Abstract
The African trypanosome Trypanosoma brucei, which causes sleeping sickness in humans and Nagana disease in livestock, is spread via blood-sucking Tsetse flies. In the fly's intestine, the trypanosomes survive digestive and trypanocidal environments, proliferate, and translocate into the salivary gland, where they become infectious to the next mammalian host. Here, we show that for successful survival in Tsetse flies, the trypanosomes use trans-sialidase to transfer sialic acids that they cannot synthesize from host's glycoconjugates to the glycosylphosphatidylinositols (GPIs), which are abundantly expressed on their surface. Trypanosomes lacking sialic acids due to a defective generation of GPI-anchored trans-sialidase could not survive in the intestine, but regained the ability to survive when sialylated by means of soluble trans-sialidase. Thus, surface sialic acids appear to protect the parasites from the digestive and trypanocidal environments in the midgut of Tsetse flies.
Keywords: Trypanosoma brucei, trypanosomiasis, glycosylphosphatidylinositol, trans-sialidase, GPI transamidase
Introduction
African trypanosomes, Trypanosoma brucei, generate serious medical and agroeconomical problems in many sub-Saharan African countries by causing sleeping sickness in humans and Nagana disease in livestock. The unicellular parasites are transmitted by blood-sucking Tsetse flies. In the fly's midgut, the trypanosomes survive digestive and trypanocidal environments (1–3), proliferate, and translocate into the salivary gland, where they become infectious to the next mammalian host (4, 5). To interfere with spreading of the parasites, molecular mechanisms of these events should be clarified, but little is known about how the parasites manage this process in Tsetse flies.
The T. brucei cells of the Tsetse fly stage, termed procyclic form, are covered by 3 × 106 molecules of glycosylphosphatidylinositol (GPI)-anchored proteins, procyclins (6–9). Procyclins are products of a small multigene family containing characteristic amino acid repeats. Glutaminyl-prolyl procyclins have repeated glutaminyl-prolyl dipeptides, whereas GPEET procyclins have repeated glycyl-prolyl-glutaminyl-glutaminyl-threonyl pentapeptides. Gene-manipulated procyclic parasites bearing only one intact GPEET gene had significantly decreased infectivity to Tsetse flies, indicating an important role of procyclins during infection (8).
GPI-anchored proteins, such as procyclins, have a signal peptide for GPI attachment at their COOH termini. In the ER, GPI transamidase replaces the signal peptide with a preassembled GPI. The GPI transamidase of T. brucei consists of five subunits as follows: TbGPI8, TbGAA1, TbGPI16, TTA1, and TTA2 (10). TbGPI8 is a catalytic subunit that cleaves the signal peptide (11). GPI, a complex glycolipid, is also synthesized in the ER through sequential additions of components to phosphatidylinositol. TbGPI10 gene encodes α1-2 mannosyltransferase that adds the third mannose to GPI (12). TbGPI10 knockout procyclics (GPI10KO) lost the surface expression of procyclins due to a lack of fully assembled GPI anchors capable of attachment to procyclins and other proteins (12). We showed that procyclin-less GPI10KO survived and proliferated in Tsetse flies, albeit less efficiently than the wild-type parasites, confirming that the protein portions of procyclins play some role but are not essential for their survival (see Fig. 1 A; reference 12).
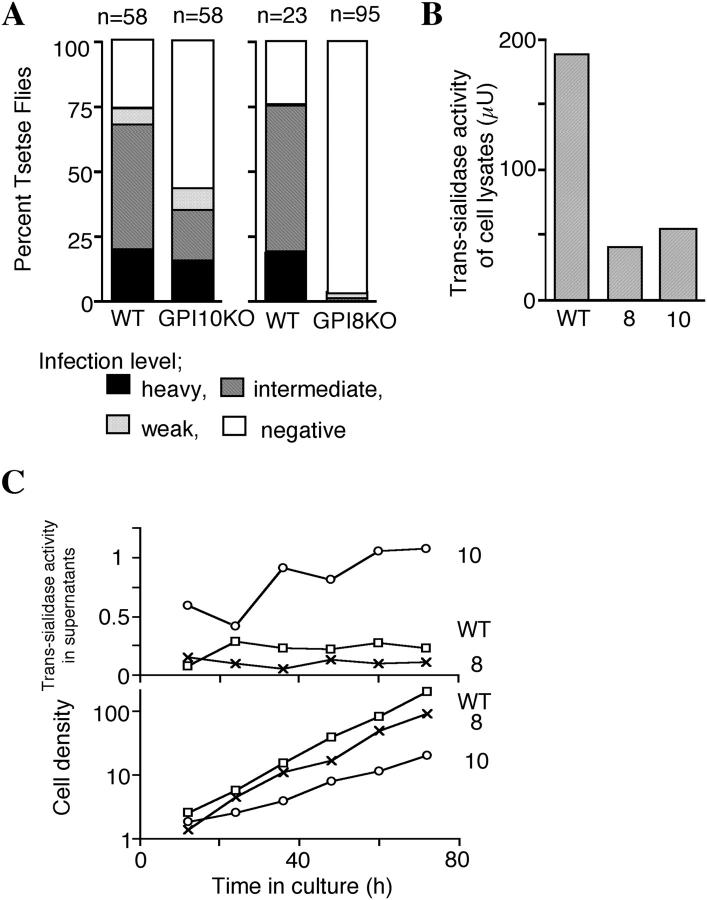
Figure 1.
