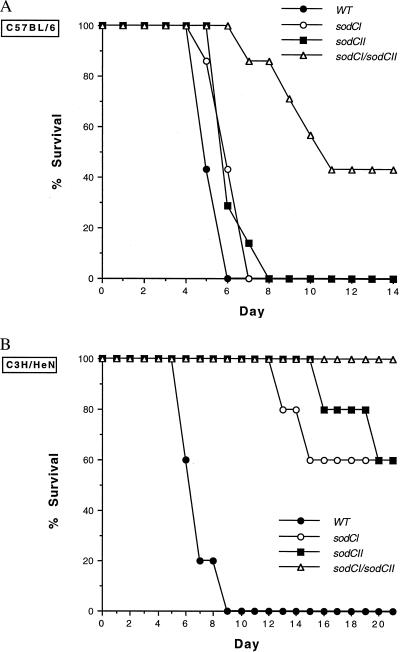
S. typhimurium strains carrying mutations in either or both sodC loci were compared with the isogenic wild-type parent strain for lethality in mice after intraperitoneal inoculation. The Salmonella-susceptible Itys C57BL/6 mice each received 600–800 organisms, and the Salmonella-resistant Ityr C3H/HeN mice each received 700–1,200 organisms. In the Salmonella-susceptible Itys C57BL/6 mouse strain, differences in virulence between wild-type S. typhimurium and strains carrying mutations in single sodC genes were scarcely discernible, but the strain with mutations in both sodC loci caused significantly less mortality (Fig. 5A). In the more resistant Ityr C3H/HeN mouse strain, all of the sodC mutant strains were significantly attenuated for virulence (Fig. 5B). Thus, the results in the resistant mouse strain demonstrate that each sodC locus contributes to virulence, whereas the results in the susceptible mouse strain show that these contributions can be additive.
Figure 5.
Mortality of C57BL/6 or C3H/HeN mice infected with sodC mutant S. typhimurium. Intraperitoneal inocula of wild-type, sodCI mutant, sodCII mutant, or sodCI/sodCII double mutant S. typhimurium were administered to C57BL/6 (Itys) mice (A) or C3H/HeN (Ityr) mice (B). Mortality was recorded daily. The virulence of S. typhimurium carrying mutations in single sodC loci was minimally attenuated in susceptible C57BL/6 mice, but the loss of both sodC loci had a marked effect on virulence. In contrast, mutations in either or both sodC loci significantly reduced virulence in resistant C3H/HeN mice.
DISCUSSION
The importance of periplasmic Cu, Zn-SOD in Salmonella pathogenesis is underscored by existence of two distinct and unlinked sodC genes. The first of these genes to be discovered is now designated sodCI, and appears to be encoded on a cryptic λ-like bacteriophage (13, 14). The second locus, sodCII, is more closely related to the E. coli sodC gene (Fig. 2).
Both SodC proteins appear to be functionally important, because a combination of mutations in both sodC genes confers a greater reduction in virulence for mice (Fig. 4A) than single mutations in either sodC gene alone. The S. typhimurium sodCII gene is maximally expressed in stationary phase (Fig. 4), similarly to the sodC genes of E. coli (17), Caulobacter crescentus (12), and Legionella pneumophila (11). This growth phase-dependent regulation is attributable to dependence on the alternative sigma factor σs (17, 24, 33). This observation further establishes σs as a coordinate regulator of Salmonella virulence determinants, because σs has been previously shown to control expression of the spv plasmid virulence genes (24). Interestingly, sodCI expression may also be enhanced during the growth transition from exponential to stationary phase (Fig. 3), despite its σs-independence, but its stationary phase expression does not appear to be as sustained as that of sodCII. A growing number of bacterial virulence genes have been found to be induced by nutrient deprivation or depletion (38–42), suggesting that the complex conditions of stationary-phase cultures may mimic the in vivo host environment encountered by pathogenic bacteria in some respects.
