Abstract
Optimum immunity against Mycobacterium tuberculosis requires both CD4+ and CD8+ T cells. In contrast with CD4+ T cells, few antigens are known that elicit CD8+ T cells during infection. CD8+ T cells specific for culture filtrate protein-10 (CFP10) are found in purified protein derivative positive donors, suggesting that CFP10 primes CD8+ T cells in vivo. Using T cells from M. tuberculosis–infected mice, we identified CFP10 epitopes recognized by CD8+ T cells and CD4+ T cells. CFP10-specific T cells were detected as early as week 3 after infection and at their peak accounted for up to 30% of CD8+ T cells in the lung. IFNγ-producing CD8+ and CD4+ T cells recognizing CFP10 epitopes were preferentially recruited to the lungs of M. tuberculosis–infected mice. In vivo cytolytic activity of CD8+ T cells specific for CFP10 and TB10.3/10.4 proteins was detected in the spleen, pulmonary lymph nodes, and lungs of infected mice. The cytolytic activity persisted long term and could be detected 260 d after infection. This paper highlights the cytolytic function of antigen-specific CD8+ T cells elicited by M. tuberculosis infection and demonstrates that large numbers of CFP10-specific cytolytic CD8+ T cells are recruited to the lung after M. tuberculosis infection.
Keywords: bacterial infection, T lymphocytes, cytotoxicity, H-2Kk haplotype, tetramers
Introduction
Cellular immunity to Mycobacterium tuberculosis plays a critical role in controlling infection. An effective T cell response determines whether immunity develops or whether the infection develops into disease. CD4+ T cells are known to be important both for the initial control of infection as well as for preventing recrudescence in both mice and humans (1). In addition to CD4+ T cells, CD8+ T cells also contribute to protection against M. tuberculosis (2, 3). Although CD4+ T cells primarily recognize antigens that enter the endocytic pathway and are presented by MHC class II, most CD8+ T cells recognize short peptides of 8–10 amino acids derived from cytosolic proteins that are presented by MHC class I molecules. Under certain conditions, MHC class I can present antigens that enter the endocytic pathway, a process known as cross-presentation (4). Both self- and foreign proteins in the cytosol are cleaved by the proteosome and the resulting peptides are translocated into the ER by the TAP1/TAP2 heterodimer. Once in the ER, the peptides assemble with the class I MHC heavy chain and β2 microglobulin (β2m) to form a trimeric complex, which is transported to the cell surface. Mice with disruptions in the β2m or TAP1 genes lack CD8+ T cells, are unable to control M. tuberculosis replication in the lung, and die prematurely compared with normal mice (5, 6). This is true after large inoculums administered intravenously, as well as after low-dose aerosol infection (7–10). Furthermore, mice lacking CD8+ T cells succumb later during the course of infection compared with mice deficient in MHC class II–restricted CD4+ T cells (9). Although MHC class II–deficient mice are more susceptible than mice lacking CD8+ T cells, it is now understood that CD4+ T cells are required for the development of CD8+ memory T cells (11).
After infection of macrophages, M. tuberculosis survives and replicates in the phagosome. Just how bacterial antigens traffic from the phagosome to the cytoplasm where they can enter the MHC class I processing pathway is a matter of controversy; several mechanisms have been proposed (12, 13). Once activated, CD8+ T cells produce cytokines including IFNγ and TNFα, which can activate macrophages and induce the production of nitrogen, oxygen radicals, and LRG47 (2, 14, 15). In addition to producing cytokines, CD8+ T cells mediate cellular cytotoxicity. Lysis of M. tuberculosis–infected macrophages could be beneficial if the released bacteria are taken up by activated macrophages that can mediate bacterial killing. In addition, human CD8+ T cells express granulysin in their cytotoxic granules that can directly kill intracellular M. tuberculosis (16). Although murine and human M. tuberculosis–specific CD8+ cytolytic T cell lines have been established in vitro, it has been difficult to demonstrate cytolytic activity of freshly isolated CD8+ T cells against infected macrophages (17–20). In addition, there has been disagreement in the literature as to whether mice lacking critical mediators of cytolysis, such as perforin, granzyme, or CD95, are more susceptible to M. tuberculosis infection (21, 22). Therefore, how CD8+ T cells contribute to host resistance against M. tuberculosis remains to be delineated.
Secreted M. tuberculosis protein antigens have been extensively studied in part because they are targets of T cell–mediated immunity (23). Antigens such as Ag85, early secretory antigen target-6 (ESAT-6), culture filtrate protein-10 (CFP10), and others elicit strong CD4+ T cells responses in both mice and humans (24, 25). In contrast, fewer M. tuberculosis antigen epitopes that are recognized by CD8+ T cells have been identified. Lewinsohn et al. cloned CD8+ T cells from a purified protein derivative positive individual using autologous M. tuberculosis–infected DCs (26). Several of the CD8+ T cell clones were MHC class I–restricted and two recognized peptide epitopes were derived from the CFP10/Mtb11 protein (27). CFP10 is a particularly interesting protein, as it is already known to be a major antigen recognized by M. tuberculosis–specific human T and B cells (28, 29). In addition, it induces a strong delayed-type hypersensitivity response in infected guinea pigs when injected intradermally (30, 31). CFP10 is encoded within the RD1 locus of the M. tuberculosis genome, a region of DNA that is present in M. tuberculosis and pathogenic Mycobacterium bovis strains, but is deleted from all bacillus Calmette-Guerin (BCG) strains (32–36). The cfp10 gene is in the same operon as the esat-6 gene, and the two genes share 40% sequence homology and belong to the ESAT-6 family of small proteins (34, 36, 37). In fact, ESAT-6 and CFP10 are secreted as heterodimers, and ESAT-6 can disrupt planar membranes (38, 39). As CFP10 and ESAT-6 are expressed by pathogenic Mycobacterium species but not by BCG, CFP10 and ESAT-6 may be virulence factors. Recent evidence suggests that specific immunity to these proteins enhance host resistance against M. tuberculosis infection (40).
To understand how CD8+ T cells contribute to host protection against M. tuberculosis infection, we sought to identify bacterial protein epitopes recognized by M. tuberculosis–specific CD8+ T cells that are elicited in vivo after respiratory infection. Using overlapping peptides based on the CFP10 protein, MHC class I– and class II–restricted epitopes were identified that were recognized by CD8+ and CD4+ T cells from infected mice. These epitopes were used to compare the generation of the pulmonary CD4+ and CD8+ T cell response to CFP10, and to determine whether cytolytic CD8+ T cells are generated in vivo after infection with M. tuberculosis.
Materials and Methods
Mice.
Age-matched female B10.BR-H2k H2-T18a/SgSnJ (B10.BR), B10.A-H2a H2-T18a/SgSnJ (B10.A), C57BL/10J (B10.J), C57BL/6 (B6), BALB/c, C3H/HeJ (HeJ), and C3H/HeSnJ (SnJ) mice were purchased from The Jackson Laboratory. Mice were housed in biosafety level three facilities under specific pathogen-free conditions at the Animal Biohazard Containment Suite (Dana Farber Cancer Institute) and were used in an approved protocol.
Aerosol Infection with Mycobacteria.
Virulent M. tuberculosis (Erdman strain) were prepared as described previously (41). Mice were infected via the aerosol route using a nose-only exposure unit (Intox Products; reference 42).
Peptides.
Overlapping peptides spanning the CFP10 protein sequence of M. tuberculosis were designed using the PeptGen web-based program (http://www.hiv.lanl.gov). The length of each peptide was 15 amino acids with a 10–amino acid overlap, but the length was adjusted to avoid C-term amino acids GPED QNTSC or the NH2-terminal amino acid Q. The 22 resulting peptides were commercially synthesized (BioSource International; Table I). The identity of each peptide was confirmed by mass spectrophotometry. The peptides were dissolved in DMSO and stored at −20°C until used. Peptides used for immunological assays were unpurified. The purity of peptides used for tetramer production was >95%.
Table I.