Both infectious ability to Tsetse flies and trans-sialidase activity are greatly decreased in GPI8KO procyclics. (A) Different infectivities to Tsetse flies of GPI10KO and GPI8KO T. brucei. Wild-type, GPI10KO, and GPI8KO procyclics (WT, 10, and 8, respectively) were fed to Tsetse flies. After 24 d, flies were dissected, and the infectivities were scored as heavy (black area, 100–300 trypanosomes per field in 10 fields with the 20× objective), intermediate (shaded area, between “heavy” and “weak”), weak (dotted area, <3 three trypanosomes per field), and negative (white area, no trypanosome detectable). The number of flies in each group is indicated above each bar. (B) Trans-sialidase activity of cell lysates from 108 of wild-type, GPI8KO, and GPI10KO parasites (WT, 8, and 10, respectively) were determined. Trans-sialidase activity is expressed in microunits. (C) Release of trans-sialidase from GPI10KO procyclics into culture supernatant. Wild-type (□), GPI8KO (×), and GPI10KO (○) procyclics were inoculated at 105 cells/ml and cultured for 3 d. Every 12 h, trans-sialidase in the culture supernatant (top) and cell density (bottom, ×105/ml) were determined. Trans-sialidase activity in the supernatant was normalized by cell density and is expressed in μU/105 cells.
The GPI anchors of procyclins are modified by sialylated poly N-acetyl-lactosamine side chains (13). T. brucei cannot synthesize sialic acid, but the procyclic form expresses GPI-anchored trans-sialidases and, by means of this enzyme, transfers sialic acids from host-sialylated glycoconjugates present in the midgut (such as the blood meal and the midgut cells) to the side chain of GPI (14–16). Therefore, the procyclin coat is thought to make a sialylated glycocalyx and has an array of procyclin proteins on top of it (13).
We (11) and others (17) recently generated procyclic form lacking the procyclin coat by disrupting the TbGPI8 gene (11). Lillico et al. reported that TbGPI8 knockout procyclic parasites (GPI8KO) lost most of the infectivity to Tsetse flies (17). In the present work, we analyzed GPI8KO and GPI10KO procyclic parasites and found that sialic acid of GPI is critical for survival in Tsetse flies.
Materials and Methods
Trypanosome.
The procyclic form of T. brucei strain 427 was used in this work. TbGPI10 and TbGPI8 knockout mutant procyclics were established as described previously (11, 12).
Tsetse Fly Infection.
The procyclic form of T. brucei grown in SDM-79 with 10% (vol/vol) heat-inactivated FCS were mixed 1:1 with washed horse red blood cells in the medium at 107 cells/ml. Tsetse flies were infected with each clone through an artificial membrane (12). On day 24 (see Fig. 1 A) or 27 (see Fig. 3, B and C) after infection, flies were dissected and scored for the infection.
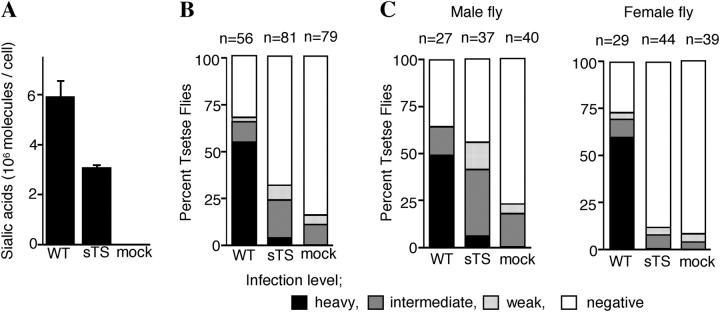
Figure 3.