The contribution of SodC to Salmonella virulence is more readily demonstrated in genetically resistant Ityr C3H/HeN mice than in genetically susceptible Itys C57BL/6 mice carrying the Nramp1 G169D allele (ref. 43 and 44; Fig. 5). Because Cu, Zn-SOD has been implicated in the resistance of Salmonella to products of nitric oxide synthase and the phagocyte NADPH oxidase (13), the reduced phagocyte production of reactive nitrogen and oxygen species associated with the G169D Nramp1 mutation (45–47) is likely to account for the diminished role of Cu, Zn-SOD for Salmonella virulence in Itys mice.
Although nearly all nontyphoidal Salmonella are considered to represent the single species S. enterica (48), it is well recognized that individual S. enterica strains can vary widely in their pathogenicity (49–52). Our analysis of the distribution of sodC alleles among various Salmonella isolates reveals that sodCII is widely conserved among Salmonella species (Table 2), but sodCI is only carried by selected strains belonging to some of the most highly pathogenic serotypes (53), including those associated with extraintestinal infection (49, 51). Thus, sodCI joins the spv plasmid virulence genes (35, 49, 54) and sopE (55) as genetic loci that can help to explain the diversity of Salmonella virulence. The association of sodCI and spvR with mobile genetic elements presumably accounts for their patchy distribution within Salmonella strains (Fig. 3).
After its divergence from E. coli approximately 100 million years ago, Salmonella acquired several “pathogenicity islands” now carried by nearly all strains (52, 55–60). However, additional horizontally acquired determinants carried by bacteriophages or plasmids appear to have enhanced the virulence of many of the strains responsible for Salmonella-associated morbidity and mortality in the world today. It is evident from our studies that classical Kaufmann–White serotype identification correlates imperfectly with the presence of important virulence loci. The analysis of the sodC genes and their distribution powerfully illustrates the ability of a genotypic perspective to provide important insights into the evolution of bacterial virulence.
Acknowledgments
This work is supported in part by grants from the National Institutes of Health (A.B., M.A.D., U.O., J.W.F., and F.F.), the United States Department of Agriculture (A.B.), and the James Biundo Foundation (F.F.). The authors are grateful to Andrés Vazquez-Torres for critical review of this manuscript prior to publication, and thank the University of Colorado Health Sciences Center Cancer Center Sequencing Facility for technical support.
ABBREVIATIONS
- SOD
superoxide dismutase
- ASP
acid shock protein
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF056931).
References
- 1.Martin J P, Jr, Fridovich I. J Biol Chem. 1981;256:6080–6089. [PubMed] [Google Scholar]
- 2.Bricker B J, Tabatabai L B, Judge B A, Deyoe B L, Mayfield J E. Infect Immun. 1990;58:1935–1939. doi: 10.1128/iai.58.9.2935-2939.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman H M, Ely B. J Bacteriol. 1990;172:2901–2910. doi: 10.1128/jb.172.6.2901-2910.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroll J S, Langford P R, Loynds B M. J Bacteriol. 1991;173:7449–7457. doi: 10.1128/jb.173.23.7449-7457.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langford P R, Loynds B M, Kroll J S. J Gen Microbiol. 1992;138:517–522. doi: 10.1099/00221287-138-3-517. [DOI] [PubMed] [Google Scholar]
- 6.Benov L T, Fridovich I. J Biol Chem. 1994;269:25310–25314. [PubMed] [Google Scholar]