Amino Acid Sequence of the 22 Overlapping Peptides
| NH2-MAEMKTDAATLAQEAGNFERISGDLKTQIDQ-VESTAGSLQGQWRGAAGTAAQAAVVRFQEAANKQK-QELDEISTNIRQAGVQYSRADEEQQQALSSQMGF-COOHa | ||
|---|---|---|
| p1b | aa 1–15c | MAEMKTDAATLAQEA (15)d |
| p2 | aa 6–20 | TDAATLAQEAGNFER (15) |
| p3 | aa 11–25 | LAQEAGNFERISGDL (15) |
| p4 | aa 16–30 | GNFERISGDLKTQI (14) |
| p5 | aa 20–32 | RISGDLKTQIDQV (13) |
| p6 | aa 23–36 | GDLKTQIDQVESTA (14) |
| p7 | aa 27–39 | TQIDQVESTAGSL (13) |
| p8 | aa 30–44 | DQVESTAGSLQGQWR (15) |
| p9 | aa 35–47 | TAGSLQGQWRGAA (13) |
| p10 | aa 38–51 | SLQGQWRGAAGTAA (14) |
| p11 | aa 41–55 | GQWRGAAGTAAQAAV (15) |
| p12 | aa 46–58 | AAGTAAQAAVVRF (13) |
| p13 | aa 49–62 | TAAQAAVVRFQEAA (14) |
| p14 | aa 53–66 | AAVVRFQEAANKQK (14) |
| p15 | aa 57–69 | RFQEAANKQKQEL (13) |
| p16 | aa 60–72 | EAANKQKQELDEI (13) |
| p17 | aa 63–77 | NKQKQELDEISTNIR (15) |
| p18 | aa 68–81 | ELDEISTNIRQAGV (14) |
| p19 | aa 72–86 | ISTNIRQAGVQYSRA (15) |
| p20 | aa 77–93 | RQAGVQYSRADEEQQQA (17) |
| p21 | aa 84–98 | SRADEEQQQALSSQM (15) |
| p22 | aa 89–100 | EQQQALSSQMGF (12) |
In Vitro Restimulation Assays.
Single cell suspensions were prepared from spleens and pulmonary LNs (PLNs) of infected mice. The RBCs were lysed using lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM Na EDTA, pH 7.3). After washing, the cells were resuspended in complete medium (RPMI 1640, 10% FCS, 2% Hepes, 1% l-glutamine, 1% penicillin-streptomycin, 0.1% β-mercaptoethanol). Lung mononuclear cells were obtained by digesting tissue with collagenase type IV (Sigma-Aldrich) followed by filtration through a 60-mesh metal strainer and 70-μM nylon strainer (Fisher Scientific). RBC lysis was performed as described before. CD8+ T cells were purified from infected spleens, PLNs, or lungs using immunomagnetic beads and MACS LS+ columns (Miltenyi Biotec) in two steps. First, using the Pan–T cell isolation kit, total T cells were purified by negative selection, followed by a second step using immunomagnetic beads to positively purify either CD4+ or CD8+ T cells. In all experiments, the purity of the cells was 90–95% as determined by flow cytometry. The purified CD4+ or CD8+ T cells were stimulated with synthetic peptides and irradiated naive splenocytes as APCs for 48 h in vitro (42). Recombinant IL-2 (Chiron Corp.) and IL-15 (R&D Systems) were added to the assay medium at 100 U/ml and 20 ng/ml, respectively, to promote CD8+ T cell growth. Culture supernatants were assayed for IFNγ by an ELISA, using antibody pairs and cytokines obtained from BD Biosciences.
ELISPOT Assay for IFNγ.
The ELISPOT method was used to detect IFNγ secretion by individual CD8+ T cells from infected mice after stimulation with peptides in vitro. In brief, using the ELISPOT kit and protocol (BD Biosciences): ELISPOT plates were coated with capture IFNγ antibody overnight at 4°C. The capture antibody was discarded, and the plates were washed and blocked with complete media for 2 h at room temperature. Purified CD8+ T cells were added along with CFP10 peptides and irradiated naive splenocytes as APCs and cultured for 36–40 h at 37°C. The cells were discarded, and plates were washed with de-ionized water and PBS/Tween 20. Secondary biotinylated antibody was added for 2 h and incubated at room temperature followed by washing with PBS/Tween 20. Streptavidin–alkaline phosphatase was added to the plates for 1 h followed by washing and development of a color reaction using the substrate AEC substrate reagent kit (BD Biosciences). The reaction was stopped when the spots developed by running the plate under water. The spots were enumerated using a dissecting microscope.
In Vivo Cytotoxicity Assay.
Splenocytes from naive C3H or BALB/c mice were prepared as described before. Half of the splenocytes were labeled with 0.5 μM (CFSElow) and the other half with 5 μM (CFSEhigh) CFSE (Molecular Probes) in PBS for 10 min at room temperature, followed by extensive washing. For experiments using C3H mice, the CFSElow population was pulsed with 10 μM of the H-2Kk–specific peptide, VESTAGSL. For experiments with BALB/c mice, the CFSElow cells were pulsed with 10 μM of the H-2Kd–specific peptide, GYAGTLQSL. In both cases, the cells were cultured with the peptide in complete medium for 1 h at 37°C, washed, and resuspended in complete medium. Both the CFSE populations were mixed at a 1:1 concentration and injected intravenously into naive or infected C3H or BALB/c mice at a concentration of 107 cells/mouse. After 18 h, the spleens, PLNs, and lungs were harvested, and single cell suspensions were made as described before. Each experimental group consisted of three to five mice, and each mouse was analyzed individually. The CFSElow or CFSEhigh populations of lymphocytes were detected in all the organs via flow cytometry using the FACSort (Becton Dickinson) and analyzed using FlowJo software (Treestar Inc.). Percent of peptide-specific lysis was determined by the formula: % lysis = 100 − 100 × [(%CFSElow/%CFSEhigh infected)/(%CFSElow/%CFSEhigh uninfected)].
Flow Cytometry and Tetramer Staining.
Purified lymphocytes from the spleens, PLNs, and lungs were resuspended at a concentration of 106 cells/sample in FACS buffer and stained with an isotype-matched control IgG or antibodies specific for mouse CD4, CD8, CD22, conjugated to FITC, PE, or streptavidin-Cy (BD Biosciences). Tetramers were produced using VESTAGSL-loaded H-2Kk or GYAGTLQSL-loaded H-2Kd, both complexed with PE (National Institute of Allergy and Infectious Diseases tetramer facility). Cells were stained with the tetramers at an optimum concentration (1:200) for 20 min on ice, washed, and fixed in 1% paraformaldehyde overnight. Cells were analyzed using a FACSort and FlowJo software was used to analyze the data.
Peptide-binding Assay.
RMA-S peptide-binding assay was performed essentially as described previously (43). In brief, RMA-S/Kk cells (provided by P. Cresswell, Yale University, New Haven, CT) were incubated overnight at 23°C in complete media. Cells were pulsed for 1–2 h with peptide CFP1032-39 at 23°C, washed, and incubated at 37°C without peptide for 4 h. Cells were washed, stained for H-2Kk expression using biotinylated H2-Kk monoclonal antibody and streptapavidin-PE-Cy5 (BD Biosciences), and analyzed using a FACSort and FlowJo software.
Statistics.
One-way analysis of variance (ANOVA) with Dunnett's posttest and two-way ANOVA with Bonferroni's posttest were performed using Prism version 4.02 for Windows (GraphPad Software).
Results
CFP10-specific MHC Class I–restricted CD8+ T Cells Are Elicited after Respiratory Infection with M. tuberculosis.