Complementation of sialylation deficiency of TbGPI8-KO mutant by soluble form trans-sialidase. (A) Partial restoration of sialic acids on the surface of GPI8KO by expressing soluble form trans-sialidase. The amount of sialic acids on wild-type (WT), GPI8KO transfected with an empty vector (mock), and GPI8KO-expressing soluble form trans-sialidase (sTS) were determined. Standard deviation of three independent measurements is indicated. (B) Restoration of the surface sialic acids rescued infectivity to Tsetse flies. Infectivity was scored in a similar way as described in Fig. 1 A. The number of flies in each group is indicated above each bar. (C) Different infectivities of sTS-transfected trypanosomes in male and female flies.
Purification of Glycoconjugates.
Free GPIs were extracted with organic solvent followed by hydrophobic chromatography. In brief, freeze-dried parasites (∼3 × 1010 of GPI8KO cells and ∼3 × 109 of GPI10KO cells) were extracted with 2.5 ml chloroform/methanol (2:1, by volume) in a sonicating water bath for 10 min. After centrifugation (5,000 g, 10 min), the insoluble material was reextracted twice more, and the insoluble pellets were further extracted with 2.0 ml chloroform/methanol/water (10:10:3, by volume) under sonication. The latter procedure was repeated twice more, and the pool of soluble material was dried under an N2 stream and partitioned by adding 500 μl 1-butanol and 500 μl water. The lower (aqueous) phases were washed twice more with 500 μl of water-saturated butanol, dried in the speed-vac, resuspended in 500 μl 0.1 M ammonium acetate in 5% 1-propanol (vol/vol; buffer A), and loaded on an octyl-sepharose column (1.0 × 0.5 cm; Sigma-Aldrich) previously equilibrated in buffer A (18, 19). Glycolipids were eluted with a linear gradient over 50 ml at a flow rate of 12 ml/h, starting with buffer A and ending with 60% (vol/vol) 1-propanol in water. Samples were detected by orcinol staining after spotting 5 μl onto a TLC plate. Procyclins were purified from wild-type cells by organic solvent and octyl-sepharose chromatography, and characterized as mainly containing fully glycosylated glutaminyl-prolyl procyclins (18).
Analyses of Sugars and Trans-Sialidase.
Monosaccharides and myo-inositol contents were determined by gas chromatography–mass spectrometry as described previously (20). Monosaccharides were measured after methanolysis, re–N-acetylation, and trimethylsilyl derivatization (20). The myo-inositol contents were measured using selected ion monitoring after strong acid hydrolysis and trimethylsilyl derivatization (20). Sialic acids were quantified as follows: cells were washed six times, suspended in 0.1 NH2SO4, and hydrolyzed at 80°C for 1 h; and free sialic acids were determined with thiobarbituric acid method (21). Trans-sialidase was assessed by measuring its activity to hydrolyze the fluorogenic substrate 2′-(4-methylumbelliferyl)α-d-N-acetylneuraminic acid (4MU-NANA; Sigma-Aldrich; reference 22). In brief, the enzyme was incubated with 0.5 mM 4 MU-NANA in 20 mM Hepes, pH 7.2, at 28°C in a final volume of 20 μl. The reaction was terminated by adding 200 μl of 0.2 M Tris-HCl, pH 9.5, and the fluorescence was measured with a Fluoroskan II (Labsystems; Flow Laboratories Inc.). 1 U of enzyme is defined as the activity to hydrolyze 1 μmol of the substrate in 1 min.
Cloning and Expression of a Soluble Form of Trans-Sialidase.
We generated a soluble form of T. brucei trans-sialidase (GenBank/EMBL/DDBJ accession no. AF310232) by deleting COOH-terminal 20 amino acids (16). For this, we amplified the corresponding sequence by PCR from DNA of T. brucei strain 427 and cloned it into the AflII-ClaI site of an expression vector pPPMCS. The expression vector pPPMCS was constructed from pHD590 (23) by replacing its promoter and luciferase gene with the normal PARP promoter and a multicloning site having a sequence 5′-AAGCTTAAGGTACCGTACGACCATGGTATCGATACAATTGAGCTCCTAGGATCC-3′.