- 7.Kroll J S, Langford P R, Wilks K E, Keil A D. Microbiology. 1995;141:2271–2279. doi: 10.1099/13500872-141-9-2271. [DOI] [PubMed] [Google Scholar]
- 8.Canvin J, Langford P R, Wilks K E, Kroll J S. FEMS Microbiol Lett. 1996;136:215–220. doi: 10.1111/j.1574-6968.1996.tb08052.x. [DOI] [PubMed] [Google Scholar]
- 9.Lainson F A, Thomson N, Rowe H A, Langford P R, Aitchison K D, Donachie W, Kroll J S. FEMS Microbiol Lett. 1996;142:11–17. doi: 10.1111/j.1574-6968.1996.tb08400.x. [DOI] [PubMed] [Google Scholar]
- 10.Stevens M K, Hassett D J, Radolf J D, Hansen E J. Gene. 1996;183:35–40. doi: 10.1016/s0378-1119(96)00417-9. [DOI] [PubMed] [Google Scholar]
- 11.St. John G, Steinman H M. J Bacteriol. 1996;178:1578–1584. doi: 10.1128/jb.178.6.1578-1584.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnell S, Steinman H M. J Bacteriol. 1995;177:5924–5929. doi: 10.1128/jb.177.20.5924-5929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeGroote M A D, Ochsner U A, Shiloh M U, Nathan C, McCord J M, Dinauer M C, Libby S J, Vazquez-Torres A, Xu Y, Fang F C. Proc Natl Acad Sci USA. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrant J L, Sansone A, Canvin J R, Pallen M J, Langford P R, Wallis T S, Dougan D, Kroll J S. Mol Microbiol. 1997;25:785–796. doi: 10.1046/j.1365-2958.1997.5151877.x. [DOI] [PubMed] [Google Scholar]
- 15.Wilks K E, Dunn K L, Farrant J L, Reddin K M, Gorringe A R, Langford P R, Kroll J S. Infect Immun. 1998;66:213–217. doi: 10.1128/iai.66.1.213-217.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.San Mateo L R, Hobbs M M, Kawula T H. Mol Microbiol. 1998;27:391–404. doi: 10.1046/j.1365-2958.1998.00687.x. [DOI] [PubMed] [Google Scholar]
- 17.Gort A S, Ferber D M, Imlay J. Mol Microbiol. 1999;32:179–191. doi: 10.1046/j.1365-2958.1999.01343.x. [DOI] [PubMed] [Google Scholar]
- 18.Bochner B R. BioTechniques. 1984;2:234–240. [Google Scholar]
- 19.Blomfield I C, Vaughn V, Rest R F, Eisenstein B I. Mol Microbiol. 1991;5:1447–1457. doi: 10.1111/j.1365-2958.1991.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 20.Vogel H J, Bonner D M. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 21.Curtiss R I, Porter S B, Munson M, Tinge S A, Hassan J O, Gentry-Weeks C, Kelly S M. In: Colonization Control of Human Bacterial Enteropathogens in Poultry. Blankenship L C, Bailey J H S, Cox N A, Stern N J, Meinersmann R J, editors. New York: Academic; 1981. pp. 169–198. [Google Scholar]
- 22.Bearson S M D, Benjamin W H J, Swords W E, Foster J W. J Bacteriol. 1996;178:2572–2579. doi: 10.1128/jb.178.9.2572-2579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boyd E F, Wang F S, Beltran P, Plock S A, Nelson K, Selander R K. J Gen Microbiol. 1993;139:1125–1132. doi: 10.1099/00221287-139-6-1125. [DOI] [PubMed] [Google Scholar]
- 24.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon R, O’Connell M, Labes M, Pühler A. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- 26.Miller V L, Mekalanos J J. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaniga K, Delor I, Cornelis G R. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 28.Simons R W, Houman F, Kleckner N. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 30.Mahon J, Lax A J. Epidemiol Infect. 1993;111:455–464. doi: 10.1017/s0950268800057186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spector M P, Aliabadi Z, Gonzalez T, Foster J W. J Bacteriol. 1986;168:420–424. doi: 10.1128/jb.168.1.420-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller J H. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab. Press; 1972. [Google Scholar]
- 33.Lee I S, Lin J, Hall H K, Bearson B, Foster J W. Mol Microbiol. 1995;17:155–167. doi: 10.1111/j.1365-2958.1995.mmi_17010155.x. [DOI] [PubMed] [Google Scholar]
