Abstract
Humoral immunity is maintained by long-lived plasma cells, constitutively secreting antibodies, and nonsecreting resting memory B cells that are rapidly reactivated upon antigen encounter. The activation requirements for resting memory B cells, particularly the role of T helper cells, are unclear. To analyze the activation of memory B cells, mice were immunized with human cytomegalovirus, a complex human herpesvirus, and tick-born encephalitis virus, and a simple flavivirus. B cell populations devoid of Ig-secreting plasma cells were adoptively transferred into T and B cell–deficient RAG-1−/− mice. Antigenic stimulation 4–6 d after transfer of B cells resulted in rapid IgG production. The response was long lasting and strictly antigen specific, excluding polyclonal B cell activation. CD4+ T cells were not involved since (a) further depletion of CD4+ T cells in the recipient mice did not alter the antibody response and (b) recipient mice contained no detectable CD4+ T cells 90 d posttransfer. Memory B cells could not be activated by a soluble viral protein without T cell help. Transfer of memory B cells into immunocompetent animals indicated that presence of helper T cells did not enhance the memory B cell response. Therefore, our results indicate that activation of virus-specific memory B cells to secrete IgG is independent of cognate or bystander T cell help.
Keywords: antigen-specific immunity, immunological memory, B lymphocyte memory, cytomegalovirus, adoptive transfer
Introduction
During a primary humoral immune response, interaction between different cell types of the adaptive and innate immune system is essential. In peripheral lymphoid organs, APCs such as DCs and macrophages make contact with antigen-specific T cells, resulting in T cell activation. B cells that have encountered their antigen in the blood, the lymph nodes, or on follicular DCs (FDCs) present antigen on MHC class II molecules and migrate to the borders of the T cell–rich areas of the lymphoid organs where cognate T–B cell interaction occurs (1, 2). This interaction can result either in differentiation of activated B cells into low affinity IgM-secreting plasma cells or into memory B cell precursors (3). These precursors are recruited into the germinal center (GC) reactions. They form GCs where proliferation, somatic mutation of the Ig genes, and antigenic selection by FDCs takes place (4). The B cells differentiate into high affinity IgG-, IgA-, or IgE-secreting plasma cells or they enter into the memory B cell pool, carrying high affinity immunoglobulin as surface receptors (4). A second encounter with the same antigen leads to a much quicker activation of memory B cells, resulting in the appearance of high affinity IgG-secreting plasma cells within a few days (3).
The requirements for the memory B cell activation upon this second antigen encounter and the involvement of T cell help are not clear. A second round of T–B cell interaction seemed to be essential for the activation of memory B cells into IgG-secreting plasma cells in experimental protocols involving either immunization with soluble proteins and hapten-carrier conjugates or viral infection of mice (5, 6). Here, we analyzed the memory B cell activation in response to two different enveloped viruses using adoptive transfer of highly purified B cells from immunized donors into recombinase-activating gene (RAG)-1−/− mice. We show that memory B cells can be activated by viral particles to produce specific IgG with virus-neutralizing capacity in the absence of helper T cells.
Materials and Methods
Mice.
8–12-wk-old C57BL/6 mice were obtained from Charles River Laboratories. C57BL/6-RAG-1−/− mice were obtained from Irmgard Förster. C57BL/6-TCRβ/δ−/− mice were obtained from The Jackson Laboratory. C57BL/6-TNF−/−, C57BL/6-TNF/LTα−/− mice (7) and C57BL/6 Ly5.1 mice were a gift from H. Körner (Nikolaus Fiebiger Center). All mice were maintained free of specific pathogens in isolated ventilated cages.
Antigens and Immunizations.
Human cytomegalovirus (HCMV) strain AD169 was propagated in primary human foreskin fibroblasts grown in MEM supplemented with 5% FCS, glutamine (100 mg/L), and gentamycine (350 mg/L). Virions and dense bodies (DBs; enveloped particles missing the viral core) were isolated via glycerol-tartrate gradient centrifugation as described (8).
Tick-born encephalitis virus (TBEV) particles and HCMV glycoprotein B (gB) were gifts from Chiron Behring and Aventis Pasteur, respectively. Both preparations corresponded to the antigen used for vaccination in humans. C57BL/6 mice were immunized twice with 20 μg HCMV-DBs, 2 μg TBEV particles, or 10 μg soluble gB in aluminum hydroxide i.p. at intervals of 4 wk and with 10 μg HCMV-DBs, 2 μg TBEV, and 10 μg gB, respectively, in PBS i.v., again after 4 wk. B cells for adoptive transfer were isolated at least 6 wk after the last immunization.
Flow Cytometry and Adoptive Transfer of B Lymphocytes.
Single cell suspensions of spleens from immunized mice were stained with PE-conjugated anti-CD19 and FITC-conjugated anti-CD8 and anti-CD4 antibodies (all antibodies were obtained from BD Biosciences). CD19+ cells were isolated by two rounds of cell sorting using a MoFlo® cell sorter (Cytomation) and analyzed for purity by flow cytometry using a FACSCalibur® (Becton Dickinson). For negative selection of B lymphocytes, single cell suspensions of spleens from HCMV-DB–immunized mice were stained with FITC-conjugated antibodies against CD4, CD8, TCRβ, TCR γ/δ, NK1.1, CD11b, CD11c, and c-kit and propidium iodine. Positive cells were removed by two rounds of cell sorting. In general, a purity >99.8% was achieved by either procedure.
5 × 106 purified CD19+ B cells were adoptively transferred into the tail vein of C57BL/6–RAG-1−/− mice. For adoptive cotransfer of primed helper T cells, 5 × 106 purified CD4+ T cells from spleens and lymph nodes of C57BL/6 mice previously immunized with HCMV-DBs were negatively selected after staining with antibodies against CD19, CD8, NK1.1, CD11b, and CD11c.
Antigen Injection and In Vivo Depletion.
On day 6 posttransfer or at later time points, RAG-1−/− mice were challenged with 10 and 2 μg of HCMV-DBs, TBEV, or gB i.v., respectively. For the in vivo depletion of potentially contaminating CD4+ T cells after adoptive transfer, mice were injected twice (2 d before and on the day of challenge) with 500 μg each of purified GK1.5 antibody (a gift from T. Brocker, Institute for Immunology, Munich, Germany). The same regimen was used for depletion of CD4+ cells before transfer of memory B cells into immunocompetent hosts.
Detection of Specific Antibodies.
