Abstract
Because of different cytokine responsiveness, surface receptor, and transcription factor expression, human CD11c− natural type I interferon–producing cells (IPCs) and CD11c+ dendritic cells were thought to derive through lymphoid and myeloid hematopoietic developmental pathways, respectively. To directly test this hypothesis, we used an in vitro assay allowing simultaneous IPC, dendritic cell, and B cell development and we tested lymphoid and myeloid committed hematopoietic progenitor cells for their developmental capacity. Lymphoid and common myeloid and granulocyte/macrophage progenitors were capable of developing into both functional IPCs, expressing gene transcripts thought to be associated with lymphoid lineage development, and into dendritic cells. However, clonal progenitors for both populations were about fivefold more frequent within myeloid committed progenitor cells. Thus, in humans as in mice, natural IPC and dendritic cell development robustly segregates with myeloid differentiation. This would fit with natural interferon type I–producing cell and dendritic cell activity in innate immunity, the evolutionary older arm of the cellular immune system.
Keywords: human hematopoietic progenitors, plasmacytoid dendritic cells, dendritic cells, development, hematopoietic lineage commitment
Introduction
Differentiation of hematopoietic stem cells (HSCs) to mature cells of the hematopoietic system involves loss of the self-renewal potential followed by sequential restriction of developmental options; i.e., the gradual commitment to different hemato-lymphoid lineages. In both mice and man, non–self-renewing progenitor cell populations with high expansion potential into exclusively lymphoid or myeloid lineages were identified, thus suggesting a lympho–myeloid dichotomy as one of the earliest steps in this hierarchical differentiation process (1).
DCs are cells of the hematopoietic system. Originally defined by their capacity to initiate primary T cell responses (2), DCs are also involved in maintaining peripheral tolerance and in regulating innate immunity (3, 4). Given their importance in immune regulation, understanding development and maintenance of DCs may thus lead to novel, targeted therapies in conditions such as immune deficiencies or autoimmune diseases.
Multiple subtypes of DCs that differ in phenotype, localization in lymphoid and nonlymphoid tissues, and in biological function have been described previously (3). With the exception of subpopulations such as epidermal Langerhans cells, DCs are continuously replenished from bone marrow HSCs. In vitro, human DCs can be generated from CD34+ hematopoietic precursor cells and from monocytes (5–7). In addition, it was shown recently that type I interferon–producing cells (IPCs) are capable of maturing to DCs (also called plasmacytoid DCs), at least in vitro (8, 9). IPCs can be generated from CD34+ hematopoietic progenitors (10–13). Although CD34+ cells expand and require proliferative and inflammatory stimuli to differentiate into DCs, monocytes and IPCs show little or no proliferative capacity, and DC maturation is triggered by distinct survival and inflammatory signals (3, 9, 14, 15).
IPCs and their offspring, mature DCs, display the following features commonly associated with lymphoid, but not myeloid, cell lineages: IPCs express CD2, CD5, and CD7 (3); T cell development associated pre-TCRα transcripts (12, 16, 17); B cell development associated Igλ-like 14.1; and Spi-B (17, 18). Furthermore, IPCs lack markers as CD11c, CD13, CD33 and mannose receptors (3); GM-CSF does not promote IPC and plasmacytoid DC development (11, 19); and differentiation of IPCs and B cells but not CD11c+ dendritic cells is inhibited by ectopic expression of inhibitor of DNA binding Id2 and Id3 (12). Thus, it was suggested that IPCs and their offspring CD11c− plasmacytoid DCs segregate with early lymphoid development and therefore are lymphoid lineage derived (3, 12).
To formally test this hypothesis, we used human early lymphoid and myeloid committed progenitor cells (20, 21) and evaluated their developmental potential in an assay system simultaneously promoting IPC, CD11c+ DC, and B cell differentiation.
Materials and Methods
Cell Samples.
Cord blood from healthy full-term newborns was obtained with written informed parental consent. The use of cord blood was approved by the Cantonal Ethics Board of Ticino, Switzerland. Peripheral blood mononuclear cells were obtained from healthy blood donors.
Cell Preparation and Flow Cytometry.