- 34.Fields P I, Swanson R V, Haidaris C G, Heffron F. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmer B M, Tran M, Heffron F. J Bacteriol. 1999;181:1364–1368. doi: 10.1128/jb.181.4.1364-1368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine W C, Buehler J W, Bean N H, Tauxe R V. J Infect Dis. 1991;164:81–87. doi: 10.1093/infdis/164.1.81. [DOI] [PubMed] [Google Scholar]
- 37.Boyd E F, Hartl D L. Genetics. 1998;149:1183–1190. doi: 10.1093/genetics/149.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeMaio J, Zhang Y, Ko C, Young D B, Bishai W R. Proc Natl Acad Sci USA. 1996;93:2790–2794. doi: 10.1073/pnas.93.7.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badger J L, Miller V L. J Bacteriol. 1998;180:793–800. doi: 10.1128/jb.180.4.793-800.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Byrne B, Swanson M S. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Engelbrecht F, Dominguez-Bernal G, Hess J, Dickneite C, Greiffenberg L, Lampidis R, Raffelsbauer D, Daniels J J, Dreft J, Kaufmann S H, et al. Mol Microbiol. 1998;30:405–417. doi: 10.1046/j.1365-2958.1998.01076.x. [DOI] [PubMed] [Google Scholar]
- 42.Kullik I, Giachino P, Fuchs T. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vidal S M, Malo D, Vogan K, Skamene E, Gros P. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 44.Vidal S M, Pinner E, Lepage P, Gauthier S, Gros P. J Immunol. 1996;157:3559–3568. [PubMed] [Google Scholar]
- 45.Denis M, Forget A, Pelletier M, Skamene E. Clin Exp Immunol. 1988;73:370–375. [PMC free article] [PubMed] [Google Scholar]
- 46.Rojas M, Barrera L F, Puzo G, Garcia L F. J Immunol. 1997;159:1352–1361. [PubMed] [Google Scholar]
- 47.Olivier M, Cook P, Desanctis J, Hel Z, Wojciechowski W, Reiner N E, Skamene E, Radzioch D. Eur J Biochem. 1998;251:734–743. doi: 10.1046/j.1432-1327.1998.2510734.x. [DOI] [PubMed] [Google Scholar]
- 48.Le Minor L, Popoff M Y. Int J Syst Bacteriol. 1987;37:465–468. [Google Scholar]
- 49.Roudier C, Krause M, Fierer J, Guiney D G. Infect Immun. 1990;58:1180–1185. doi: 10.1128/iai.58.5.1180-1185.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fierer J, Krause M, Tauxe R, Guiney D. J Infect Dis. 1992;166:639–642. doi: 10.1093/infdis/166.3.639. [DOI] [PubMed] [Google Scholar]
- 51.Ochman H, Soncini F C, Solomon F, Groisman E A. Proc Natl Acad Sci USA. 1996;93:7800–7804. doi: 10.1073/pnas.93.15.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bäumler A J, Tsolis R M, Ficht T A, Adams L G. Infect Immun. 1998;66:4579–4587. doi: 10.1128/iai.66.10.4579-4587.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goldberg M B, Rubin R H. Infect Dis Clin North Am. 1988;2:571–598. [PubMed] [Google Scholar]
- 54.Gulig P A, Danbara H, Guiney D G, Lax A J, Norel F, Rhen M. Mol Microbiol. 1993;7:825–830. doi: 10.1111/j.1365-2958.1993.tb01172.x. [DOI] [PubMed] [Google Scholar]
- 55.Hardt W D, Urlaub H, Galan J E. Proc Natl Acad Sci USA. 1998;95:2574–2579. doi: 10.1073/pnas.95.5.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galan J E, Curtiss R I. Proc Natl Acad Sci USA. 1989;86:6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 58.Blanc-Potard A B, Groisman E A. EMBO J. 1997;16:5376–5385. doi: 10.1093/emboj/16.17.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collazo C M, Galan J E. Gene. 1997;192:51–59. doi: 10.1016/s0378-1119(96)00825-6. [DOI] [PubMed] [Google Scholar]
- 60.Wong K K, McClelland M, Stillwell L C, Sisk E C, Thurston S J, Saffer J D. Infect Immun. 1998;66:3365–3371. doi: 10.1128/iai.66.7.3365-3371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]