Sera from mice were analyzed by ELISA for virus-specific IgG. Sera were diluted 1:100 and compared with a dilution series of a hyperimmune serum from Balb/c mice, which was included on each individual ELISA plate. 2 μg/ml HCMV virions and 1 μg/ml TBEV particles, respectively, were coated to ELISA plates. Relative intensity (RI) corresponds to percentage of OD reached by a 1:400 dilution of the hyperimmune serum.
The IgG subclass composition of sera was analyzed by ELISA using subclass-specific, biotinylated antibodies (Southern Biotechnology Associates) and horseradish peroxidase–coupled Streptavidin (Amersham Biosciences). For the detection of specific antibodies on Western blots, lysates of HCMV virions (2 μg/lane) were separated by SDS-PAGE, and Western blotting was performed by standard procedures using horseradish peroxidase–conjugated mouse IgG–specific antibodies (Southern Biotechnology Associates).
Virus Neutralization Assay.
Virus neutralization assays were performed as described (9).
Immunofluorescence Microscopy.
Tissue blocks from spleens were embedded in optimal cutting temperature compound (Diatec) and stored at −80°C. Tissue sections (10 μm) were thawed onto gelatin-coated glass slides, air dried, fixed in acetone (for 10–15 min at −20°C), and rehydrated with PBS and 0.01% Tween 20 (Sigma-Aldrich). Nonspecific binding sites were blocked for 30 min at room temperature (RT) with PBS containing 0.1% BSA and 10% FCS. Each incubation step lasted 30 min at RT alternating with five washing steps in PBS/Tween (0.01%, 10 min, RT). Sections were incubated with goat anti–mouse IgG-Cy3 (Jackson Laboratories). After an additional blocking step with mouse serum, slides were incubated with FITC-conjugated anti-B220 (BD Biosciences) or alternatively with FITC-conjugated peanut agglutinin (Vector Laboratories) and biotinylated anti-CD45.2 (clone 104; BD Biosciences) followed by streptavidin-conjugated Cy5 (Amersham Biosciences). Sections were analyzed using an immunofluorescence microscope (Carl Zeiss MicroImaging, Inc.) equipped with high sensitivity gray scale digital camera (Openlab system; Improvision). Separate color images were collected for each section, analyzed, and merged afterwards. Final image processing was performed using Corel Photo-Paint software.
Online Supplemental Material.
Fig. S1 (available at http://www.jem.org/cgi/content/full/jem.20030091/DC1) shows the purity of B cell populations after two rounds of cell sorting. Single cell suspensions of spleens taken from C57BL/6 mice immunized with HCMV-DBs were stained with PE-conjugated anti-CD19 and FITC-conjugated anti-CD8 and anti-CD4 antibodies. CD19+ cells were isolated by two rounds of cell sorting and analyzed for purity by FACS®.
Results
Memory B Cells Can Be Activated by Antigen after Adoptive Transfer into Mice Lacking T Lymphocytes.
We chose to investigate the requirements for memory B cell activation using RAG-1−/− mice, which are deficient of T and B cells, as a host for adoptive transfer of memory B cells from mice immunized with HCMV. The virus particle is composed of >30 structural proteins, including at least eight envelope glycoproteins. As the prototypic β-herpesvirus, it shows strict species specificity, and therefore viral replication is restricted to cells of human origin (10). To generate memory B cells, C57BL/6 mice were immunized with HCMV-DBs using standard procedures. HCMV-DBs represent noninfectious enveloped particles that mainly consist of viral tegument proteins contained in a complete viral envelope (11). Mice immunized with HCMV-DBs mount a humoral and cellular immune response including CD4+ and CD8+ T cells (12).
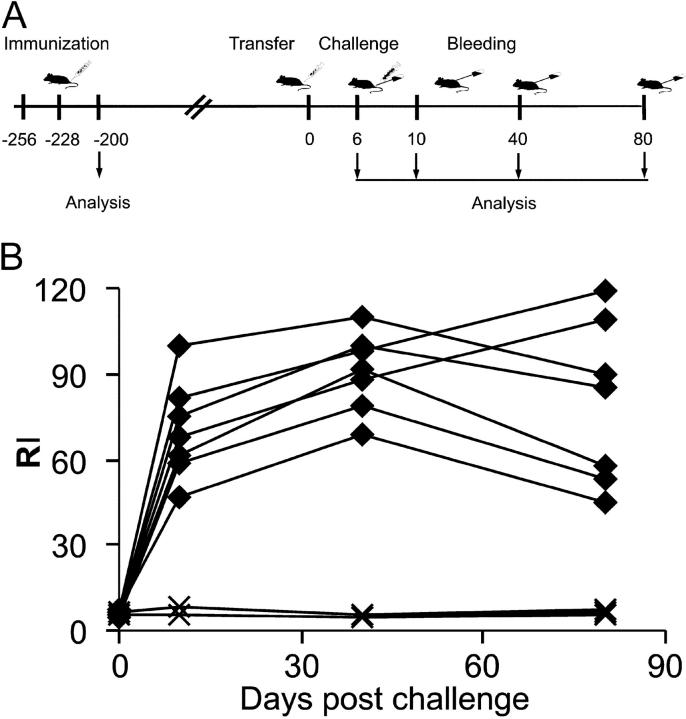
4 wk after the last immunization, blood was taken and serum was analyzed for HCMV-specific IgG and neutralizing antibodies (a schematic outline of the experimental protocol is provided in Fig. 1 A). Sera from all immunized mice exhibited high titers of virus-specific IgG, as determined by ELISA, and considerable virus-neutralizing activity (range 1:1,000–1:3,200; a representative analysis is shown in Fig. 1 D). At different time points (42–200 d) after the last immunization, CD19-positive small resting B cells were isolated from the spleen by two rounds of cell sorting. In general, a purity of >99.8% was achieved by this procedure (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20030091/DC1). Individual RAG-1−/− mice were infused with 5 × 106 purified B cells, and 6 d later the animals were either injected with 10 μg HCMV-DBs i.v. or left untreated. Sera were analyzed for HCMV-specific IgG between days 10 and 80 after transfer of B cells. 10 d after antigen stimulation, HCMV-specific IgG was clearly detectable (Fig. 1 B). The titers remained elevated during the observation period of 80 d. In the adoptively transferred RAG-1−/− mice, HCMV-specific IgG titers were 2–3 log2 dilutions lower than in immunized animals, which was expected since only a fraction of the B cells normally present in mice were used for the transfer. In fact, transferring higher numbers of CD19+ B cells to recipient mice resulted in elevated antibody titers (not depicted). In contrast, sera from RAG-1−/− mice that had been infused with B cells from naive donors and challenged with HCMV-DBs remained negative for HCMV-specific IgG during the entire observation period (Fig. 1 B). The use of CD19, which is not present on plasma cells, as the target for B cell isolation and the absence of HCMV-specific IgG in RAG-1−/− mice on day 6 after transfer of immune B cells argued against the presence of IgG-producing plasma cells in the transplant (Fig. 1 B). The lack of plasma cells in the transplant was further corroborated by the fact that RAG-1−/− mice, which had received B cells from immune donors but were not challenged with HCMV-DBs for up to 90 d remained negative for HCMV-specific IgG (see Fig. 6 A and not depicted).