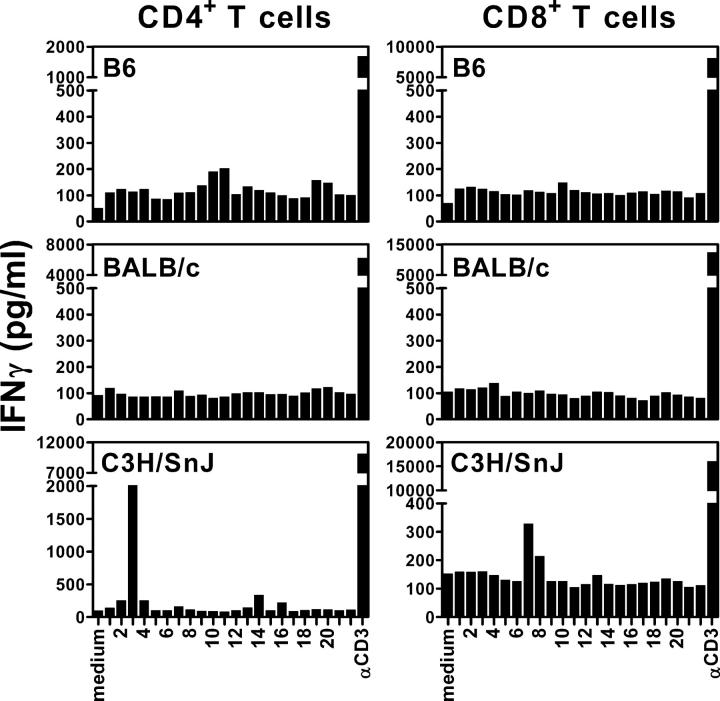
Overlapping peptides spanning the CFP10 protein (Table I) were screened to identify antigen epitopes recognized by CD8+ T cells that are elicited during M. tuberculosis infection. B6 (H-2b), C3H (H-2k), and BALB/c (H-2d) mice were infected with virulent M. tuberculosis via the aerosol route and their spleens were harvested 4 wk after infection at the peak of the immune response (41). Purified CD4+ or CD8+ T cells were cultured with the individual peptides and naive irradiated syngeneic splenocytes, and after 48 h, the culture supernatants were assayed for IFNγ as a measure of T cell activation. Peptide 3 (LAQEAGNFERISGDL; herein referred to as CFP1011-25) was recognized by CD4+ T cells and the overlapping peptides 7 (amino acid [aa] 27–39: TQIDQVESTAGSL) and 8 (aa 30–44: DQVESTAGSLQGQWR) were recognized by CD8+ T cells from C3H mice, but not B6 or BALB/c mice (Fig. 1). Peptides 7 and 8 were only recognized by purified CD8+ T cells, but not CD4+ T cells from infected C3H mice (Fig. 2 A). To identify the minimal antigenic epitope, three overlapping peptides were synthesized, IDQVESTA (aa 29–36), DQVESTAGSL (aa 30–39), and VESTAGSL (aa 32–39), that spanned the sequence common to peptides 7 and 8. The 8mer VESTAGSL was identified as the minimal antigenic epitope recognized by CD8+ T cells from infected C3H mice (Fig. 2, A and B). To identify which MHC class I molecule presents VESTAGSL, naive congenic B10 mice were used as APCs. Purified CD8+ T cells from infected C3H (H-2k) mice were tested for their ability to recognize the 8mer VESTAGSL presented by either B10.J (H-2b), B10.A (H-2a), or B10.BR (H-2Kk) irradiated splenocytes. CD8+ T cells recognized VESTAGSL presented by B10.BR splenocytes (KkDkLk) and B10.A (KkDdLd) splenocytes, indicating that H-2Kk is the restricting element (Fig. 2 C). The VESTAGSL peptide contains the H-2Kk binding motif (http://syfpeithi.bmi-heidelberg.com), and the VESTAGSL peptide stabilized the expression of H-2Kk by RMA-S transfectants, verifying that it binds to H-2Kk (reference 44 and unpublished data). Not only did the CFP10 epitope prime CD8+ T cells in vivo but also a CD8+ T cell line specific for the VESTAGSL peptide recognized peritoneal macrophages infected in vitro with M. tuberculosis (unpublished data). These data indicate that after infection of C3H mice with M. tuberculosis, H-2Kk–restricted CD8+ T cells are primed that recognize the CFP10 epitope VESTAGSL (herein referred to as CFP1032-39).
Figure 1.
Identification of a new MHC class I–restricted M. tuberculosis epitope recognized by CD8+ T cells. Purified CD4+ and CD8+ T cells from splenocytes of week 4–infected C57BL/6, BALB/c, and C3H/HeSnJ mice were cultured with CFP10 peptides at a concentration of 10 μM and syngeneic naive irradiated splenocytes as APCs. The amount of IFNγ released into the supernatants was measured by ELISA after 48 h. The data represent one out of three independent experiments with similar results
Figure 2.
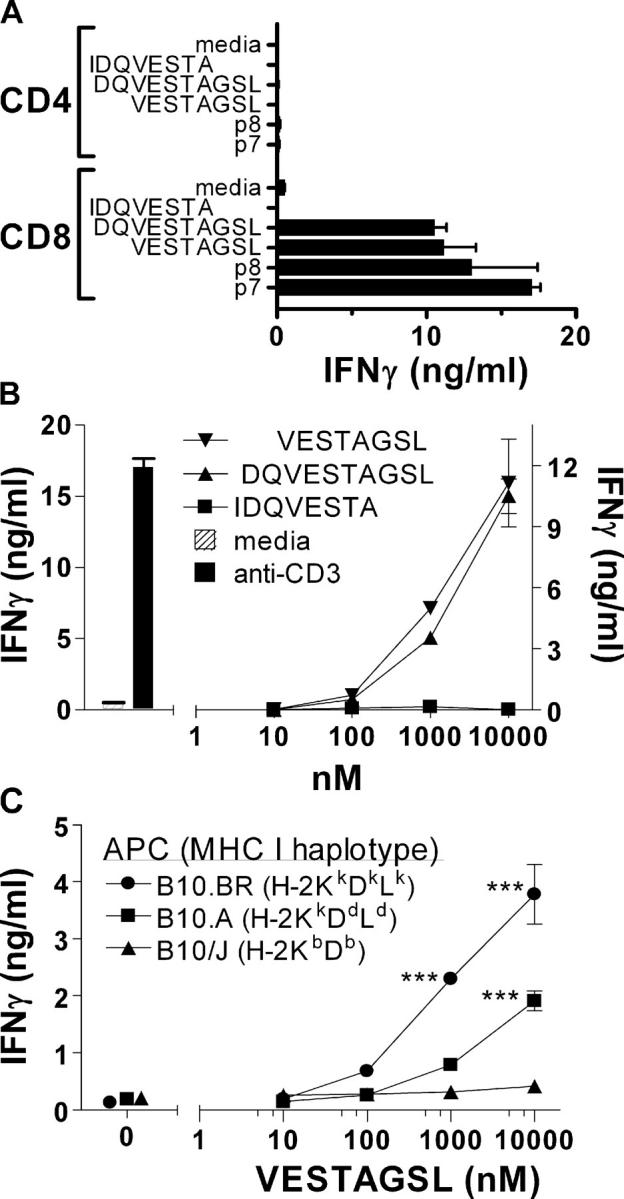
Identification of the minimal epitope and restricting element recognized by CD8+ T cells. (A) Definition of the minimal epitope recognized by CFP10-specific CD8+ T cells. Purified CD4+ and CD8+ splenic T cells obtained from C3H/HeSnJ mice 4 wk after infection were cultured with p7 or p8 from the CFP10 peptide library, or the overlapping peptides VESTAGSL, DQVESTAGSL, and IDQVESTA (all at 10 μM). IFNγ in the culture supernatants was measured after 48 h by ELISA. The CD8+ T cell responses to VESTAGSL, DQVESTAGSL, p7, and p8 are all statistically significant (P < 0.05) compared with media alone when analyzed by one-way ANOVA. (B) Dose–response of CFP10-specific CD8+ T cells to specific peptides. Splenic CD8+ T cell responses to overlapping peptides VESTAGSL, DQVESTAGSL, and IDQVESTA. The responses to VESTAGSL and DQVESTAGSL were similar to each other and both are significantly different from the response to IDQVESTA (P < 0.01, by two-way ANOVA). (C) Recognition of CFP10 by CD8+ T cells was restricted by H-2Kk. IFNγ production was used to indicate splenic CD8+ T cell recognition of the VESTAGSL peptide when presented by irradiated splenocytes obtained from B10.BR, B10.A, or B10.J mice. ***, P < 0.001 by two-way ANOVA with Bonferroni's posttest. Results shown are representative of at least three independent experiments. Error bars represent SD.
Priming of CFP10-specific CD4+ and CD8+ T Cells Occurs Rapidly after Infection with M. tuberculosis.
To determine the frequency of CFP1032-39-specific CD8+ T cells, an IFNγ ELISPOT assay was developed. Purified splenic CD8+ T cells from either infected or uninfected C3H/HeJ mice were cultured with the H-2Kk–restricted CFP1032-39 peptide and irradiated naive splenocytes as APCs. To demonstrate the specificity of the ELISPOT assay, an H-2Kd–restricted epitope, GYAGTLQSL, from the TB10.3 and TB10.4 proteins of M. tuberculosis was used (herein referred to as TB10.3/420-28; reference 45). CD8+ T cells from infected C3H mice recognized the CFP1032-39 peptide, but not the TB10.3/420-28 peptide. Conversely, CD8+ T cells from infected BALB/c mice recognized the H-2Kd–restricted epitope, but not the H-2Kk–restricted CFP1032-39 peptide. CD8+ T cells from uninfected BALB/c mice and C3H/HeJ mice did not recognize either peptide as expected (Fig. 3 A). These results demonstrate the specificity of the ELISPOT in detecting CFP1032-39-specific H-2Kk–restricted CD8+ T cells, and independently confirm that H-2Kd–restricted CD8+ T cells specific for the TB10.3/10.4 protein are elicited by M. tuberculosis infection.
Figure 3.