Mononuclear cells were isolated by density gradient centrifugation (Ficoll-Paque; ICN Biomedicals). CD34+ cells were immunomagnetically enriched according to the manufacturer's instructions (CD34+ selection kit; Miltenyi Biotec). Hematopoietic progenitor cells were isolated as described previously (20, 21). In brief, combined HSCs and progenitors were sorted as lin− (CD2, RPA-2.10; CD3, S4.1; CD4, S3.5; CD8, 3B5; CD11b, ICRF44; CD14, TUK4; CD19, SJ25-C1; CD20, 2H7; CD56, B159; and GPA, GA-R2) CD34+ (HPCA-2) IL-3Rα+/− (CD123, 9F5) cells. Lymphoid committed cells were sorted as lin−CD34+CD38+ (HIT2) CD10+ (5-1B4) and/or CD7+ (CD7-6B7). Myeloid committed cells were sorted as lin− (as aforementioned with the addition of CD7 and CD10) CD34+CD38+, and IL-3Rα+ (CD123, 9F5) CD45RA− (MEM56) (common myeloid progenitors [CMPs]), or IL-3Rα+CD45RA+ (granulocyte/macrophage progenitors [GMPs]), or IL-3Rα−CD45RA− (megakaryocyte/erythrocyte progenitors [MEPs]). When lymphoid and myeloid progenitor cells were sorted simultaneously from the same cord blood sample, CD45RA was omitted from the staining, and CMP and GMP were sorted as a combined cell population. In all cases, IL-3Rαhi cells were carefully excluded. Antibodies against the following antigens were used as indicated: CD11c (B-ly6), HLA-DR (TÜ36), TLR-2 (TL2.1), TLR-4 (HTA125), CD3 (UCHT1), CD14 (RM052), CD40 (MAB89), CD62L (DREG56), CD80 (MAB104), CD83 (HB15a), CD86 (HA5.2B7), CD4 (13B8.2), CD19 (J4.119), BDCA-1 (AD5-8E7), BDCA-2 (AC144), and BDCA-4 (AD5-17F6). Antibodies were purchased from BD Biosciences, Caltag, Ancell, Miltenyi Biotec, or Immunotech. Dead cells were excluded by propidium iodide staining and appropriate isotype-matched control mAbs were used to determine the level of background staining. Cells were sorted and analyzed using a 488-nm argon and a 633-nm helium-neon laser FACS Vantage SE (Becton Dickinson). For single cell and limiting dilution assays, cells were double sorted to achieve virtually pure cell populations.
Cell Culture.
Cells were cultured for 10–20 d on irradiated (30 Gy) murine Sys-1 (Ac6) (SyStemix Inc.) stroma cell monolayers in RPMI 1640 (GIBCO BRL) supplemented with 100 U/ml penicillin/streptomycin, 100 U/ml l-glutamine, 20 nM 2-mercaptoethanol, 10% FCS (GIBCO BRL), and 10 ng/ml recombinant human flt3 ligand (R&D Systems).
To determine cellular divisions in culture, input populations were labeled for 10 min with 5 μM carboxyfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes) at 37°C.
For limiting dilution experiments, cells were sorted directly into 96-well plates on irradiated stroma cells at numbers of 300, 100, 30, 10, and 1 cell per well. Frequency of progenitors reading-out as both IPC and DC was determined using “Loi de Poisson” statistics. The percentage of detection failure for both IPCs and DCs was calculated as 100 × (1 − k/n), where k is the number of positive wells for both cell types, and n is the number of repeats.
To generate DCs from monocytes, immunomagnetically selected (Miltenyi Biotec) peripheral blood CD14+ cells were cultured in RPMI 1640 supplemented with 10% FCS, 100 ng/ml GM-CSF (Leukomax; Novartis), and 20 ng/ml human IL-4 (R&D Systems) over 5 d.
For DC maturation, 0.1 μg/ml LPS (Sigma-Aldrich) was added to CD34+ and monocyte-derived DC cultures overnight.
Mixed lymphocyte reactions were performed using CFSE-labeled CD4+ peripheral blood responder T cells plated with stimulator cells in U-bottom 96-well plates in a 10:1 ratio.
Cytospin Preparations.
Cells were spun on microscope slides and were fixed and stained with May-Grünwald and Giemsa's reagent.
INF-α Production.
Sorted cells were infected overnight with 40 HAU/ml of Influenza virus (strain A/Beijing/353/89, gift of I. Julkunen) in U-bottom 96-well plates and INF-α was measured in supernatants (human INF-α ELISA kit, PBL Biomedical Laboratories).
PCR Analysis.