Results and Discussion
GPI8KO Procyclic Form Has a Severely Decreased Ability to Survive in Tsetse Flies.
GPI8KO mutant grew faster in vitro than GPI10KO mutant (Fig. 1 C). Nevertheless, GPI8KO had a greatly decreased ability to survive in the midgut than wild-type parasite or the GPI10KO mutant (Fig. 1 A), which was consistent with a paper by Lillico et al. (17).
GPI8KO Procyclic Form Lacks Cell Surface Sialic Acid.
A significant difference between GPI10KO and GPI8KO cells should be in the structures of their GPIs. TbGPI10 encodes a mannosyltransferase that adds the third mannose to GPI (12), which suggests that GPI10KO cells have truncated “nonprotein-linked” GPI with only two mannoses (Fig. 2 B). TbGPI8 encodes a catalytic subunit of GPI transamidase (11), suggesting that GPI8KO cells have nonprotein-linked GPI with a complete core (Fig. 2 C). Vassella et al. reported that procyclic trypanosomes completely lacking all procyclin genes express free GPI on their surface (24). Lillico et al. reported that some of the nonprotein-linked GPIs are on the surface of the GPI8KO (17). We found that GPI10KO as well as GPI8KO trypanosomes express abundant nonprotein-linked GPIs on their cell surface (unpublished data). To determine the carbohydrate compositions of these free GPIs, we isolated them from GPI10KO and GPI8KO cells, and as a reference, intact procyclins from the wild-type cells. The procyclins had the carbohydrate composition expected from their known structures (Table I) . The free GPI from GPI10KO cells had ∼2 mol of mannose per mole of myo-inositol and, in addition, galactose, N-acetylglucosamine, and sialic acid. The latter sugars are components of the sialylated poly-N-acetyl-lactosamine side chains found on procyclins (25) and free GPIs from procyclin null mutant (24) and GPI10KO (unpublished data). In contrast, the free GPIs from GPI8KO cells had mannose, galactose, and N-acetylglucosamine, but no sialic acid. Because it was reported that the GPI anchors are the major sialylated molecules on procyclic form T. brucei (14, 15), we determined the total sialic acid contents of those cells. Wild-type and GPI10KO cells had 5–6 × 106 and 2.5 × 106 sialic acid molecules per cell, respectively, whereas GPI8KO cells had no detectable sialic acid. Therefore, a lack of sialic acid is a major phenotype of GPI8KO cells.
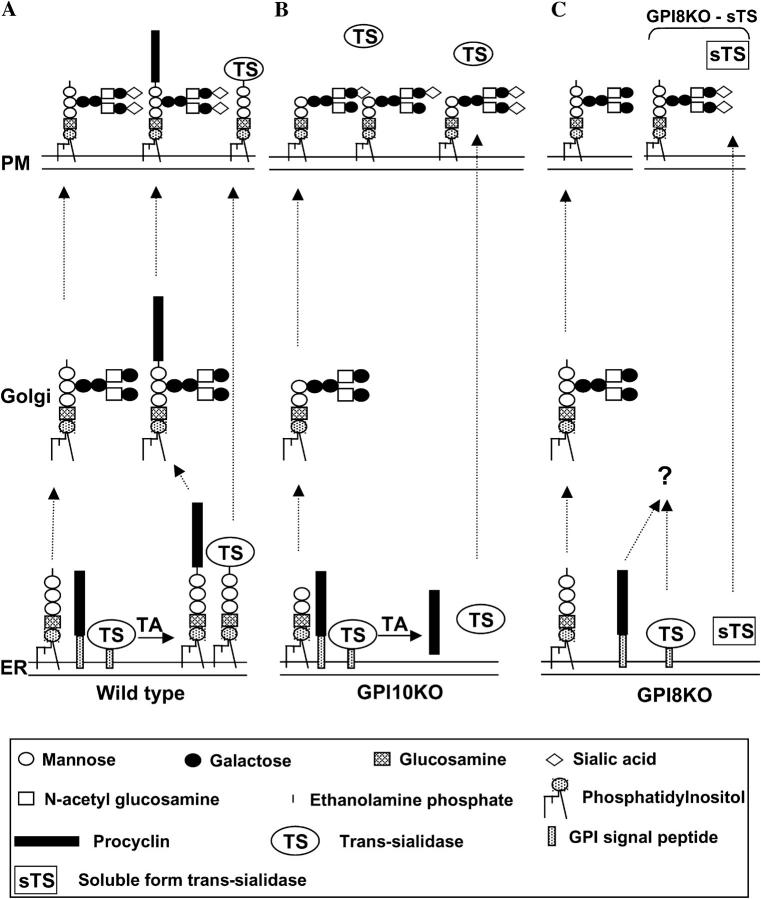
Figure 2.