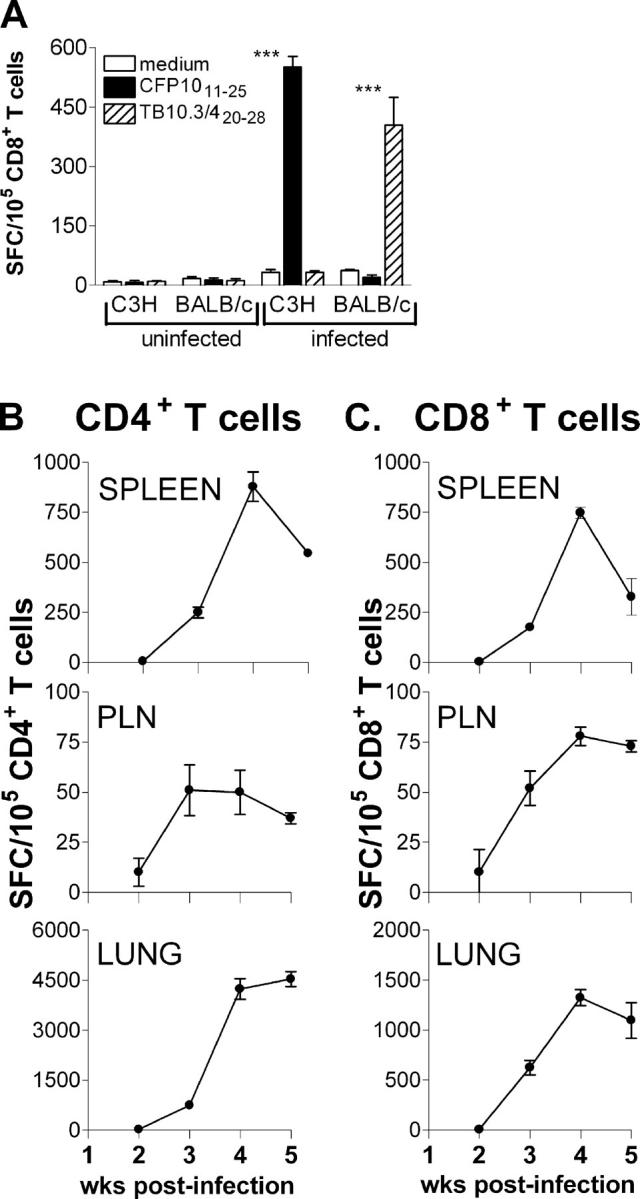
Enumeration of the peptide-specific CD4+ and CD8+ T cells after M. tuberculosis infection using the ELISPOT assay. (A) Epitope specificity and H-2 restriction of the ELISPOT assay. Purified splenic CD8+ T cells from uninfected or infected C3H/HeJ or BALB/c mice were cultured with syngeneic APCs in the presence of the H-2Kk–restricted CFP1032-39 or H-2Kd–restricted TB10.3/420-28 peptides and an IFNγ ELISPOT assay was performed. ***, P < 0.001 by two-way ANOVA with Bonferroni's posttest. (B and C) The kinetics of the CFP10-specific T cell response. Purified CD4+ T cells (B) or CD8+ T cells (C) from infected C3H/HeJ mice were cultured with the I-Ak–restricted CFP1011-25 peptide or the H-2Kk–restricted CFP1032-39 peptide in the presence of syngeneic irradiated splenocytes as APCs. The T cells were purified from pooled organs of six infected mice at each time point. Controls without peptide produced a minimal background level of IFNγ+ spots and this was subtracted from the number of spots in the presence of the peptide. The data are representative of two independent experiments and shown as the number of spot-forming cells/100,000 CD4+ or CD8+ T cells. Error bars represent SD.
To compare the priming of CD4+ and CD8+ T cells specific for CFP10 protein, we used the MHC class II– and class I–restricted CFP10 epitopes and measured the T cell response to CFP10 using the IFNγ ELISPOT assay. The frequency of CFP1011-25-specific CD4+ T cells and CFP1032-39-specific CD8+ T cells in the PLNs, lung, and spleen was compared after infection of C3H mice with aerosolized M. tuberculosis. CD8+ or CD8− (e.g., CD4+) T cells were purified from the infected organs 2–5 wk after infection and cultured with irradiated splenocytes from uninfected C3H mice with either the CFP1032-39 or CFP1011-25 peptides, respectively.
2 wk after infection, a small number of peptide-specific IFNγ spots could be detected in the PLNs, spleen, and lung. Although a significant increase in the frequency of CFP10-specific CD4+ and CD8+ T cells in the PLNs was observed by week 3, it never reached a frequency >0.05–0.075%. This indicates that antigen-specific T cells fail to accumulate in the PLNs, despite the presence of bacteria at this site (42). In contrast, a rapid increase in the frequency of CFP10-specific T cells occurred between 2 and 3 wk after infection in the lung and spleen (Fig. 3 B). The number of CFP10-specific CD4+ and CD8+ T cells steadily increased in the spleen and reached a peak between weeks 4 and 5. In the lungs of infected mice, there was a noticeable enrichment of CFP10-specific T cells. The frequency of CFP1011-25-specific CD4+ T cells in the lung increased ∼180-fold between week 2 (25 spots/105 CD4+ T cells) and week 5 (4538 spots/105 CD4+ T cells) (Fig. 3 B). A high frequency of CFP1032-39-specific CD8+ T cells was also detected in the lungs, representing ∼1% of the CD8+ T cells (Fig. 3 C). Therefore, priming of the CFP10-specific CD4+ and CD8+ T cells occurs with similar kinetics and, once elicited, undergo expansion and accumulate particularly in the spleen and lung.
Antigen-specific CD8+ T Cells Are Enriched in the Lung after Infection with M. tuberculosis.
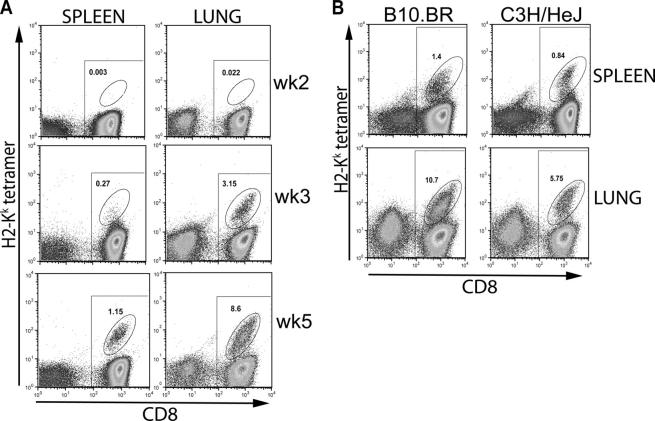
The IFNγ ELISPOT revealed an unexpectedly high frequency of CFP10-specific CD8+ and CD4+ T cells in the lungs of C3H mice. To corroborate the ELISPOT assay results, flow cytometry was performed using H-2Kk tetramers loaded with the CFP1032-39 peptide and H-2Kd tetramers made with the TB10.3/420-28 peptide. These tetrameric complexes were used to stain CD8+ T cells from uninfected or M. tuberculosis–infected C3H/HeJ and BALB/c mice. The H-2Kk tetramer specifically stained splenic CD8+ T cells from infected, but not uninfected, C3H/HeJ mice (Fig. 4). Conversely, the H-2Kd tetramers detected TB10.3/420-28-specific CD8+ T cells in the spleens of infected, but not uninfected, BALB/c mice. Neither tetramer stained CD8+ T cells from uninfected C3H/HeJ or BALB/c mice. These data confirm with reasonable certainty that the H-2Kk and H-2Kd tetramers specifically stain CD8+ T cells specific for their respective MHC–peptide complexes.
Figure 4.
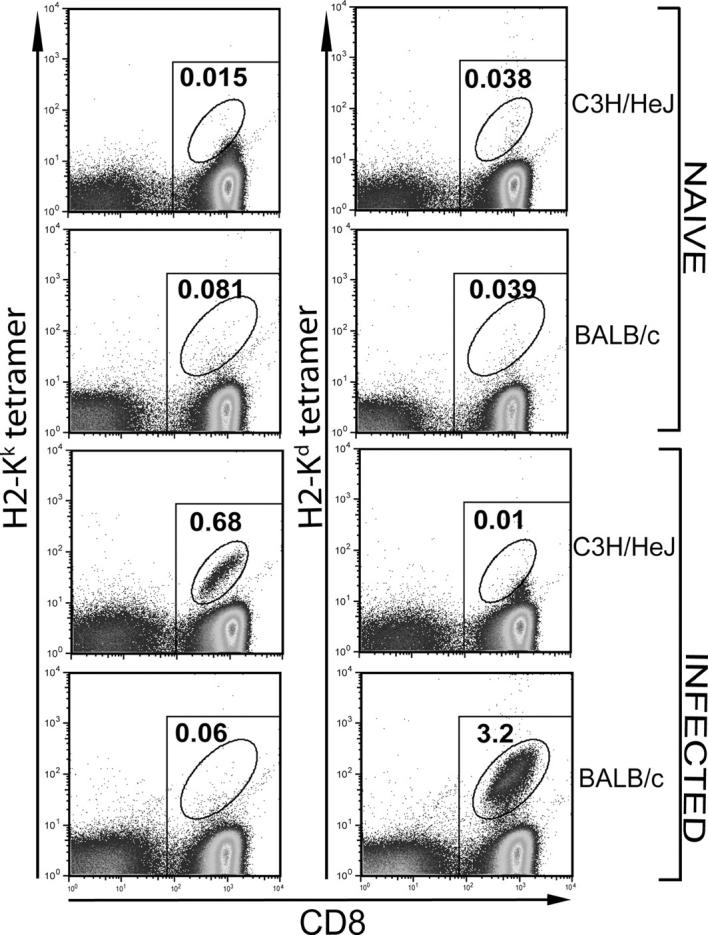
H-2Kk and H-2Kd peptide tetramer staining of CD8+ T cells in M. tuberculosis–infected C3H/HeJ or BALB/c mice. Purified CD8+ T cells from splenocytes of naive or 4 wk–infected C3H/HeJ or BALB/c mice were stained with the CFP1032-39/H-2Kk or TB10.3/420-28/H-2Kd tetramers. A significant population of H-2Kk and the H-2Kd tetramer+ CD8+ T cells was found only in cells obtained from the infected C3H/HeJ and BALB/c mice, respectively. All of the CD8+ T cells are within the rectangular gate and all of the tetramer+ cells are within the oval gate. The number represents the percentage of CD8+ T cells that are tetramer+.