RNA was isolated from sorted cells using TRIzol reagent (Invitrogen) followed by DNase I (Invitrogen) treatment. cDNA was synthesized using random hexamers and Superscript II reverse transcriptase (Invitrogen).
Real-time PCR was performed using a sequence detector (model ABI PRISM 7700; PerkinElmer) and TaqMan target mixes (Assay-on-Demand Gene expression reagents; Applied Biosystems): Flt3 (Hs 00174690 m1), c-mpl (Hs 00180489 m1), IL-7Rα (Hs 00233682 m1), pre-TCRα (Hs 00300125 m1), Spi-B (Hs 00162150 m1), and β-actin (Hs 99999903 m1). TLR transcripts were detected using the following primer and probe sequences: TLR2 (F, CAGCACTGGTGTCTGGCATG; Probe, CTGTGCTCTGTTCCTGCTGATCCTGC; and R, GAGCCAGGCCCACATCATT), TLR4 (F, GTTTCCTGCAATGGATCAAGGA; Probe, TCGTTCAACTTCCACCAAGAGCTGCCT; and R, TGCTTATCTGAAGGTGTTGCACAT) TLR7 (F, TTACCTGGATGGAAACCAGCTACT; Probe, AGATACCGCAGGGCCTCCCGC; and R, TCAAGGCTGAGAAGCTGTAAGCTA), TLR9 (F, TGAAGACTTCAGGCCCAACTG; and Probe, AGCACCCTCAACTTCACCTTGGATCTGTC).
Results and Discussion
We wanted to test CD11c− IPC and CD11c+ DC differentiation from human lineage–restricted hematopoietic progenitor cells (20, 21) to clarify their respective lymphoid and/or myeloid lineage–associated developmental pathways.
Under the influence of GM-CSF, human CD34+ hematopoietic cells and monocytes give rise to CD11c+ DCs in culture (5–7). Also, using multiple cytokines, it was shown that lymphoid progenitors differentiate into DCs, albeit with lower efficiency (20, 22). We cultured sorted human CMPs, GMPs, and MEPs in complete media supplemented with SCF, Flt3L, GM-CSF, IL-4, and TNFα. CMPs and GMPs expanded 40–50-fold; and at 2 wk, about half of cells in culture displayed DC morphology, expressed CD11c, MHC class II, CD40, CD80, CD83, and CD86, and were efficient stimulators in allogeneic mixed lymphocyte reactions (unpublished data). No CD11c+ DCs developed from MEPs, and no CD11c−IL3Rαhi IPCs were detectable from any of the progenitor populations (unpublished data).
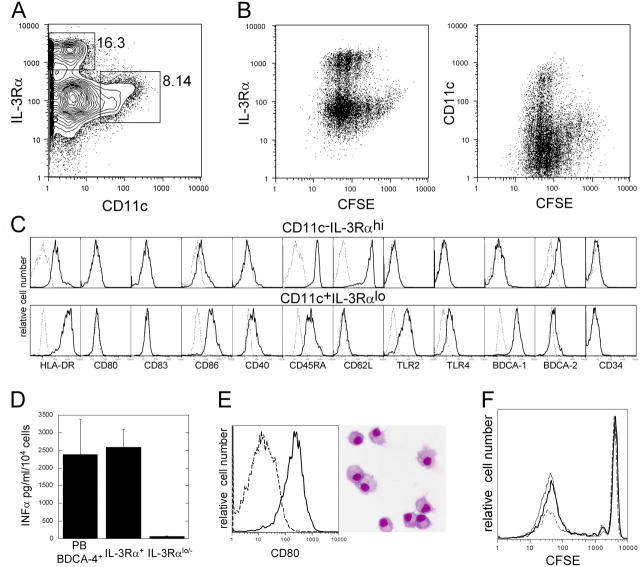
However, IPCs differentiate from human CD34+ cells in Flt3L-supplemented cultures within 10–25 d (11, 13) and on S17 stroma cells within 4 d (12). We found that sorted lin−CD34+IL-3Rα−/lo cells cultured for 8–20 d on Ac6 stroma cells supplemented with 10 ng/ml Flt3L gave rise to both CD11c−IL-3Rαhi (5–29% of total cells) and CD11c+IL-3Rαlo cells (3–14%; Fig. 1 A). To acquire IPC and DC phenotypes, input cells divided five to six times (Fig. 1 B). CD11c−IL-3Rαhi cells coexpressed HLA-DR, CD86, CD45RA, CD62L, and BDCA-2, but not CD80, CD83, CD40, and CD34 (Fig. 1 C), and, after overnight stimulation with influenza virus, produced similar amounts of INFα as peripheral blood BDCA-4+ IPCs (Fig. 1 D). Thus, culture-generated CD11c−IL-3Rαhi cells are functional IPCs. CD11c+IL-3Rαlo cells coexpressed HLA-DR, CD86, CD40, CD45RA, TLR2, TLR4, and BDCA-1, but not CD80, CD83, and CD34, which is consistent with an immature CD11c+ DC phenotype (Fig. 1 C). After overnight activation with LPS, cells up-regulated CD80 and CD83, acquired typical DC morphology (Fig. 1 E and not depicted), and became potent stimulators of allogeneic T cells (Fig. 1 F). Thus, culture-generated CD11c+ IL-3Rαlo cells are functional immature DCs.