Figure 1.
IgG reactivity and biological activity of recipient and donor sera. (A) Summary of the experimental protocol for immunization, adoptive transfer, and challenge with antigen. C57BL/6 mice were immunized twice with HCMV-dense bodies. 200 d later, sorted B cells from the spleen were adoptively transferred into RAG-1−/− recipients. On day 6 posttransfer, recipients were challenged with 10 μg HCMV-DBs i.v., and sera were analyzed on the days indicated. (B) Sera from recipients of B cells from immunized (♦) and naive (X) donors were analyzed by ELISA for HCMV-specific IgG. (C) Sera taken at day 40 postchallenge were analyzed in immunoblots and compared with sera of donor mice. (D) Neutralization activity of sera from RAG-1−/− mice adoptively transferred with immune (♦) or naive (X) B cells and of sera from donor mice (•) was analyzed in vitro. (E) IgG subclass composition of donor sera (•) (dilution 1:1,000) and recipient sera (○) (dilution, 1:200) analyzed by ELISA using subclass-specific antibodies. Values correspond to the dilution above detection threshold.
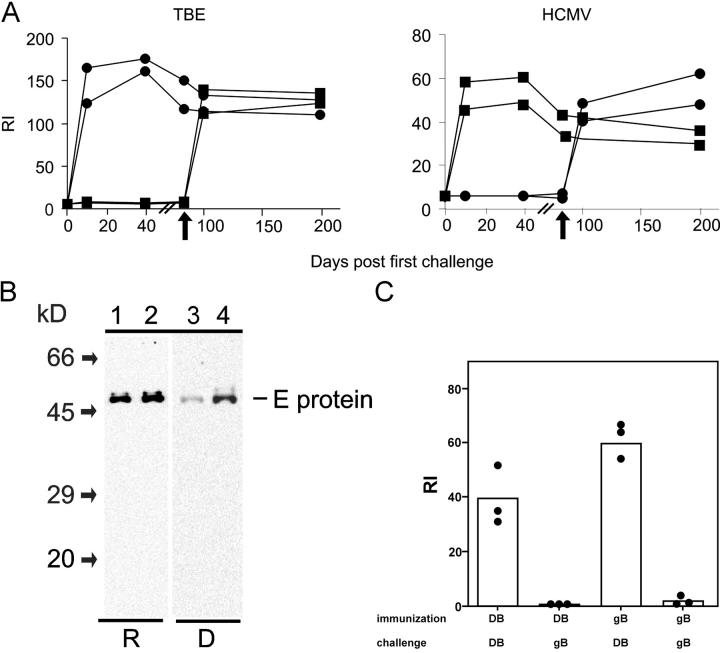
Figure 6.
Specificity of the IgG response of adoptively transferred memory B cells. (A) C57BL/6 mice were immunized with HCMV-DBs and TBEV particles, and CD19+ cells were isolated and transferred to RAG-1−/− mice according to the legend for Fig. 1 A. On day 4 posttransfer, recipients were challenged with 10 μg HCMV-DBs (▪) or 2 μg TBEV particles (•). Blood was taken at the days indicated, and sera were analyzed by ELISA for TBEV- (left) and HCMV-specific (right) IgG reactivity, respectively. On day 90 posttransfer, recipients were immunized again (arrows), this time using the reciprocal antigen, and IgG titers were determined. (B) Sera taken at day 40 poststimulation (dilution, 1:250) and sera of donor mice (dilution, 1:40,00) were analyzed by Western blot for IgG specificities against lysates of TBEV particles. (C) C57BL/6 mice were immunized with either a recombinant monomeric form of the HCMV viral envelope protein gB or HCMV-DBs, and CD19+ cells were isolated and transferred to RAG-1−/− mice according to Fig. 1 A. On day 7 posttransfer, recipients were challenged with either 2 μg HCMV-DBs or 10 μg recombinant gB. The bars represent the mean of RI values; dots are individual values. The experiment was repeated twice with similar results.
The specificity of the antibody response was analyzed by Western blot. A range of individual HCMV structural proteins was recognized by antibodies from the recipient RAG-1−/− mice, which included those known to induce an IgG response during natural infection of humans (Fig. 1 C). Most of the antibody specificities present in donor sera were also detected in the recipient sera. No additional antibody specificities were generated in the recipient mice. Occasionally the entire spectrum of donor specificities was not recovered in the recipients, most likely as a result of the low numbers of memory B cells that were transferred to the recipient animals. However, antibodies directed against the immunodominant envelope glycoprotein of HCMV, gp58, which represents the transmembrane subunit of gB (13) were detectable in all recipient sera. The antiviral activity of immunoglobulins produced in RAG-1−/− recipient mice was tested in in vitro neutralization assays using infectious HCMV as the target. Sera reached 50% neutralization titers in dilutions between 1:60 and 1:200 (Fig. 1 D). Again, neutralization titers of immunized C57BL/6 mice exceeded those of recipient RAG-1−/− mice by 2–3 log2 dilutions.
To further characterize the antibody response, the IgG subclass composition of HCMV-specific antibodies was determined. The distribution of IgG subclasses was similar between sera from donor and recipient mice with IgG1, IgG2a, and IgG2b being the dominant subclasses, whereas IgG3 was found in lower concentrations (Fig. 1 E). To exclude a potential activating effect of the anti-CD19 antibody used during cell sorting, we also applied a negative purification procedure by removing all non–B cells from the spleen cell suspension. This procedure resulted in similar purity of the B cells and in identical IgG titers upon antigen stimulation after transfer (unpublished data).