Model for processing of procyclin and trans-sialidase (TS) in wild type and GPI mutant trypanosomes. (A) The successful GPI anchoring of procyclins and TS in the ER (bottom) and subsequent addition of branched poly-N-acetyl-lactosamine side chains in the Golgi (middle) lead to the surface expression of GPI-anchored procyclins and TS. The surface-expressed TS sialylates GPI side chains on procyclins and free GPIs. (B) The lack of TbGPI10 leads to the formation of a truncated GPI precursor that cannot be transferred to nascent procyclins and TS. Therefore, the transamidase (TA) produces soluble forms of procyclins and TS that are secreted from the cell. Thus, cell surface–free GPIs that have been modified with poly-N-acetyl-lactosamine structures become sialylated by soluble TS. (C) The lack of TbGPI8, the catalytic subunit of transamidase, prevents any processing of procyclins and TS, probably resulting in their intracellular degradation. Unused intact PP1 glycolipids are processed in the Golgi with branched poly-N-acetyl-lactosamine side chains and expressed on the cell surface. The free GPIs are not sialylated due to the absence of cell surface TS. However, when GPI8KO cells are transformed with a gene-encoding, soluble, secreted form of TS (sTS) (right), the surface of GPI8KO cells becomes sialylated. Mannose, galactose, inositol, N-acetylglucosamine, glucosamine, and sialic acid are represented by open circles, closed circles, dotted circles, open squares, dotted squares, and diamonds, respectively. Procyclins and GPI attachment signal peptides are depicted as black and dotted rectangles, respectively.
Table I.
Sugar Compositions of GPIs from GPI8KO and GPI10KO Cells and Procyclina
| Monosaccharide | Procyclin GPIb |
GPI10KO GPIb |
GPI8KO GPIb |
|---|---|---|---|
| mol | mol | mol | |
| Mannose | 3.87 ± 0.26c | 1.87 ± 0.16 | 1.64 ± 0.12d |
| Galactose | 9.20 ± 0.58 | 3.37 ± 0.27 | 2.97 ± 0.16 |
| N-acetylglucosamine | 10.0 ± 0.80c | 4.40 ± 0.72 | 3.40 ± 0.24 |
| Sialic acid | 3.30 ± 0.36 | 0.67 ± 0.02 | ND |
The compositional values are the means ± SD of three independent determinations.
Normalized with respect to myo-inositol.
The mannose and N-acetylglucosamine amounts for procyclin include contributions from the N-linked oligosaccharide present on some forms of procyclins.
The terminal mannose-6-phosphate derivative is not detected with the methanolysis/trimethylsilyl method. Therefore, only two out of the three mannose residues of GPI8KO GPI were detected.
Defective Generation of Trans-sialidase Accounts for the Lack of Sialic Acid on TbGPI8KO Cells.
We measured trans-sialidase to understand the basis of the absence and the presence of sialic acids on GPI8KO and GPI10KO cells, respectively. GPI8KO and GPI10KO cells had similarly decreased trans-sialidase activity (Fig. 1 B, ∼20–30% of wild-type levels). Because trans-sialidases are GPI-anchored proteins, we measured the enzyme activity in the culture supernatants expecting possible secretion. Indeed, GPI10KO cells secreted a large amount of trans-sialidase, whereas GPI8KO and wild-type cells did only slightly (Fig. 1 C). The secreted trans-sialidase would account for the presence of sialic acid on GPI10KO cells.