Very few tetramer-specific CD8+ T cells were detected in the PLNs at all times tested (unpublished data). The finding that CFP10-specific CD4+ and CD8+ T cells do not accumulate in the draining LNs suggests that, although T cell priming may occur in the LNs (42), once activated, antigen-specific T cells rapidly traffic to other sites of disease. In contrast, a surprisingly high frequency of H-2Kk tetramer+ CD8+ T cells was detected in the lungs of M. tuberculosis–infected C3H mice. At week 2, 0.014% of CD8+ T cells stained with the H-2Kk tetramer, which increased to 2.75% at week 3 and to 8.12% by week 5, representing a 512-fold increase between weeks 2–5 (Fig. 5 A). In more chronically infected mice, up to 30% of CD8+ T cells were CFP1032-39-specific based on staining with the tetramer. CD8+ T cells obtained from bronchoalveolar lavage fluid of infected mice contained a comparable proportion of CFP1032-39-specific H-2Kk–restricted CD8+ T cells as did the lung (unpublished data). In the spleens, a similar increase in tetramer+ CD8+ T cells was observed. Although the correlation was very good, the ELISPOT tended to underestimate the frequency of antigen-specific T cells determined using the tetramers. This discrepancy could be the result of a technical problem such as nonspecific tetramer staining, although numerous controls did not identify this as a significant source of error (Fig. 4), or that the culture conditions for the ELISPOT do not activate 100% of the antigen-specific CD8+ T cells. Alternately, this discrepancy may have a biological explanation. Although the tetramer detects all of the CFP1032-39-specific H-2Kk–restricted CD8+ T cells, the ELISPOT only detects the subset that secretes IFNγ. A similar discrepancy between tetramer staining and ELISPOT has also been observed in viral models and has been suggested to be a consequence of T cell exhaustion or T cell anergy (46, 47). Finally, CFP10-specific CD8+ T cells were also detected in resistant B10.BR mice, which had a greater percentage of tetramer+ CD8+ T cells in their spleens and lungs after infection with M. tuberculosis than did C3H mice (Fig. 5 B). Regardless, combining the tetramer and ELISPOT data, one observes a specific enrichment of CFP10-specific CD4+ and CD8+ T cells in the lung compared with the spleen during the first few weeks of infection.
Figure 5.
Detection of tetramer+ CD8+ T cells in the spleens and lungs of C3H/HeJ and B10.BR mice after M. tuberculosis infection. (A) Purified CD8+ T cells from splenocytes of infected C3H/HeJ mice were stained at various times after infection. Tetramer+ CD8+ T cells could be detected in the spleens and lungs of infected C3H/HeJ mice as early as 3 wk after infection. The percent of tetramer+ CD8+ T cells is shown in all panels. (B) CFP10-specific CD8+ T cells in C3H/HeJ and B10.BR mice after infection. The percentage of tetramer+ CD8+ T cells found in the spleens and lungs of resistant B10.BR mice and susceptible C3H/HeJ mice were compared 4 wk after infection with M. tuberculosis. All of the CD8+ T cells are within the rectangular gate and all of the tetramer+ cells are within the oval gate. The number represents the percentage of CD8+ T cells that are tetramer+.
Peptide-specific Cytotoxic CD8+ T Cells Are Recruited to the Lung after M. tuberculosis Infection.
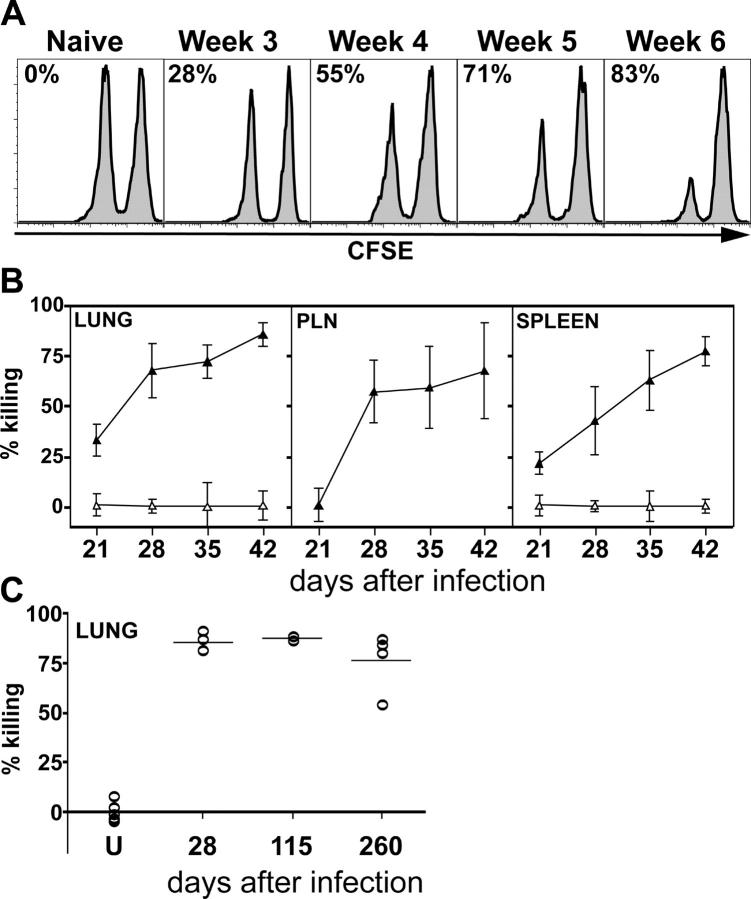
An important question concerning the function of M. tuberculosis–specific CD8+ T cells is whether they mediate any unique functions in host defense against infection. One possibility is that these CD8+ T cells function as cytolytic T cells in vivo. Bulk CD8+ T cells stimulated in vitro acquire the capacity to lyse infected macrophages in vitro; however, it is unclear whether they have this function in vivo (48, 49). To address whether CD8+ T cells elicited during infection are cytolytic in vivo, we adoptively transferred peptide-pulsed or unpulsed, CFSE-labeled splenocytes into uninfected or infected mice to demonstrate in vivo cytotoxicity (50). This approach has been used to demonstrate viral CTL activity in vivo and allowed us to determine whether the CFP1032-39 peptide sensitized splenocytes to lysis by CTL elicited after M. tuberculosis infection (51–54). Killing of peptide-pulsed splenocytes by CD8+ T cells in vivo was detected as early as week 3 after infection in the spleen and lung and 1 wk later in the PLNs when compared with the lungs and spleens of naive mice (Fig. 6, A and B). These data correlate with the results of the ELISPOT assay depicted in Fig. 3, in which very few antigen-specific CD8+ T cells were detected at week 2, but increased dramatically at weeks 3 and 4. Strikingly, the ability of the T cells to remain cytotoxic was stable over time up to 6 wk after infection, at which time the majority of mice succumbed to infection. To determine how long the in vivo CTL activity persisted, we turned to BALB/c mice, which survive for up to 1 yr after infection. CD8+ T cells from Mycobacterium-infected BALB/c mice killed TB10.3/420-28-pulsed P815 targets after in vitro stimulation (45). After adoptive transfer of CFSE-labeled unpulsed and TB10.3/420-28-pulsed splenocytes into uninfected and infected BALB/c mice, we observed killing of TB10.3/420-28-pulsed splenocytes in the lungs of infected BALB/c mice (Fig. 6 C). Upon M. tuberculosis infection, in vivo cytolytic activity was detected as early as 4 wk after infection and persisted at least to 37 wk. This is the first paper showing in vivo cytolytic activity mediated by pulmonary CD8+ T cells specific for the CFP10 and TB10.3/10.4 proteins.