In RAG-1−/− mice, a homeostatic expansion of a fraction of B cells has been observed, which show an activated phenotype (14). To exclude potential influences of the complete lack of competition in the RAG-1−/− system, we used additional experimental settings. In a first set of experiments, we used TCRβ/δ−/− mice devoid of any T lymphocytes (15) as recipients for adoptively transferred memory B cells from HCMV-DB–immunized C57BL/6 animals. These mice reacted equally well to antigenic stimulation with HCMV-DBs as RAG-1−/− recipients (Fig. 2). In a second set of experiments, immunocompetent C57BL/6 mice were treated with the monoclonal antibody GK1.5 to eliminate endogenous CD4+ T cells. These mice were then used as hosts for memory B cells from HCMV-DB–immunized donors. Recipient mice were stimulated with antigen on day 1 after transfer. Whereas GK1.5 antibody treatment completely prevented an IgG response against HCMV-DBs, it allowed production of IgG in animals that had received memory B cells (Fig. 2). Thus, the T cell–independent memory B cell response that we observe in RAG−/− recipients is not a consequence of B cell expansion and homeostatic differentiation.
Figure 2.
IgG memory response in different host mice lacking functional CD4+ cells. 5 × 106 sorted B cells from hyperimmunized C57BL/6 mice were transferred as described in Fig. 1, and mice were challenged with antigen 1 d after adoptive transfer of cells. The IgG antibody response in different recipient mice was measured 7 d after challenge with 2 μg HCMV-DBs i.v. Controls received no memory B cell preparations. The bars represent the mean of RI values; dots are individual values.
These results indicated that, after adoptive transfer into mice devoid of T or T and B cells, highly purified B cells from HCMV-DB–immunized C57BL/6 mice can be activated to produce specific IgG by antigenic stimulation. Moreover, the lack of antibody production in mice receiving B cells from naive donors demonstrated that the primary antibody response against HCMV-DBs in mice is T cell dependent.
Antibody Responses in Recipient Mice Are Generated in the Absence of T Cells.
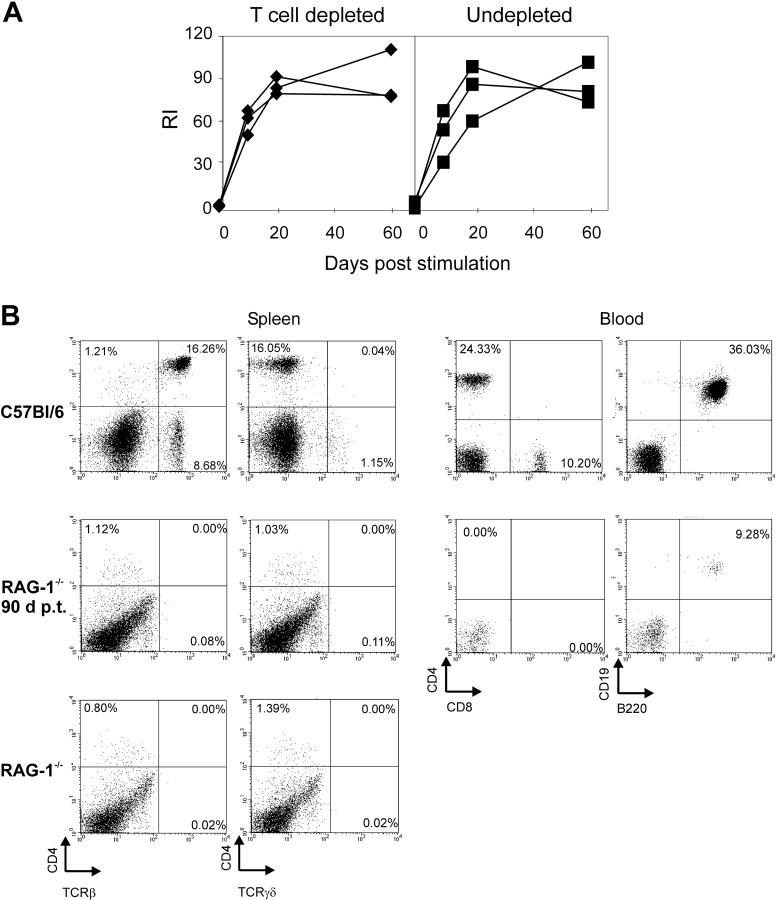
Although the purity of our B cell preparations argued against a participation of CD4+ T cells in the observed memory response, we further investigated a potential involvement of small numbers of CD4+ T cells in this process. First, potentially contaminating CD4+ T cells were depleted in recipient RAG-1−/− mice after transfer and before challenge. To this end, recipient mice were treated with depleting CD4-specific antibody GK1.5. The antibody concentration used was sufficient for a complete abrogation of a primary antibody response to HCMV-DBs in C57BL/6 mice (Fig. 2). When HCMV-DB–specific IgG was measured between days 10 and 60 postchallenge, no difference in antibody titers was observed between animals that had received GK1.5 and the control mice (Fig. 3 A). Second, spleens and blood of recipient mice were analyzed for the presence of CD4+ T helper cells 90 d after transfer. This time period is expected to be sufficient for homeostatic expansion of potentially contaminating T cells to detectable numbers (16). Neither the spleen nor the blood of recipient RAG-1−/− mice harbored cells, which were CD4+/TCR+, indicating that the transferred B cell population contained no or negligible numbers of CD4+ and CD8+ or γ/δ-TCR+ T cells (Fig. 3 B).
Figure 3.
T cell depletion and T cell numbers in recipient mice. (A) RAG-1−/− mice adoptively transferred with immune B cells were injected with 500 μg GK1.5 antibody 2 d before and on the day of stimulation with 10 μg HCMV-DBs. Blood was taken on the days indicated, and sera were analyzed for HCMV-specific IgG (♦) compared with sera of untreated recipients (▪). (B) Spleens and blood were taken from RAG-1−/− mice, RAG-1−/− mice 90 d post adoptive transfer and from donor mice. Single cell suspensions were stained with FITC- or PE-conjugated antibodies and analyzed by FACScan. The specificities of the staining antibodies are indicated at the axes.