We propose a mechanism to explain the aforementioned phenotypes. In the ER of wild-type cells, procyclin and trans-sialidase precursors are recognized by the GPI transamidase complex and their COOH-terminal GPI signal peptides are replaced by the intact GPI precursor, PP1 (Fig. 2 A; reference 26). The GPI-anchored procyclins and some excess PP1 leave the ER and are processed in the Golgi apparatus to bear branched poly-N-acetyl-lactosamine side chains, which become terminally sialylated upon reaching the plasma membrane via the action of the cell surface trans-sialidase together with surrounding host sialoglycoconjugate donors (Fig. 2 A). In the GPI10KO cells, the GPI precursor is truncated and does not contain the ethanolamine group necessary for transfer to procyclin and trans-sialidase precursors (Fig. 2 B). Alternately, the entire GPI transamidase complex is present and will process the procyclin and trans-sialidase precursors by removing their GPI signals and transferring them to water instead of the GPI precursor (27). The latter results in the formation of soluble procyclins (12) and trans-sialidases that are secreted out of the cell (Fig. 1 C). In contrast, the GPI8KO cells make intact PP1 molecules, but cannot use them for transfer to procyclins and trans-sialidases because they lack the catalytic subunit of the GPI transamidase. In this case, the unprocessed procyclins and trans-sialidases remain associated with the ER membrane via their uncleaved GPI signal peptides and presumably undergo intracellular degradation (Fig. 2 C), which has been demonstrated for unprocessed GPI protein precursors in mammalian cells (28).
If this hypothesis is true, a truncated trans-sialidase lacking the COOH-terminal GPI-attachment signal sequence might be secreted and complement the lack of sialic acids on the surface of GPI8KO cells (Fig. 2 C). T. brucei has multiple trans-sialidase genes, and one of them has been shown to be functional (16). We cloned the functional isoform of trans-sialidase, constructed a truncated form (soluble form trans-sialidase [sTS]) and transfected GPI8KO cells with sTS expression plasmid (GPI8KO-sTS). The transfectant obtained sialic acids from a culture medium to a level approximately half that of the wild-type (Fig. 3 A, sTS and WT), a level similar to that of GPI10KO cells. Therefore, we partially restored sialic acids on GPI8KO cells. Most of the sialic acids on GPI8KO-sTS were found in the GPI-containing 1-butanol-saturated water phase derived from the extract with chloroform/methanol/water (10:10:3).
A Lack of Sialic Acid Is a Basis of Decreased Ability of TbGPI8KO to Survive In Tsetse Flies.
We examined the ability of GPI8KO-sTS cells to survive and proliferate in Tsetse flies. On day 27 after infection with procyclic trypanosomes, only 15% of flies had mock-transfected GPI8KO parasites all at weak (5%) or intermediate (10%) levels, whereas 31% of flies had GPI8KO-sTS parasites with more intermediate (21%) or even heavy levels (Fig. 3 B, 3%). This difference was significant (P < 0.02) as assessed by the Mann-Whitney U test. Therefore, the partial recovery of sialic acids resulted in the partial recovery of the ability to survive in Tsetse flies. We found that survival of GPI8KO-sTS in male flies was restored efficiently (Fig. 3 C, left, 13.5% weak, 35.1% intermediate, and 5.4% heavy in sTS vs. 5.0% weak, 17.5% intermediate, and 0% heavy in mock; P < 0.01), indicating that the addition of sialic acid by trans-sialidase is essential for the survival of procyclic forms of T. brucei. Trypanocidal lectins and antimicrobial peptides in addition to digestive enzymes are mechanisms of the resistance to trypanosomes in the Tsetse fly midgut (1–3). Sialic acids might be important for procyclics to evade one or both of these mechanisms. In female flies, sTS increased survival significantly, but less efficiently (Fig. 3 C, right, 2.3% weak, 9.1% intermediate, and 0% heavy in sTS vs. 5.1% weak, 2.6% intermediate, and 0% heavy in mock; P = 0.53). The difference between male and female flies was also significant (P < 0.01). A level of sialic acid required for survival might be higher in female flies and the level obtained by sTS would have not been enough to efficiently restore survival. There is an analysis that antimicrobial activity in the hemolymph of female Tsetse flies was stronger than that of male flies (29). If antimicrobial activity in midgut is also stronger, a higher level of sialic acid may be required for trypanosomes to survive in the female midgut, perhaps accounting for the inefficient recovery of survival in female flies.
Concluding Remarks.
The results of this work indicate that sialo-glycocalyx coat plays an important role for the survival of procyclic form T. brucei in the midgut of Tsetse fly. Therefore, trans-sialidase, side-chain modification of GPI, GPI core biosynthesis, and GPI transamidase are good targets for the development of antiprocyclic compounds. Inhibitors of these steps might act as a transmission blocking chemotherapeutics. Inhibitors of biosynthesis of GPI core or GPI transamidase should also work against the mammalian disease because the GPI biosynthesis pathway is essential for the bloodstream form (12).