Figure 6.
Generation of peptide-specific cytotoxic CD8+ T cells after M. tuberculosis infection. (A) In vivo cytolytic activity of CFP1032-39-specific CD8+ T cells. A 1:1 mixture of CFSEhigh and CFP1032-39-pulsed CFSElow splenocytes were injected i.v. into C3H/HeJ that were uninfected or infected 3–6 wk previously with M. tuberculosis. The spleens of the recipient mice were removed after 18 h and analyzed by flow cytometry. A histogram gated on CFSE+ events is shown for a representative mouse from each group. The number in each plot represents the percent killing of the CFP1032-39-pulsed CFSElow splenocytes, which was calculated as described in Materials and Methods. (B) The relative killing of the peptide-pulsed targets in the lungs, PLNs, and spleens of infected mice compared with uninfected mice was calculated as described in Materials and Methods. Each point represents the mean ± SD of five mice per group. The experiment was repeated twice with similar results. (C) Uninfected (U) or infected BALB/c mice were injected as described before with TB10.3/420-28-pulsed and unpulsed splenocytes. The percent killing in the lungs of individual mice from 28 to 260 after infection is shown. Each symbol represents an individual mouse.
Discussion
Based on a variety of data, including the increased susceptibility of β2m, TAP1, KdDb, and CD8 knockout mice to infection, it has become clear that CD8+ T cells play a critical role in mediating protective immunity to M. tuberculosis (1, 5–10). CD8+ T cells can recognize infected cells, produce cytokines such as TNFα and IFNγ, lyse infected cells, and kill M. tuberculosis. Although CD8+ T cells specific for mycobacterial proteins can be elicited by vaccination using antigen 85A (aa 144–152), 38kD (aa 225–234), and MPT4 (aa 190–198), MHC class I–presented bacterial epitopes that prime CD8+ T cells during infection remain elusive (55–57). Some progress has been made in identifying the antigens recognized by human CD8+ T cells, and several of these are proteins secreted by M. tuberculosis. In fact, it has been proposed that antigens secreted early during the course of M. tuberculosis infection account for the ability of live, but not heat-killed, M. tuberculosis to confer protection in animal models (58). One of these secreted proteins is the 10-kD protein (CFP10), which is a major antigen recognized by human T and B cells after infection (27–29, 59). We reasoned that CFP10 would be recognized by murine CD8+ T cells after infection in vivo, and that the immune response to CFP10 may be an important component of host immunity to M. tuberculosis. Therefore, we screened overlapping peptides spanning the sequence of the CFP10 protein to determine whether murine CD8+ T cells specific for CFP10 are elicited by M. tuberculosis infection.
A minimal epitope (VESTAGSL; CFP1032-39) was identified that is presented by H-2Kk to CD8+ T cells primed during infection. From the same peptide library, a second epitope was identified (LAQEAGNFERISGDL; CFP1011-25) that is recognized by CD4+ T cells. Both the CD4+ and CD8+ T cell responses to CFP10 evolved similarly in the spleen, lung, and draining LNs of infected C3H/HeJ mice. The CFP10-specific response was detected as early as 2 wk and peaked at weeks 4 and 5. Using CFP1032-39-loaded H-2Kk tetramers, antigen-specific CD8+ T cells could be detected ex vivo in the lungs, spleens, and PLNs of infected mice, although antigen-specific CD8+ T cells were selectively enriched in the lungs of infected mice. We found up to 30% of pulmonary CD8+ T cells in infected mice stained with the CFP1032-39–H-2Kk tetramers. This high frequency of antigen-specific T cells is similar to what is seen in Listeria monocytogenes infection (60). Pope et al. showed that the magnitude of the CD8+ T cell response measured by MHC class I tetramers is increased in the nonlymphoid organs when compared with the secondary lymphoid organs in L. monocytogenes infection (61). In our current analysis, we too detected an increase in tetramer+ CD8+ T cells in the lungs when compared with the spleen and PLNs. After short-term stimulation with infected DCs, lung CD8+ T cells produce IFNγ, although CD4+ T cells appear to be the major source of this cytokine (14). We have extended these results to demonstrate that antigen-specific CD8+ T cells that produce IFNγ are recruited to the lung early after infection. Furthermore, our finding that CFP1032-39-specific CD8+ T cells are greatly enriched in the lung compared with other sites of infection such as the spleen or LNs provides the first evidence that antigen-specific CD8+ T cells specifically accumulate in the lung, as opposed to the nonspecific recruitment of CD8+ T cells because of the production of chemokines, proinflammatory cytokines, and the up-regulation of cell adhesion molecules in the lung.
The function of CD8+ T cells in tuberculosis is not entirely clear, and there is conflicting data whether mice with targeted deletion of cytotoxic granule proteins are more susceptible to tuberculosis. Furthermore, significant cytolytic activity of CD8+ T cells against infected macrophages has been difficult to demonstrate ex vivo and usually requires stimulation of the T cells in vitro before lysis of target cells can be observed. Having identified an MHC class I–restricted epitope, we were able to demonstrate that CFP10-specific CD8+ T cells were cytolytic in vivo. Other M. tuberculosis antigens have recently been reported to be recognized by MHC class I–restricted CD8+ T cells. The epitope GYAGTLQSL that is derived from the TB10.3 and TB10.4 proteins are recognized by H-2Kd–restricted CD8+ T cells from M. tuberculosis and BCG-infected mice (45). By using H-2Kd tetramers and ELISPOT assay, we have extended these results to show that a significant number of TB10.3/420-28-specific CD8+ T cells are present in the lungs of infected mice. Furthermore, the CD8+ T cells that recognize this peptide are cytolytic in vivo and their cytolytic activity could be detected even as late as 260 d after infection. An epitope from the phosphate-binding protein PstS-3 also contains an epitope recognized by CD8+ T cells from infected C57BL/6 mice (62). In contrast with CFP10 and TB10.3/10.4, PstS3-specific CD8+ T cells in the peripheral blood could not be detected using peptide-loaded Db-tetramers, probably because their frequency, <0.02% in the spleen, is too low (62). Nevertheless, cytolytic T cells specific for this peptide appear to exist in the spleen, although the induction of in vivo CTL activity only appeared 8–10 wk after infection. These data suggest that the cytolytic activity appears to be related to the frequency of antigen-specific CD8+ T cells.
CD8+ T cells can kill target cells by several mechanisms, including the release of cytotoxic granules containing perforin and granzymes, CD95/CD95L-dependent killing, and the production of TNFα that induces apoptosis in some sensitive targets. Although disagreement exists about the requirement for cytotoxic molecules, perforin and CD95 appear to be needed under some conditions for optimum host resistance in murine models of tuberculosis (7, 21, 22). One reason for the discrepancies in published studies is that the requirement for CD8+ T cells may be more critical late during the course of infection. Studies that focused on early time points generally did not observe significant differences, whereas survival studies more typically reported a requirement for cytotoxic molecules. This possibility is supported by the finding that CD8+ T cell depletion leads to greater disease exacerbation when performed late during the course of aerosol infection (63). Interestingly, mice lacking CD8+ T cells (i.e., β2m, TAP1, and CD8 knockout mice) die more rapidly than mice lacking perforin, suggesting there may be some redundancy for cytotoxic pathways, as has been observed in certain viral systems (64, 65). Alternately, CD8+ T cells may mediate other functions, and at least one paper has emphasized that IFNγ production by CD8+ T cells can be protective (66). After in vitro stimulation of pulmonary CD8+ T cells with M. tuberculosis–infected DCs, CD8+ T cells acquire the ability to lyse infected macrophages in vitro, and the lysis is dependent on granule exocytosis (67). The establishment of a robust in vivo CTL assay will allow the dissection of the molecular pathways used by CTLs generated in M. tuberculosis–infected mice.
The continuing HIV/AIDS epidemic and the spread of multidrug-resistant M. tuberculosis has contributed to the perpetuation of the global epidemic of tuberculosis. Although M. bovis BCG is used universally as a vaccine, its efficacy in preventing pulmonary tuberculosis in adults is controversial (68). Antigen-specific CD8+ T cells are considered to be important for vaccine-induced immunity, and several of the vaccine strategies currently being evaluated are ones that potentially elicit CD8+ T cells. However, the paucity of defined human and murine epitopes recognized by MHC class I–restricted CD8+ T cells has impaired their immunological evaluation. Our paper has defined epitopes of the CFP10 protein that are presented by MHC class I and class II. Although we cannot say whether these are immunodominant epitopes, it clearly elicits a vigorous CTL response that is recruited to the lungs, and accounts for up to 30% of the pulmonary CD8+ T cells. These epitopes will make possible further insight into how CD8+ T cells mediate host resistance to tuberculosis and allow comparison of different vaccination strategies that elicit CD8+ T cell responses.