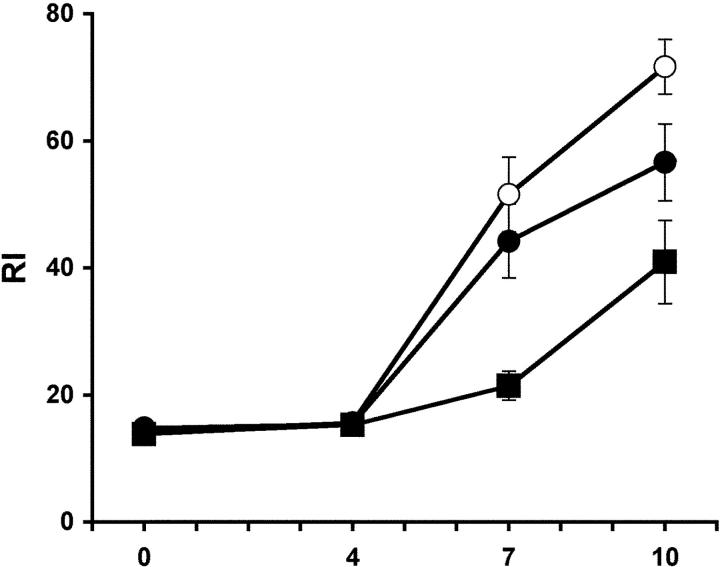
Finally, we wanted to analyze a potential enhancing effect of virus-specific memory T cells on virus-specific memory B cell responses by an adoptive cotransfer of CD4+ helper T cells into RAG-1−/− mice. However, a rapid and dramatic inflammatory bowel disease developing after the transfer of sorted CD4+ cells into syngeneic C57BL/6 RAG-1−/− mice prevented the analysis of the influence of CD4+ cells in the adoptive transfer experiments involving RAG-1−/− mice. For this reason, we decided to adoptively transfer B cells from HCMV-DB–immunized C57BL/6 mice into naive C57BL/6 mice with or without CD4+ T cells from the spleen and lymph node of HCMV-DB–immunized mice as a source of primed T cell help. As expected, the challenge of recipient and control mice with HCMV-DBs yielded faster IgG antibody responses with higher titer in recipients of sorted B cells containing virus-specific memory B cells when compared with naive control mice. Naive C57BL/6 mice responded to a single i.v. immunization with HCMV-DBs since the mice were not irradiated (Fig. 4). Importantly, the transfer of CD4+ cells from HCMV-DB–immunized C57BL/6 mice together with sorted B cells did not enhance the virus-specific antibody response in the recipient mice (Fig. 4).
Figure 4.
Antibody responses after adoptive transfer of memory B and memory T cells. C57BL/6 mice received 5 × 106 B cells from C57BL/6 mice immunized with HCMV-DBs (○), 5 × 106 immune B cells together with 5 × 106 CD4+ T cells from C57BL/6 mice previously immunized with HCMV-DBs (•) or no cells (▪). On day 6 posttransfer, the mice were challenged with 2 μg HCMV-DBs i.v. Blood was taken at the days indicated, and sera were analyzed by ELISA for HCMV-specific IgG. The values represent the mean values (± SD) of four recipient mice, and one of two similar experiments is shown.
Virus-specific Memory B Cells Are Not Participating in Germinal Center Reactions after Challenge with Virus.
The lack of enhancement of the memory IgG response in the presence of primed T cell help suggested that virus-specific memory B cells are not participating in GC reactions upon challenge with virus. Therefore, we assessed the formation of GCs in naive C57BL/6-Ly5.1 mice after adoptive transfer of memory B cell preparations together with primed CD4+ cells, both from C57BL/6-Ly5.2 mice and after challenge with HCMV-DBs. GC reactions in the spleen were detectable at day 8–10 after challenge, with 9–14 GCs per section, whereas naive mice did not contain detectable GCs (not depicted). Donor-derived cells did not participate in any of more than 50 GCs analyzed (Fig. 5, B and C). Instead, clusters of donor-derived cells that were IgGhigh and had partially down-regulated CD45 appeared as early as 4 d after challenge outside B cell follicles and the T cell zone at the border of the white pulp to the red pulp (Fig. 5, A and B) at or close to the marginal zone.
Figure 5.

Localization of the IgG response in the presence of T cell help. Memory B cell preparations (5 × 106 CD19+ cells) together with primed CD4+ cells (2.5 × 106 cells), both from C57BL/6-Ly5.2 mice, were transferred into naive C57BL/6-Ly5.1 mice and challenged with HCMV-DBs 1 d after adoptive transfer. (A) 4 d after challenge, clusters of donor-derived (CD45.2 in blue), IgGhigh (red) cells were observed outside the follicles (thick arrows). B220 is stained in green. (B) 10 d after challenge, clusters of donor-derived (CD45.2, blue), IgGhigh (red) cells are still persistent (thick arrows); in addition IgG+ GCs could be observed inside the follicles (thin arrows). The cells participating in the GC reaction are CD45.2 negative. (C) A GC from day 10 after challenge is stained with peanut agglutinin (green). Donor CD45.2 cells are blue; IgG-positive cells are red. Magnification: (A and B) 50×; (C) 200×.
The Memory B Cell Response Is Antigen Specific.
It remained a possibility that HCMV-DBs have the capacity for B cell receptor–independent activation of B cells or a fraction thereof. Since the antigen-specific memory compartment contains an increased frequency of specific B cells, this activation could result in the secretion of detectable amounts of HCMV-specific IgG in recipients of memory B cells.
To investigate this possibility, we included TBEV particles as a second viral antigen in the experimental protocol. TBEV is a member of the Flaviviridae. The envelope consists of a lipid bilayer containing the two viral proteins M and E. The E protein is the target for the neutralizing humoral immune response (17). C57BL/6 mice were immunized with both antigens simultaneously, and the development of a humoral immune response against both viruses was confirmed at least 4 wk after the last immunization (not depicted). CD19+ B cells were isolated and adoptively transferred to RAG-1−/− mice as described above. Between 4 and 7 d posttransfer, mice were challenged with either HCMV-DBs or TBEV i.v. On day 10 after challenge, only recipients injected with TBEV developed TBEV-specific IgG titers, whereas serum from mice that had received HCMV-DBs had no detectable TBEV-specific antibody titer and vice versa (Fig. 6 A). The specific antibody titers remained elevated for the observation period of 40 d. In immunoblots, sera from TBEV recipient animals reacted exclusively with the E protein, which corresponded to the specificity found in sera of the B cell donor mice (Fig. 6 B). To determine if B cells retained the capacity to be activated with either antigen, recipient mice were stimulated with the reciprocal antigen 90 d after adoptive cell transfer. IgG titers similar to those produced after stimulation at early time points posttransfer developed within 1 wk (Fig. 6 A). We conclude from these experiments that neither HCMV-DBs nor TBEV induce a B cell receptor–independent activation of memory B cells in the recipient mice. The responding memory B cells seem to have a life span of at least 90 d.
To investigate whether the particulate structure of the antigen is of importance for the activation of memory B cells, we adoptively transferred cells from C57BL/6 mice immunized with a recombinant monomeric form of the viral envelope protein gB. This protein has been used for immunization in humans (18) but is also highly immunogenic in mice (19). When recipient mice were challenged with gB, no antibody titer developed (Fig. 6 C). Likewise, gB was not capable of stimulating memory B cells from HCMV-DB–immunized animals. In contrast, stimulation of memory B cells from gB-immunized mice with HCMV-DBs resulted in IgG production similar to mice that had been primed with HCMV-DBs.