Figure 1.
Lin−CD34+IL3Rαlo cells develop in vitro to functional IPCs and immature DCs. (A) Sorted lin−CD34+IL3Rαlo cells develop to both CD11c−IL3Rαhi and CD11c+IL3Rαlo cells within 13 d and (B) five to six divisions of input cells as determined by CFSE dilution in flt3L-supplemented Ac6 stroma cell cultures. Percentages of gated populations are indicated in contour plots. (C) CD11c−IL3Rαhi and CD11c+IL3Rαlo cells express IPC- and DC-associated surface markers (bold lines) on indicated cell populations as gated in A. (D) Influenza-stimulated CD11c−IL3Rαhi cells but not CD11c+/−IL3Rαlo/− cells from culture produce similar amounts of IFNα as peripheral blood BDCA-4+ IPCs. Mean and standard deviation of 10 (cultured cells) and 5 (BDCA-4+ cells) experiments are shown. (E) LPS-activated CD11c+IL3Rαlo cells up-regulate CD80 (bold line), show typical DC morphology, and (F) are potent stimulators of allogeneic CD4+ T cells as determined by CFSE dilution at day 7. Contour plot shows overlay of CD3+ gated T cell proliferation in response to activated CD11c+IL3Rαlo cells (bold line), CD14+ monocyte-derived dendritic cells (thin line), and CD11c− cells (dashed line). Cells were plated in a 1:10 stimulator to responder cell ratio. Data are representative of two experiments.
Ac6 cells produce mouse stem cell factor (SCF), flt3L, IL-7, and IL-15, and supported human IPC, DC, as well as B cell differentiation (unpublished data). However, addition of 10 ng/ml human flt3L led to an about threefold increase of total cell numbers without obviously changing the percentage of differentiated cell fractions (unpublished data). Therefore, this culture system provides a tool to simultaneously evaluate IPC, DC, and B cell differentiation from defined human hematopoietic progenitor cells in vitro.
Lymphoid progenitors, CMPs, and GMPs (as well as combined CMP and GMP) gave rise to both CD11c−IL-3Rαhi and CD11c+IL-3Rαlo cells (Fig. 2 A); however, the mean expansion of CMPs and GMPs was higher than that of lymphoid progenitors (Table I). CD11c−IL-3Rαhi cells derived from either progenitors produced high amounts of IFNα upon viral stimulation, thus were functional IPCs (two experiments each: 4,100 and 1,440 pg IFNα/ml/104 CD11c−IL-3Rαhi cells from lymphoid progenitors; and 3,470 and 1,720 pg IFNα/ml/104 CD11c−IL-3Rαhi cells from CMPs and GMPs). Similarly, lymphoid- and myeloid-derived CD11c+ cells became functional DCs after overnight LPS maturation, as determined by surface marker expression and capacity to stimulate allogeneic T cells (unpublished data). MEPs showed only limited, if any, CD11c−IL-3Rαhi cell differentiation capacity and did not generate CD11c+IL-3Rαlo cells (Fig. 2 A), demonstrating that, as in mice (23), DC differentiation potential is lost, once megakaryocyte/erythrocyte commitment occurs. As demonstrated previously (21), myeloid progenitors did not differentiate into B cells, whereas lymphoid progenitors simultaneously generated IPCs, DCs, and B cells (Fig. 2 A).
Figure 2.