Acknowledgments
The authors thank S. Jean and the staff of the Animal Biohazard Containment Suite at the Dana Farber Cancer Institute for the help in facilitating these experiments.
This work was supported by National Institutes of Health grant no. R01 HL645450 and American Lung Association grant Career Investigator Award to S.M. Behar.
The authors have no conflicting financial interests.
Abbreviations used in this paper: β2m, β2 microglobulin; aa, amino acid; ANOVA, analysis of variance; BCG, bacillus Calmette-Guerin; CFP10, culture filtrate protein-10; ESAT-6, early secretory antigen target-6; PLN, pulmonary LN.
References
- 1.Flynn, J.L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129. [DOI] [PubMed] [Google Scholar]
- 2.Feng, C.G., A.G. Bean, H. Hooi, H. Briscoe, and W.J. Britton. 1999. Increase in gamma interferon-secreting CD8(+), as well as CD4(+), T cells in lungs following aerosol infection with Mycobacterium tuberculosis. Infect. Immun. 67:3242–3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith, S.M., and H.M. Dockrell. 2000. Role of CD8 T cells in mycobacterial infections. Immunol. Cell Biol. 78:325–333. [DOI] [PubMed] [Google Scholar]
- 4.Ackerman, A.L., and P. Cresswell. 2004. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 5:678–684. [DOI] [PubMed] [Google Scholar]
- 5.Flynn, J.L., M.M. Goldstein, K.J. Triebold, B. Koller, and B.R. Bloom. 1992. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. USA. 89:12013–12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behar, S.M., C.C. Dascher, M.J. Grusby, C.R. Wang, and M.B. Brenner. 1999. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J. Exp. Med. 189:1973–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner, J., C.D. D'Souza, J.E. Pearl, P. Marietta, M. Noel, A.A. Frank, R. Appelberg, I.M. Orme, and A.M. Cooper. 2001. CD8- and CD95/95L-dependent mechanisms of resistance in mice with chronic pulmonary tuberculosis. Am. J. Respir. Cell Mol. Biol. 24:203–209. [DOI] [PubMed] [Google Scholar]
- 8.Rolph, M.S., B. Raupach, H.H. Kobernick, H.L. Collins, B. Perarnau, F.A. Lemonnier, and S.H. Kaufmann. 2001. MHC class Ia-restricted T cells partially account for beta2-microglobulin-dependent resistance to Mycobacterium tuberculosis. Eur. J. Immunol. 31:1944–1949. [DOI] [PubMed] [Google Scholar]
- 9.Mogues, T., M.E. Goodrich, L. Ryan, R. LaCourse, and R.J. North. 2001. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 193:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urdahl, K.B., D. Liggitt, and M.J. Bevan. 2003. CD8+ T cells accumulate in the lungs of Mycobacterium tuberculosis-infected Kb−/−Db−/− mice, but provide minimal protection. J. Immunol. 170:1987–1994. [DOI] [PubMed] [Google Scholar]
- 11.Sun, J.C., M.A. Williams, and M.J. Bevan. 2004. CD4+ T cells are required for the maintenance, not programming, of memory CD8+ T cells after acute infection. Nat. Immunol. 5:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens, D.L., and M.A. Horwitz. 1995. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181:257–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreira, A.L., J. Wang, L. Tsenova-Berkova, W. Hellmann, V.H. Freedman, and G. Kaplan. 1997. Sequestration of Mycobacterium tuberculosis in tight vacuoles in vivo in lung macrophages of mice infected by the respiratory route. Infect. Immun. 65:305–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serbina, N.V., and J.L. Flynn. 1999. Early emergence of CD8(+) T cells primed for production of type 1 cytokines in the lungs of Mycobacterium tuberculosis-infected mice. Infect. Immun. 67:3980–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, C.G., C.M. Collazo-Custodio, M. Eckhaus, S. Hieny, Y. Belkaid, K. Elkins, D. Jankovic, G.A. Taylor, and A. Sher. 2004. Mice deficient in LRG-47 display increased susceptibility to mycobacterial infection associated with the induction of lymphopenia. J. Immunol. 172:1163–1168. [DOI] [PubMed] [Google Scholar]
- 16.Stenger, S., D.A. Hanson, R. Teitelbaum, P. Dewan, K.R. Niazi, C.J. Froelich, T. Ganz, S. Thoma-Uszynski, A. Melian, C. Bogdan, et al. 1998. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 282:121–125. [DOI] [PubMed] [Google Scholar]
- 17.Lalvani, A., R. Brookes, R.J. Wilkinson, A.S. Malin, A.A. Pathan, P. Andersen, H. Dockrell, G. Pasvol, and A.V. Hill. 1998. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA. 95:270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann, S.H., U. Vath, J.E. Thole, J.D. Van Embden, and F. Emmrich. 1987. Enumeration of T cells reactive with Mycobacterium tuberculosis organisms and specific for the recombinant mycobacterial 64-kDa protein. Eur. J. Immunol. 17:351–357. [DOI] [PubMed] [Google Scholar]
- 19.De Libero, G., I. Flesch, and S.H. Kaufmann. 1988. Mycobacteria-reactive Lyt-2+ T cell lines. Eur. J. Immunol. 18:59–66. [DOI] [PubMed] [Google Scholar]
- 20.Mohagheghpour, N., D. Gammon, L.M. Kawamura, A. van Vollenhoven, C.J. Benike, and E.G. Engleman. 1998. CTL response to Mycobacterium tuberculosis: identification of an immunogenic epitope in the 19-kDa lipoprotein. J. Immunol. 161:2400–2406. [PubMed] [Google Scholar]
- 21.Cooper, A.M., C. D'Souza, A.A. Frank, and I.M. Orme. 1997. The course of Mycobacterium tuberculosis infection in the lungs of mice lacking expression of either perforin- or granzyme-mediated cytolytic mechanisms. Infect. Immun. 65:1317–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laochumroonvorapong, P., J. Wang, C.C. Liu, W. Ye, A.L. Moreira, K.B. Elkon, V.H. Freedman, and G. Kaplan. 1997. Perforin, a cytotoxic molecule which mediates cell necrosis, is not required for the early control of mycobacterial infection in mice. Infect. Immun. 65:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orme, I.M. 1988. Induction of nonspecific acquired resistance and delayed-type hypersensitivity, but not specific acquired resistance in mice inoculated with killed mycobacterial vaccines. Infect. Immun. 56:3310–3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasan, A., A. Childerstone, K. Pervin, T. Shinnick, Y. Mizushima, R. Van der Zee, R. Vaughan, and T. Lehner. 1995. Recognition of a unique peptide epitope of the mycobacterial and human heat shock protein 65-60 antigen by T cells of patients with recurrent oral ulcers. Clin. Exp. Immunol. 99:392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arend, S.M., P. Andersen, K.E. van Meijgaarden, R.L. Skjot, Y.W. Subronto, J.T. van Dissel, and T.H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early-secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850–1854. [DOI] [PubMed] [Google Scholar]
- 26.Lewinsohn, D.M., A.L. Briden, S.G. Reed, K.H. Grabstein, and M.R. Alderson. 2000. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J. Immunol. 165:925–930. [DOI] [PubMed] [Google Scholar]
- 27.Lewinsohn, D.M., L. Zhu, V.J. Madison, D.C. Dillon, S.P. Fling, S.G. Reed, K.H. Grabstein, and M.R. Alderson. 2001. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J. Immunol. 166:439–446. [DOI] [PubMed] [Google Scholar]
- 28.Dillon, D.C., M.R. Alderson, C.H. Day, T. Bement, A. Campos-Neto, Y.A. Skeiky, T. Vedvick, R. Badaro, S.G. Reed, and R. Houghton. 2000. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J. Clin. Microbiol. 38:3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skjot, R.L., T. Oettinger, I. Rosenkrands, P. Ravn, I. Brock, S. Jacobsen, and P. Andersen. 2000. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect. Immun. 68:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brusasca, P.N., R. Colangeli, K.P. Lyashchenko, X. Zhao, M. Vogelstein, J.S. Spencer, D.N. McMurray, and M.L. Gennaro. 2001. Immunological characterization of antigens encoded by the RD1 region of the Mycobacterium tuberculosis genome. Scand. J. Immunol. 54:448–452. [DOI] [PubMed] [Google Scholar]
- 31.Colangeli, R., J.S. Spencer, P. Bifani, A. Williams, K. Lyashchenko, M.A. Keen, P.J. Hill, J. Belisle, and M.L. Gennaro. 2000. MTSA-10, the product of the Rv3874 gene of Mycobacterium tuberculosis, elicits tuberculosis-specific, delayed-type hypersensitivity in guinea pigs. Infect. Immun. 68:990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brosch, R., S.V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, et al. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA. 99:3684–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pym, A.S., P. Brodin, R. Brosch, M. Huerre, and S.T. Cole. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46:709–717. [DOI] [PubMed] [Google Scholar]