Together, these data show that two unrelated enveloped viruses are capable of activating memory B cells in the absence of T helper cells. The physical nature of the challenging antigen seems to be of importance for the differentiation of memory B cells into IgG-secreting cells.
An Organized Lymphoid Tissue Is Required for T Cell–independent Differentiation of Virus-specific Memory B Cells.
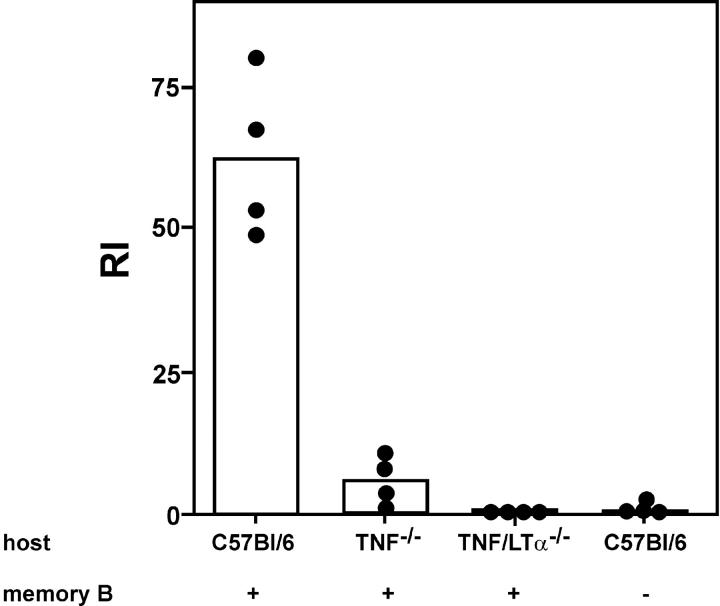
In a series of experiments, we noted that transferred memory B cells were unresponsive to challenge with HCMV-DBs when the antigen was applied within minutes after the injection of memory B cell preparations, a protocol that is widely used for the assessment of memory B cell function. This effect was observed in RAG-1−/− recipients, T cell−/− mice, and anti-CD4–treated recipients. In contrast, when virus particles were given 24 h after adoptive transfer, a full response was measurable in T cell−/− mice (unpublished data). These findings suggested that memory B cells needed to migrate to a specific lymphoid compartment in order to become fully responsive to antigenic stimulation in a T cell–independent way. To test this, we used TNF−/− and TNF/LTα−/− mice as recipients in which the organization of the lymphoid architecture is severely altered (20). Wild-type, TNF−/−, and TNF/LTα−/− C57BL/6 mice were treated with anti-CD4–specific antibody, and memory B cell preparations were adoptively transferred. An IgG memory response was clearly detectable in anti-CD4–treated wild-type mice, whereas only a minute IgG response was detected in TNF−/− recipients (Fig. 7). In TNF/LTα−/− recipients, the IgG memory response was completely absent, likewise in anti-CD4–treated C57BL/6 mice that had not received memory cells (Fig. 7). These results indicate that homing of memory B cells to secondary lymphoid tissue is required for the T cell–independent memory B cell response.
Figure 7.
IgG memory response in recipient mice with altered lymphoid architecture. 5 × 106 sorted B cells from a pool of hyperimmunized C57BL/6 mice were transferred as described in the legend for Fig. 1, and mice were challenged with antigen 1 d after adoptive transfer of cells. Control C57BL/6 mice were treated with GK1.5 antibody but received no memory B cell preparations. The IgG antibody response in different recipient mice treated with GK1.5 antibody was measured 7 d after challenge with 2 μg HCMV-DBs i.v. The bars represent the mean of RI values; dots are individual values. The experiment was repeated, and similar results were obtained.
Discussion
Our results show that virus-specific memory B cell responses do not require T cell help. After transfer of B cells from immunized mice, RAG-1−/− mice and mice that are devoid of T cells but contain a normal B cell compartment developed a specific IgG titre. The responding cell population in the recipient mice consisted of antigen-specific, resting B memory cells, since (a) only small, CD19+ B cells were sorted from the donor mice, (b) antigen-specific IgG was undetectable after adoptive transfer in the recipient mice even after a prolonged period of time in the absence of antigen, and (c) the response after challenge with antigen exhibited all the characteristics of a memory response with regard to both kinetics and Ig isotype. Furthermore and in accordance with previous findings (21, 22), the transferred memory B cells appear to be long-lived in the RAG-1−/− mice in the absence of T cells, since specific responses with similar titers could be induced even 3 mo after transfer (Fig. 6 A). Interestingly, the presence of primed T cell help in immunocompetent C57/BL6 mice did not enhance antibody production after antigenic stimulation of adoptively transferred memory B cells, indicating that in virus-specific humoral memory responses cognate or bystander T cell help plays a minor role.
Our experiments with the viral glycoprotein gB as a soluble monomer clearly indicate that, as expected, memory B cell responses against monomeric protein antigens cannot be induced in the absence of T cell help. In this experimental system that provides a model for a physiological secondary response against exotoxins, T cell help apparently is a strict requirement. The presentation of the antigen during challenge seems to dictate whether a T-independent memory response can be induced, but not the presentation of the antigen during generation of B memory cells, as memory B cells generated by immunization with gB in adjuvant could be activated by virus particles.
Some virus infections can elicit an IgG response in the absence of T cell help. Requirements seem to be a repetitive and highly organized antigenic structure that occurs in structurally simple nonenveloped viruses, such as vesicular stomatitis virus or polyomavirus (23). However, when inactivated viruses or viral proteins are used for immunization the switch to IgG production is strictly dependent on T cell help (24–26). In accordance with these findings, we observed T cell dependence for a primary B cell response against HCMV particles in CD4+ T cell–depleted mice and undetectable IgM responses after immunization of RAG-1−/− mice that had been adoptively transferred with naive B cells (unpublished data). These results rule out a T cell–independent primary antibody response against our antigens. Activation of virus-specific memory B cells has also been reported to be strictly dependent on specific T cell help (6). This is in contrast to our study where transferred memory B cells were effectively stimulated to differentiate into antibody-secreting cells in the absence of T cells, indicating that neither specific nor bystander T cell help is required for the activation of memory B cells. One possible explanation for the difference could be the experimental protocol (6). In the adoptive transfer experiments reported by Ochsenbein et al. (6), antigen was given 20 min after transfer of memory B cells into anti-CD4–treated recipients. In our experiments in RAG-1−/− mice or anti-CD4–treated immunocompetent recipients challenged with virus, shortly after transfer of purified B cells an IgG antivirus response was also undetectable. However, challenge with antigen 24 h after transfer of memory cells induced a response. Interestingly, challenge with antigen shortly after transfer of memory cells is widely and traditionally used in experiments assessing the function of memory B cells (5, 27).