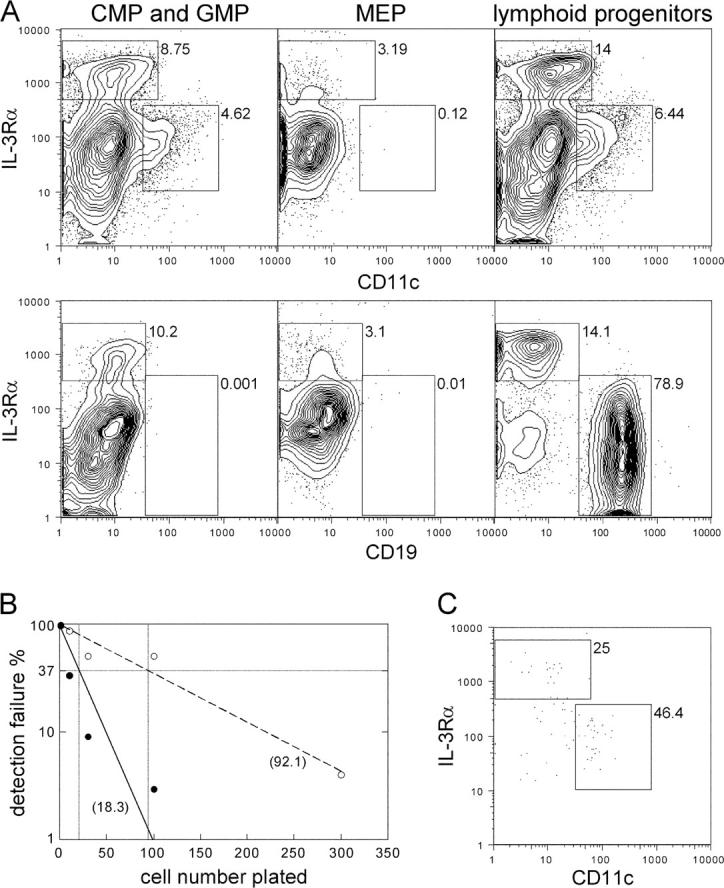
Lymphoid progenitors, CMPs, and GMPs develop in vitro to both IPCs and immature DCs and contain clonal progenitors for both populations. (A) Lymphoid progenitors, CMPs, and GMPs generate comparable relative numbers of both CD11c−IL3Rαhi and CD11c+ IL3Rαlo cells, whereas MEPs generate few, if any, CD11c−IL3Rαhi and no CD11c+IL3Rαlo cells (top). Only lymphoid progenitors developed to CD19+ B cells (bottom). Representative contour plots of three experiments analyzed at day 14 of culture. (B) Frequencies of myeloid (solid line) and lymphoid (dashed line) progenitors simultaneously differentiating to IPCs and DCs were determined by limiting dilution. The x axis depicts the number of plated cells and the y axis detection failure for combined IPC and DC read-out. Horizontal and vertical bars mark the 37% negative read-out predicting progenitor frequencies (numbers in brackets). Statistics were calculated on the basis of mean values of each dilution step from three and six independent experiments for myeloid and lymphoid progenitors, respectively. Correlation coefficients for curve extrapolations were r = 0.9372 and r = 0.93996 for myeloid and lymphoid progenitors, respectively. (C) Dot plot shows typical combined IPC and DC read-out from a single myeloid progenitor at day 14 d of culture. Percentages of gated populations are indicated in all plots.
Table I.
Cellular Expansion of CD34+ IL-3Rαlo Cells and Lymphoid and Myeloid Progenitors
| Total | IPCs | DCs | |
|---|---|---|---|
| CD34+ IL-3Rαlo | 129.7 (96–160) | 18.5 (10.6–29.7) | 10.4 (8.6–13.5) |
| CMPs and GMPs | 55.8 (30–88) | 5.5 (0.9-12) | 5.2 (1.8–14.9) |
| Lymphoid progenitors | 24.9 (10–60) | 2.7 (0.6–8.9) | 3.9 (1.0–9.9) |
Mean fold expansion of total cells, IPCs and DCs relative to input progenitor cell numbers at day 13 of cultures. Numbers in parentheses represent the range. Data represent results from three independent experiments for CD34+ IL-3Rαlo cells, nine for CMPs and GMPs, and seven for lymphoid progenitors.