Acknowledgments
We thank Dr. H. Ashida for discussion and K. Kinoshita and F. Ishii for assistance.
The work was supported by the Ministry of Education, Science, Sports, Culture and Technology of Japan (to T. Kinoshita, K. Nagamune, and Y. Maeda); the Wellcome Trust (to M.A.J. Ferguson and A. Acosta-Serrano); and the Human Frontier Science Program (to T. Kinoshita and M.A.J. Ferguson). A. Acosta-Serrano was also supported by a Wellcome Trust Travelling Research Fellowship.
The present address of A. Acosta-Serrano is Wellcome Centre for Molecular Parasitology, Glasgow University, Glasgow G11 6NU, Scotland, UK.
References
- 1.Welburn, S.C., and I. Maudlin. 1999. Tsetse-trypanosome interactions: rites of passage. Parasitol. Today. 15:399–403. [DOI] [PubMed] [Google Scholar]
- 2.Hao, Z., I. Kasumba, M.J. Lehane, W.C. Gibson, J. Kwon, and S. Aksoy. 2001. Tsetse immune responses and trypanosome transmission: implications for the development of tsetse-based strategies to reduce trypanosomiasis. Proc. Natl. Acad. Sci. USA. 98:12648–12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulanger, N., R. Brun, L. Ehret-Sabatier, C. Kunz, and P. Bulet. 2002. Immunopeptides in the defense reactions of Glossina morsitans to bacterial and Trypanosoma brucei brucei infections. Insect Biochem. Mol. Biol. 32:369–375. [DOI] [PubMed] [Google Scholar]
- 4.Vickerman, K. 1985. Developmental cycles and biology of pathogenic trypanosomes. Br. Med. Bull. 41:105–114. [DOI] [PubMed] [Google Scholar]
- 5.Vickerman, K., L. Tetley, K.A. Hendry, and C.M. Turner. 1988. Biology of African trypanosomes in the tsetse fly. Biol. Cell. 64:109–119. [DOI] [PubMed] [Google Scholar]
- 6.Pays, E., and D.P. Nolan. 1998. Expression and function of surface proteins in Trypanosoma brucei. Mol. Biochem. Parasitol. 91:3–36. [DOI] [PubMed] [Google Scholar]
- 7.McConville, M.J., and M.A. Ferguson. 1993. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem. J. 294:305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruepp, S., A. Furger, U. Kurath, C.K. Renggli, A. Hemphill, R. Brun, and I. Roditi. 1997. Survival of Trypanosoma brucei in the tsetse fly is enhanced by the expression of specific forms of procyclin. J. Cell Biol. 137:1369–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roditi, I., and M. Liniger. 2002. Dressed for success: the surface coats of insect-borne protozoan parasites. Trends Microbiol. 10:128–134. [DOI] [PubMed] [Google Scholar]
- 10.Nagamune, K., K. Ohishi, H. Ashida, Y. Hong, J. Hino, K. Kangawa, N. Inoue, Y. Maeda, and T. Kinoshita. 2003. GPI transamidase of Trypanosoma brucei has two previously uncharacterized (trypanosomatid transamidase 1 and 2) and three common subunits. Proc. Natl. Acad. Sci. USA. 100:10682–10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohishi, K., K. Nagamune, Y. Maeda, and T. Kinoshita. 2003. Two subunits of glycosylphosphatidylinositol transamidase, GPI8 and PIG-T, form a functionally important intermolecular disulfide bridge. J. Biol. Chem. 278:13959–13967. [DOI] [PubMed] [Google Scholar]
- 12.Nagamune, K., T. Nozaki, Y. Maeda, K. Ohishi, T. Fukuma, T. Hara, R.T. Schwarz, C. Sutterlin, R. Brun, H. Riezman, and T. Kinoshita. 2000. Critical roles of glycosylphosphatidylinositol for Trypanosoma brucei. Proc. Natl. Acad. Sci. USA. 97:10336–10341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehlert, A., N. Zitzmann, J.M. Richardson, A. Treumann, and M.A. Ferguson. 1998. The glycosylation of the variant surface glycoproteins and procyclic acidic repetitive proteins of Trypanosoma brucei. Mol. Biochem. Parasitol. 91:145–152. [DOI] [PubMed] [Google Scholar]