Our findings strongly suggest that virus-specific memory B cells must home to specific sites within the lymphoid system to become antigen responsive in a T cell–independent way. The observation that a T cell–independent differentiation of virus-specific memory B cells could not be induced in TNF/Ltα−/− or TNF−/− recipient mice supports this view and confirms earlier data that lymphoid architecture, which depends on the expression of LTα for its development and maintenance, is needed to support a memory Ig response after antigenic challenge (28). Experimentally provided T cell help might overcome the requirement for homing to specific sites in secondary lymphoid organs (6). The bone marrow has been shown recently to be a location for T cell priming for blood-born antigens (29), and it has been suggested that memory B cells might interact with primed T helper cells in the bone marrow of splenectomized ALY/ALY mice that lack lymph nodes and Peyer's patches (6).
What are the costimulatory requirements for a virus-specific memory B cell in the absence of T cell help? One possibility is that FDCs that are crucial for the generation of memory cells (30) might also be essential for the differentiation after antigen challenge. Since B lymphocytes are sufficient to induce a FDC network upon transfer in SCID mice (31), this possibility cannot be ruled out. The failure to induce a T cell–independent memory B cell response against virus particles after transfer of cells into TNF- and TNF/LTα-deficient recipients (Fig. 7) might imply a fundamental role of FDC networks for memory B cell activation, as FDC networks are absent in these mice (7, 32, 33). An alternative explanation for the absence of a memory response in TNF- and TNF/LTα-deficient recipients might be the lack of a detectable marginal sinus in the spleen of these mice (7). For rats and humans, it has been shown that memory B cells can reside in the marginal zone of the spleen (34, 35). The localization of clusters of cells expressing high levels of IgG after challenge with virus (Fig. 5) is compatible with an activation of these cells in the marginal zone. Virus-specific memory cells could potentially receive important costimuli while located in the marginal zone, similar to IgM+ marginal zone B cells reactive to blood-born bacteria (36). The exact localization and the nature of costimuli for memory B cells to become activated in a T cell–independent way need to be further elucidated. Other possible costimulatory signals for memory B cells could originate from the innate immune system, particularly complement components (37), type I interferons (38), or pattern recognition receptors on B cells (39).
Understanding the mechanism(s) of memory B cell activation will not only provide fundamental information about this important cell type of the humoral immune system but could also be of clinical importance. HCMV represents an important pathogen in bone marrow or stem cell transplant recipients in the early phases after transplantation, at times when T cell–dependent virus-specific humoral responses are not possible. Expanding the pool of memory B cells or vaccination strategies to activate residual virus memory B cells might be of benefit for the course of the infection in these patients.
Acknowledgments
We thank Dr. Heiner Körner for providing the RAG-1−/−, C57BL/6 Ly5.1, TNF−/−, and TNF/LTα−/− mice, for helpful suggestions, and fruitful discussions; Chiron Behring for the TBEV; Aventis Pasteur for gB preparations; and Drs. Fritz Melchers, William Britt, and Martin Turner for critical reading of our manuscript.
This work was supported in part by the Deutsche Forschungsgemeinschaft with grants to M. Mach and T.H. Winkler.
The online version of this article includes supplemental material.
B. Hebeis' present address is Babraham Institute, Babraham, Cambridge CB2 4AT, UK.
Abbreviations used in this paper: DB, dense body; FDC, follicular DC; gB, glycoprotein B; GC, germinal center; HCMV, human cytomegalovirus; RAG, recombinase-activating gene; RI, relative intensity; TBEV, tick-born encephalitis virus.
References
- 1.Garside, P., E. Ingulli, R.R. Merica, J.G. Johnson, R.J. Noelle, and M.K. Jenkins. 1998. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 281:96–99. [DOI] [PubMed] [Google Scholar]
- 2.Cyster, J.G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science. 286:2098–2102. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science. 272:54–60. [DOI] [PubMed] [Google Scholar]
- 4.MacLennan, I.C. 1994. Germinal centers. Annu. Rev. Immunol. 12:117–139. [DOI] [PubMed] [Google Scholar]
- 5.Vieira, P., and K. Rajewsky. 1990. Persistence of memory B cells in mice deprived of T cell help. Int. Immunol. 2:487–494. [DOI] [PubMed] [Google Scholar]
- 6.Ochsenbein, A.F., D.D. Pinschewer, S. Sierro, E. Horvath, H. Hengartner, and R.M. Zinkernagel. 2000. Protective long-term antibody memory by antigen-driven and T help-dependent differentiation of long-lived memory B cells to short-lived plasma cells independent of secondary lymphoid organs. Proc. Natl. Acad. Sci. USA. 97:13263–13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korner, H., M. Cook, D.S. Riminton, F.A. Lemckert, R.M. Hoek, B. Ledermann, F. Kontgen, B. Fazekas de St Groth, and J.D. Sedgwick. 1997. Distinct roles for lymphotoxin-alpha and tumor necrosis factor in organogenesis and spatial organization of lymphoid tissue. Eur. J. Immunol. 27:2600–2609. [DOI] [PubMed] [Google Scholar]
- 8.Talbot, P., and J.D. Almeida. 1977. Human cytomegalovirus: purification of enveloped virions and dense bodies. J. Gen. Virol. 36:345–349. [DOI] [PubMed] [Google Scholar]
- 9.Klein, M., K. Schoppel, N. Amvrossiadis, and M. Mach. 1999. Strain-specific neutralization of human cytomegalovirus isolates by human sera. J. Virol. 73:878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mocarsky, E.S. 2001. Cytomegaloviruses and their replication. Virology. B.N. Fields, editor. Lippincott Williams and Wilkins, Philadelphia, PA. 2629-2673.