- 34.Cole, S.T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S.V. Gordon, K. Eiglmeier, S. Gas, C.E. Barry III, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 393:537–544. [DOI] [PubMed] [Google Scholar]
- 35.Harboe, M., T. Oettinger, H.G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sorensen, A.L., S. Nagai, G. Houen, P. Andersen, and A.B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berthet, F.X., P.B. Rasmussen, I. Rosenkrands, P. Andersen, and B. Gicquel. 1998. A Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiol. 144:3195–3203. [DOI] [PubMed] [Google Scholar]
- 38.Renshaw, P.S., P. Panagiotidou, A. Whelan, S.V. Gordon, R.G. Hewinson, R.A. Williamson, and M.D. Carr. 2002. Conclusive evidence that the major T-cell antigens of the Mycobacterium tuberculosis complex ESAT-6 and CFP-10 form a tight, 1:1 complex and characterization of the structural properties of ESAT-6, CFP-10, and the ESAT-6*CFP-10 complex. Implications for pathogenesis and virulence. J. Biol. Chem. 277:21598–21603. [DOI] [PubMed] [Google Scholar]
- 39.Hsu, T., S.M. Hingley-Wilson, B. Chen, M. Chen, A.Z. Dai, P.M. Morin, C.B. Marks, J. Padiyar, C. Goulding, M. Gingery, et al. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. USA. 100:12420–12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pym, A.S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K.E. Griffiths, G. Marchal, C. Leclerc, and S.T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9:533–539. [DOI] [PubMed] [Google Scholar]
- 41.Chackerian, A.A., T.V. Perera, and S.M. Behar. 2001. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 69:2666–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chackerian, A.A., J.M. Alt, T.V. Perera, C.C. Dascher, and S.M. Behar. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 70:4501–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Belz, G.T., W. Xie, and P.C. Doherty. 2001. Diversity of epitope and cytokine profiles for primary and secondary influenza a virus-specific CD8+ T cell responses. J. Immunol. 166:4627–4633. [DOI] [PubMed] [Google Scholar]
- 44.Gold, M.C., M.W. Munks, M. Wagner, U.H. Koszinowski, A.B. Hill, and S.P. Fling. 2002. The murine cytomegalovirus immunomodulatory gene m152 prevents recognition of infected cells by M45-specific CTL but does not alter the immunodominance of the M45-specific CD8 T cell response in vivo. J. Immunol. 169:359–365. [DOI] [PubMed] [Google Scholar]
- 45.Majlessi, L., M.J. Rojas, P. Brodin, and C. Leclerc. 2003. CD8+-T-cell responses of Mycobacterium-infected mice to a newly identified major histocompatibility complex class I-restricted epitope shared by proteins of the ESAT-6 family. Infect. Immun. 71:7173–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wherry, E.J., J.N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reignat, S., G.J. Webster, D. Brown, G.S. Ogg, A. King, S.L. Seneviratne, G. Dusheiko, R. Williams, M.K. Maini, and A. Bertoletti. 2002. Escaping high viral load exhaustion: CD8 cells with altered tetramer binding in chronic hepatitis B virus infection. J. Exp. Med. 195:1089–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho, S., V. Mehra, S. Thoma-Uszynski, S. Stenger, N. Serbina, R.J. Mazzaccaro, J.L. Flynn, P.F. Barnes, S. Southwood, E. Celis, et al. 2000. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc. Natl. Acad. Sci. USA. 97:12210–12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva, C.L., and D.B. Lowrie. 2000. Identification and characterization of murine cytotoxic T cells that kill Mycobacterium tuberculosis. Infect. Immun. 68:3269–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aichele, P., K. Brduscha-Riem, S. Oehen, B. Odermatt, R.M. Zinkernagel, H. Hengartner, and H. Pircher. 1997. Peptide antigen treatment of naive and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity. 6:519–529. [DOI] [PubMed] [Google Scholar]
- 51.Nelson, D., C. Bundell, and B. Robinson. 2000. In vivo cross-presentation of a soluble protein antigen: kinetics, distribution, and generation of effector CTL recognizing dominant and subdominant epitopes. J. Immunol. 165:6123–6132. [DOI] [PubMed] [Google Scholar]
- 52.Barber, D.L., E.J. Wherry, and R. Ahmed. 2003. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 171:27–31. [DOI] [PubMed] [Google Scholar]
- 53.Marzo, A.L., B.F. Kinnear, R.A. Lake, J.J. Frelinger, E.J. Collins, B.W. Robinson, and B. Scott. 2000. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J. Immunol. 165:6047–6055. [DOI] [PubMed] [Google Scholar]
- 54.Oehen, S., and K. Brduscha-Riem. 1998. Differentiation of naive CTL to effector and memory CTL: correlation of effector function with phenotype and cell division. J. Immunol. 161:5338–5346. [PubMed] [Google Scholar]
- 55.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T.P. van den Berg, A. Vanonckelen, J. Ooms, E. Saman, J.B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 66:1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu, X., H.J. Stauss, J. Ivanyi, and H.M. Vordermeier. 1997. Specificity of CD8+ T cells from subunit-vaccinated and infected H-2b mice recognizing the 38 kDa antigen of Mycobacterium tuberculosis. Int. Immunol. 9:1669–1676. [DOI] [PubMed] [Google Scholar]
- 57.Feng, C.G., C. Demangel, A.T. Kamath, M. Macdonald, and W.J. Britton. 2001. Dendritic cells infected with Mycobacterium bovis bacillus Calmette Guerin activate CD8(+) T cells with specificity for a novel mycobacterial epitope. Int. Immunol. 13:451–458. [DOI] [PubMed] [Google Scholar]
- 58.Roberts, A.D., M.G. Sonnenberg, D.J. Ordway, S.K. Furney, P.J. Brennan, J.T. Belisle, and I.M. Orme. 1995. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 85:502–508. [PMC free article] [PubMed] [Google Scholar]
- 59.Lalvani, A., P. Nagvenkar, Z. Udwadia, A.A. Pathan, K.A. Wilkinson, J.S. Shastri, K. Ewer, A.V. Hill, A. Mehta, and C. Rodrigues. 2001. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J. Infect. Dis. 183:469–477. [DOI] [PubMed] [Google Scholar]
- 60.Busch, D.H., and E.G.P. Am. 1999. T lymphocyte dynamics during Listeria monocytogenes infection. Immunol. Lett. 65:93–98. [DOI] [PubMed] [Google Scholar]
- 61.Pope, C., S.K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402–3409. [DOI] [PubMed] [Google Scholar]
- 62.Romano, M., O. Denis, S. D'Souza, X.M. Wang, T.H. Ottenhoff, J.M. Brulet, and K. Huygen. 2004. Induction of in vivo functional Db-restricted cytolytic T cell activity against a putative phosphate transport receptor of Mycobacterium tuberculosis. J. Immunol. 172:6913–6921. [DOI] [PubMed] [Google Scholar]
- 63.van Pinxteren, L.A., J.P. Cassidy, B.H. Smedegaard, E.M. Agger, and P. Andersen. 2000. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur. J. Immunol. 30:3689–3698. [DOI] [PubMed] [Google Scholar]
- 64.Topham, D.J., R.A. Tripp, and P.C. Doherty. 1997. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J. Immunol. 159:5197–5200. [PubMed] [Google Scholar]
- 65.Topham, D.J., R.C. Cardin, J.P. Christensen, J.W. Brooks, G.T. Belz, and P.C. Doherty. 2001. Perforin and Fas in murine gammaherpesvirus-specific CD8(+) T cell control and morbidity. J. Gen. Virol. 82:1971–1981. [DOI] [PubMed] [Google Scholar]
- 66.Tascon, R.E., E. Stavropoulos, K.V. Lukacs, and M.J. Colston. 1998. Protection against Mycobacterium tuberculosis infection by CD8+ T cells requires the production of gamma interferon. Infect. Immun. 66:830–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Serbina, N.V., C.C. Liu, C.A. Scanga, and J.L. Flynn. 2000. CD8+ CTL from lungs of Mycobacterium tuberculosis-infected mice express perforin in vivo and lyse infected macrophages. J. Immunol. 165:353–363. [DOI] [PubMed] [Google Scholar]
- 68.Sterne, J.A., L.C. Rodrigues, and I.N. Guedes. 1998. Does the efficacy of BCG decline with time since vaccination? Int. J. Tuberc. Lung Dis. 2:200–207. [PubMed] [Google Scholar]