- 14.Pontes de Carvalho, L.C., S. Tomlinson, F. Vandekerckhove, E.J. Bienen, A.B. Clarkson, M.S. Jiang, G.W. Hart, and V. Nussenzweig. 1993. Characterization of a novel trans-sialidase of Trypanosoma brucei procyclic trypomastigotes and identification of procyclin as the main sialic acid acceptor. J. Exp. Med. 177:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engstler, M., G. Reuter, and R. Schauer. 1993. The developmentally regulated trans-sialidase from Trypanosoma brucei sialylates the procyclic acidic repetitive protein. Mol. Biochem. Parasitol. 61:1–13. [DOI] [PubMed] [Google Scholar]
- 16.Montagna, G., M.L. Cremona, G. Paris, M.F. Amaya, A. Buschiazzo, P.M. Alzari, and A.C. Frasch. 2002. The trans-sialidase from the african trypanosome Trypanosoma brucei. Eur. J. Biochem. 269:2941–2950. [DOI] [PubMed] [Google Scholar]
- 17.Lillico, S., M.C. Field, P. Blundell, G.H. Coombs, and J.C. Mottram. 2003. Essential roles for GPI-anchored proteins in African trypanosomes revealed using mutants deficient in GPI8. Mol. Biol. Cell. 14:1182–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acosta-Serrano, A., R.N. Cole, A. Mehlert, M.G. Lee, M.A. Ferguson, and P.T. Englund. 1999. The procyclin repertoire of Trypanosoma brucei. Identification and structural characterization of the Glu-Pro-rich polypeptides. J. Biol. Chem. 274:29763–29771. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson, M.A.J., P. Murray, H. Rutherford, and M.J. McConville. 1993. A simple purification of procyclic acidic repetitive protein and demonstration of a sialylated glycosyl-phosphatidylinositol membrane anchor. Biochem. J. 291:51–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferguson, M.A.J. 1993. GPI membrane anchors: isolation and analysis. Glycobiology: A Practical Approach. M. Fukuda and A. Kobata, editors. IRL Press, Oxford. 349–383.
- 21.Warren, L. 1963. Thiobarbituric acid assay of sialic acids. Methods Enzymol. 6:463–465. [Google Scholar]
- 22.Schenkman, S., L. Pontes de Carvalho, and V. Nussenzweig. 1992. Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. J. Exp. Med. 175:567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biebinger, S., L.E. Wirtz, P. Lorenz, and C. Clayton. 1997. Vectors for inducible expression of toxic gene products in bloodstream and procyclic Trypanosoma brucei. Mol. Biochem. Parasitol. 85:99–112. [DOI] [PubMed] [Google Scholar]
- 24.Vassella, E., P. Bütikofer, M. Engstler, J. Jelk, and I. Roditi. 2003. Procyclin null mutants of Trypanosoma brucei express free glycosylphosphatidylinositols on their surface. Mol. Biol. Cell 14:1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treumann, A., N. Zitzmann, A. Hulsmeier, A.R. Prescott, A. Almond, J. Sheehan, and M.A. Ferguson. 1997. Structural characterisation of two forms of procyclic acidic repetitive protein expressed by procyclic forms of Trypanosoma brucei. J. Mol. Biol. 269:529–547. [DOI] [PubMed] [Google Scholar]
- 26.Field, M.C., A.K. Menon, and G.A. Cross. 1991. A glycosylphosphatidylinositol protein anchor from procyclic stage Trypanosoma brucei: lipid structure and biosynthesis. EMBO J. 10:2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma, D.K., J. Vidugiriene, J.D. Bangs, and A.K. Menon. 1999. A cell-free assay for glycosylphosphatidylinositol anchoring in African trypanosomes. Demonstration of a transamidation reaction mechanism. J. Biol. Chem. 274:16479–16486. [DOI] [PubMed] [Google Scholar]
- 28.Field, M.C., P. Moran, W. Li, G.A. Keller, and I.W. Caras. 1994. Retention and degradation of proteins containing an uncleaved glycosylphosphatidylinositol signal. J. Biol. Chem. 269:10830–10837. [PubMed] [Google Scholar]
- 29.Kaaya, G.P., and N. Darji. 1988. The humoral defense system in tsetse: differences in response due to age, sex and antigen types. Dev. Comp. Immunol. 12:255–268. [DOI] [PubMed] [Google Scholar]