In limiting dilution analysis, ∼1 out of 92 lymphoid progenitors and 1 out of 18 combined CMP and GMP progenitors gave rise to both IPCs and DCs (Fig. 2, B and C). These data prove that both lymphoid- and myeloid-committed cell populations contain single cells with IPC and DC potential, and shows that these progenitors are more frequent within myeloid committed cell populations, at least as detected by this in vitro assay. Although no single lymphoid progenitor cell simultaneously generated IPCs, DCs, and B cells, clonal IPC/B and DC/B progenitors were detectable (3 in 168, and 4 in 168, respectively). Thus, the lack of combined IPC, DC, and B cell read out from single lymphoid progenitors might be caused by lineage determination before the second cell division in culture.
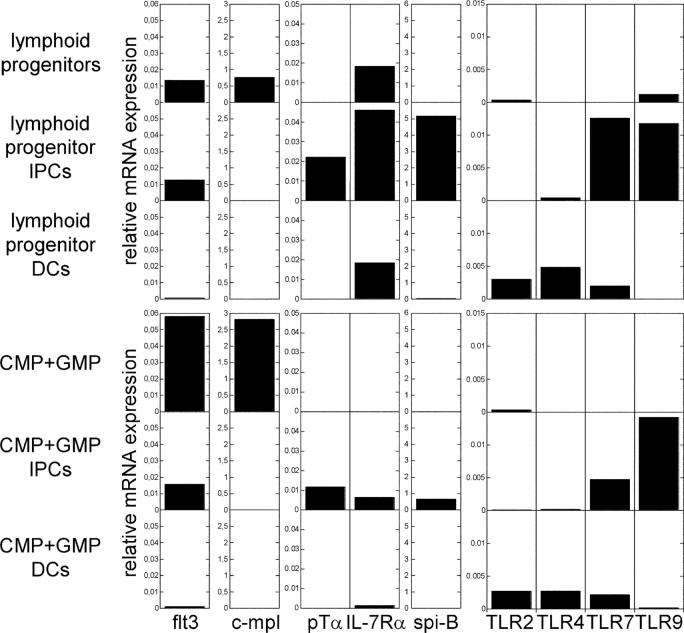
To evaluate gene transcripts associated with IPC and DC development, we tested mRNA expression in progenitor cells and in their respective differentiated IPC and DC cell fractions (Fig. 3). Flt3 was expressed in combined CMPs and GMPs at high levels, in lymphoid progenitors and both offspring IPCs at intermediate levels, and in both offspring DCs at low, but still, detectable levels (Fig. 3), thus likely paralleling mouse flt3 expression in IPC and DC development (24, 25). To formally prove this, progenitors should be isolated according to surface flt3 expression. However, currently available monoclonal antibodies to human flt3 in our hands did not provide sufficient discrimination of populations to directly address this issue. In contrast to flt3, trombopoietin (Tpo) receptor transcripts (c-mpl) were only detectable on progenitors (Fig. 3), thus suggesting that IPC gain in flt3L- and Tpo-supplemented CD34+ cell cultures is rather a result of Tpo-driven progenitor cell expansion than of Tpo-mediated IPC differentiation signals (13).
Figure 3.
Gene expression in myeloid and lymphoid progenitors and respective progenitor-derived IPCs and DCs Expression of indicated mRNA gene transcripts in progenitors and in offspring day 14 IPCs and DCs are shown in arbitrary units relative to endogenous β-actin. Data are representative of three independent experiments. c-mpl, thrombopoietin receptor; flt3, flt3 receptor; pTα, pre–T cell receptor α; IL-7Rα: IL-7 receptor α; spi-B, Spi-B transcription factor; TLR, Toll-like receptor.
Expression of lymphoid-associated transcripts as pre-Tα and Spi-B was taken as evidence for lymphoid lineage derivation of IPCs (3, 12, 16, 17). Interestingly, both lymphoid– and myeloid progenitor–derived IPCs expressed the lymphoid-associated transcripts pre-Tα, IL-7Rα, and Spi-B, albeit at slightly different levels (Fig. 3). Thus, although some of these transcripts are likely biologically relevant for IPC development and function (18), they do not allow conclusions on hematopoietic lineage origin.
Both lymphoid and myeloid progenitor derived IPCs expressed TLR7 and TLR9 and both DCs expressed TLR2, 4, and 7 as described previously (3, 14, 15) and low level expression of some TLRs could be detected in progenitor populations. Because TLR ligation provides important cues for IPC and DC maturation and function, it is tempting to speculate that TLR ligation might influence early IPC and DC differentiation, e.g., in inflammatory conditions.