- 11.Sarov, I., and I. Abady. 1975. The morphogenesis of human cytomegalovirus. Isolation and polypeptide characterization of cytomegalovirions and dense bodies. Virology. 66:464–473. [DOI] [PubMed] [Google Scholar]
- 12.Pepperl, S., J. Munster, M. Mach, J.R. Harris, and B. Plachter. 2000. Dense bodies of human cytomegalovirus induce both humoral and cellular immune responses in the absence of viral gene expression. J. Virol. 74:6132–6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Britt, W.J., and M. Mach. 1996. Human cytomegalovirus glycoproteins. Intervirology. 39:401–412. [DOI] [PubMed] [Google Scholar]
- 14.Agenes, F., and A.A. Freitas. 1999. Transfer of small resting B cells into immunodeficient hosts results in the selection of a self-renewing activated B cell population. J. Exp. Med. 189:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mombaerts, P., E. Mizoguchi, M.J. Grusby, L.H. Glimcher, A.K. Bhan, and S. Tonegawa. 1993. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell. 75:274–282. [DOI] [PubMed] [Google Scholar]
- 16.Rocha, B., N. Dautigny, and P. Pereira. 1989. Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur. J. Immunol. 19:905–911. [DOI] [PubMed] [Google Scholar]
- 17.Kreil, T.R., E. Maier, S. Fraiss, E. Attakpah, I. Burger, J.W. Mannhalter, and M.M. Eibl. 1998. Vaccination against tick-borne encephalitis virus, a flavivirus, prevents disease but not infection, although viremia is undetectable. Vaccine. 16:1083–1086. [DOI] [PubMed] [Google Scholar]
- 18.Pass, R.F., A.M. Duliege, S. Boppana, R. Sekulovich, S. Percell, W. Britt, and R.L. Burke. 1999. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J. Infect. Dis. 180:970–975. [DOI] [PubMed] [Google Scholar]
- 19.Britt, W., J. Fay, J. Seals, and C. Kensil. 1995. Formulation of an immunogenic human cytomegalovirus vaccine: responses in mice. J. Infect. Dis. 171:18–25. [DOI] [PubMed] [Google Scholar]
- 20.Fu, Y.X., and D.D. Chaplin. 1999. Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17:399–433. [DOI] [PubMed] [Google Scholar]
- 21.Schittek, B., and K. Rajewsky. 1990. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 346:749–751. [DOI] [PubMed] [Google Scholar]
- 22.Maruyama, M., K.P. Lam, and K. Rajewsky. 2000. Memory B-cell persistence is independent of persisting immunizing antigen. Nature. 407:636–642. [DOI] [PubMed] [Google Scholar]
- 23.Szomolanyi-Tsuda, E., and R.M. Welsh. 1998. T-cell-independent antiviral antibody responses. Curr. Opin. Immunol. 10:431–435. [DOI] [PubMed] [Google Scholar]
- 24.Leist, T.P., S.P. Cobbold, H. Waldmann, M. Aguet, and R.M. Zinkernagel. 1987. Functional analysis of T lymphocyte subsets in antiviral host defense. J. Immunol. 138:2278–2281. [PubMed] [Google Scholar]
- 25.Bachmann, M.F., U.H. Rohrer, T.M. Kundig, K. Burki, H. Hengartner, and R.M. Zinkernagel. 1993. The influence of antigen organization on B cell responsiveness. Science. 262:1448–1451. [DOI] [PubMed] [Google Scholar]
- 26.Szomolanyi-Tsuda, E., Q.P. Le, R.L. Garcea, and R.M. Welsh. 1998. T-cell-independent immunoglobulin G responses in vivo are elicited by live-virus infection but not by immunization with viral proteins or virus-like particles. J. Virol. 72:6665–6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sprent, J., and H. von Boehmer. 1976. Helper function of T cells depleted of alloantigen-reactive lymphocytes by filtration through irradiated F1 hybrid recipients. I. Failure to collaborate with allogeneic B cells in a secondary response to sheep erythrocytes measured in vivo. J. Exp. Med. 144:616–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu, Y.X., G. Huang, Y. Wang, and D.D. Chaplin. 2000. Lymphotoxin-alpha-dependent spleen microenvironment supports the generation of memory B cells and is required for their subsequent antigen-induced activation. J. Immunol. 164:2508–2514. [DOI] [PubMed] [Google Scholar]
- 29.Feuerer, M., P. Beckhove, N. Garbi, Y. Mahnke, A. Limmer, M. Hommel, G.J. Hammerling, B. Kyewski, A. Hamann, V. Umansky, and V. Schirrmacher. 2003. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat. Med. 9:1151–1157. [DOI] [PubMed] [Google Scholar]
- 30.Cyster, J.G., K.M. Ansel, K. Reif, E.H. Ekland, P.L. Hyman, H.L. Tang, S.A. Luther, and V.N. Ngo. 2000. Follicular stromal cells and lymphocyte homing to follicles. Immunol. Rev. 176:181–193. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez, M., F. Mackay, J.L. Browning, M.H. Kosco-Vilbois, and R.J. Noelle. 1998. The sequential role of lymphotoxin and B cells in the development of splenic follicles. J. Exp. Med. 187:997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto, M., S. Mariathasan, M.H. Nahm, F. Baranyay, J.J. Peschon, and D.D. Chaplin. 1996. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 271:1289–1291. [DOI] [PubMed] [Google Scholar]
- 33.Le Hir, M., H. Bluethmann, M.H. Kosco-Vilbois, M. Muller, F. di Padova, M. Moore, B. Ryffel, and H.P. Eugster. 1996. Differentiation of follicular dendritic cells and full antibody responses require tumor necrosis factor receptor-1 signaling. J. Exp. Med. 183:2367–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu, Y.J., S. Oldfield, and I.C. MacLennan. 1988. Memory B cells in T cell-dependent antibody responses colonize the splenic marginal zones. Eur. J. Immunol. 18:355–362. [DOI] [PubMed] [Google Scholar]
- 35.Dunn-Walters, D.K., P.G. Isaacson, and J. Spencer. 1995. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J. Exp. Med. 182:559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balazs, M., F. Martin, T. Zhou, and J. Kearney. 2002. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 17:341–352. [DOI] [PubMed] [Google Scholar]
- 37.Fischer, M.B., S. Goerg, L. Shen, A.P. Prodeus, C.C. Goodnow, G. Kelsoe, and M.C. Carroll. 1998. Dependence of germinal center B cells on expression of CD21/CD35 for survival. Science. 280:582–585. [DOI] [PubMed] [Google Scholar]
- 38.Le Bon, A., G. Schiavoni, G. D'Agostino, I. Gresser, F. Belardelli, and D.F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity. 14:461–470. [DOI] [PubMed] [Google Scholar]
- 39.Bernasconi, N.L., N. Onai, and A. Lanzavecchia. 2003. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells. Blood. 101:4500–4504. [DOI] [PubMed] [Google Scholar]