Together, our data demonstrate that both human IPCs and DCs develop from lymphoid- and myeloid-restricted progenitor cells. Compared with lymphoid progenitors, myeloid progenitors are 5–10-fold more abundant in cord blood and bone marrow (20–22). As shown here, clonal progenitors for both IPCs and DCs are more frequent in myeloid populations and, on a population basis, myeloid progenitors show higher expansion potential for both IPCs and DCs. Therefore, if IPC, DC, and B cell read out in this bone marrow stroma cell culture truly reflects in vivo steady-state IPC, DC, and B cell development, most IPCs and DCs should be derived from myeloid progenitor populations, at least in the extrathymic environment. This parallels our and others previous findings for in vitro and in vivo CD11c+ DC and IPC development in mice (23, 26, 27; unpublished data), suggesting that these pathways are conserved in different species.
Recently, a dividing precursor population for IPCs and DCs was characterized in mouse blood (28); clonal analysis to test the existence of single cells with multi-DC differentiation capacities, however, were not provided. Here, we demonstrate for the first time clonal precursors for both IPCs and DCs in lymphoid and myeloid progenitor cell populations; however, the question of a possible downstream IPC- and DC-restricted clonal progenitor is not addressed. We reason that promiscuous IPC and DC potentials in successive hematopoietic differentiation correlates with expression and responsiveness to flt3/flt3L signals and likely are terminated by concurring lineage determination events (e.g., expression of Pax5 in B cell differentiation [reference 29]). This should be tested in future experiments.
IPCs, and to some extent CD11c+ DCs, are important mediators of innate immunity, and, after consecutive maturation, initiate antigen-specific adaptive immune responses. However, innate immunity predates adaptive immunity in evolution. In this view, it is conceivable that both IPC and DC differentiation robustly segregates with myeloid development; then, IPCs might primarily use genetic programs that later were adopted in adaptive immune system development.
Acknowledgments
We thank L. Bronz and the staff of obstetrics, Ospedale San Giovanni, Bellinzona for cord blood collection.
This study was supported in part by a Swissbridge and a Deutsche Forschungsgemeinschaft grant (MA 2159/2-1) to M.G. Manz.
The authors have no conflicting financial interests.
References
- 1.Kondo, M., A.J. Wagers, M.G. Manz, S.S. Prohaska, D.C. Scherer, G.F. Beilhack, J.A. Shizuru, and I.L. Weissman. 2003. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu. Rev. Immunol. 21:759–806. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau, J., and R.M. Steinman. 1998. Dendritic cells and the control of immunity. Nature. 392:245–252. [DOI] [PubMed] [Google Scholar]
- 3.Liu, Y.J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 106:259–262. [DOI] [PubMed] [Google Scholar]
- 4.Steinman, R.M., D. Hawiger, and M.C. Nussenzweig. 2003. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21:685–711. [DOI] [PubMed] [Google Scholar]
- 5.Caux, C., B. Vanbervliet, C. Massacrier, C. Dezutter-Dambuyant, B. de Saint-Vis, C. Jacquet, K. Yoneda, S. Imamura, D. Schmitt, and J. Banchereau. 1996. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNFα. J. Exp. Med. 184:695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony–stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179:1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ardavin, C., G. Martinez del Hoyo, P. Martin, F. Anjuere, C.F. Arias, A.R. Marin, S. Ruiz, V. Parrillas, and H. Hernandez. 2001. Origin and differentiation of dendritic cells. Trends Immunol. 22:691–700. [DOI] [PubMed] [Google Scholar]
- 8.Grouard, G., M.C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y.J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 185:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kadowaki, N., S. Antonenko, J.Y. Lau, and Y.J. Liu. 2000. Natural interferon α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olweus, J., A. BitMansour, R. Warnke, P.A. Thompson, J. Carballido, L.J. Picker, and F. Lund-Johansen. 1997. Dendritic cell ontogeny: a human dendritic cell lineage of myeloid origin. Proc. Natl. Acad. Sci. USA. 94:12551–12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blom, B., S. Ho, S. Antonenko, and Y.J. Liu. 2000. Generation of interferon α-producing predendritic cell (Pre-DC)2 from human CD34(+) hematopoietic stem cells. J. Exp. Med. 192:1785–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spits, H., F. Couwenberg, A.Q. Bakker, K. Weijer, and C.H. Uittenbogaart. 2000. Id2 and Id3 inhibit development of CD34+ stem cells into predendritic cell (pre-DC)2 but not into pre-DC1: evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 192:1775–1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen, W., S. Antonenko, J.M. Sederstrom, X. Liang, A.S. Chan, H. Kanzler, B. Blom, B.R. Blazar, and Y.J. Liu. 2004. Thrombopoietin cooperates with FLT3-ligand in the generation of plasmacytoid dendritic cell precursors from human hematopoietic progenitors. Blood. 103:2547–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388–3393. [DOI] [PubMed] [Google Scholar]
- 15.Kadowaki, N., S. Ho, S. Antonenko, R.W. Malefyt, R.A. Kastelein, F. Bazan, and Y.J. Liu. 2001. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 194:863–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Res, P.C., F. Couwenberg, F.A. Vyth-Dreese, and H. Spits. 1999. Expression of pTalpha mRNA in a committed dendritic cell precursor in the human thymus. Blood. 94:2647–2657. [PubMed] [Google Scholar]
- 17.Bendriss-Vermare, N., C. Barthelemy, I. Durand, C. Bruand, C. Dezutter-Dambuyant, N. Moulian, S. Berrih-Aknin, C. Caux, G. Trinchieri, and F. Briere. 2001. Human thymus contains IFN-alpha-producing CD11c(−), myeloid CD11c(+), and mature interdigitating dendritic cells. J. Clin. Invest. 107:835–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schotte, R., M.C. Rissoan, N. Bendriss-Vermare, J.M. Bridon, T. Duhen, K. Weijer, F. Briere, and H. Spits. 2003. The transcription factor Spi-B is expressed in plasmacytoid DC precursors and inhibits T-, B-, and NK-cell development. Blood. 101:1015–1023. [DOI] [PubMed] [Google Scholar]
- 19.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X.L. Xu, G. Trinchieri, A. O'Garra, and Y.J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony–stimulating factor. J. Exp. Med. 195:953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galy, A., M. Travis, D. Cen, and B. Chen. 1995. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 3:459–473. [DOI] [PubMed] [Google Scholar]
- 21.Manz, M.G., T. Miyamoto, K. Akashi, and I.L. Weissman. 2002. Prospective isolation of human clonogenic common myeloid progenitors. Proc. Natl. Acad. Sci. USA. 99:11872–11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hao, Q.L., J. Zhu, M.A. Price, K.J. Payne, L.W. Barsky, and G.M. Crooks. 2001. Identification of a novel, human multilymphoid progenitor in cord blood. Blood. 97:3683–3690. [DOI] [PubMed] [Google Scholar]
- 23.Manz, M.G., D. Traver, T. Miyamoto, I.L. Weissman, and K. Akashi. 2001. Dendritic cell potentials of early lymphoid and myeloid progenitors. Blood. 97:3333–3341. [DOI] [PubMed] [Google Scholar]
- 24.Karsunky, H., M. Merad, A. Cozzio, I.L. Weissman, and M.G. Manz. 2003. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J. Exp. Med. 198:305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Amico, A., and L. Wu. 2003. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J. Exp. Med. 198:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Traver, D., K. Akashi, M. Manz, M. Merad, T. Miyamoto, E.G. Engleman, and I.L. Weissman. 2000. Development of CD8alpha-positive dendritic cells from a common myeloid progenitor. Science. 290:2152–2154. [DOI] [PubMed] [Google Scholar]
- 27.H. Shigematsu, B. Reizis, H. Iwasaki, S. Mizuno, D. Hu, D. Traver, P. Leder, N. Sakaguchi, and K. Akashi. 2004. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 21:43–53. [DOI] [PubMed] [Google Scholar]
- 28.del Hoyo, G.M., P. Martin, H.H. Vargas, S. Ruiz, C.F. Arias, and C. Ardavin. 2002. Characterization of a common precursor population for dendritic cells. Nature. 415:1043–1047. [DOI] [PubMed] [Google Scholar]
- 29.Nutt, S.L., B. Heavey, A.G. Rolink, and M. Busslinger. 1999. Commitment to the B-lymphoid lineage depends on the transcription factor Pax5. Nature. 401:556–562. [DOI] [PubMed] [Google Scholar]