Abstract
The pleiotropic cytokine interleukin 4 (IL-4) has been shown to regulate many processes thought to be important in the allergic diathesis. To determine the mechanism(s) by which IL-4 mediates allergic airway responses to inhaled allergens, we compared the effects of antigen sensitization and challenge on the development of allergic airway responses in mice in which the gene for the signal transducer and activator of transcription factor 6 (Stat6) was disrupted to those of their wild-type littermates. Strikingly, Stat6-deficient mice failed to develop airway hyperresponsiveness (AHR), which was observed in their wild-type littermates after allergen provocation. Moreover, antigen-induced increases in mucus-containing cells were found to be completely Stat6 dependent. Consistent with the lack of Th2 cytokine responses in Stat6-deficient mice, no ovalbumin-specific immunoglobulin (Ig)E was detected in their serum. In contrast, Stat6 signaling only partially mediated antigen-induced eosinophilia with no role in vascular adhesion molecule 1 expression. These results indicate that Stat6 signal transduction is critical in the development of allergen-induced AHR and that agents that specifically inhibit this pathway may provide a novel strategy for the treatment of allergic disorders.
Allergic asthma is characterized by airway hyperresponsiveness to specific and/or nonspecific stimuli, chronic pulmonary eosinophilia, elevated serum IgE levels, and excessive mucus production. The pathology associated with asthma is thought to be mediated by CD4+ T lymphocytes producing the type 2 cytokines, IL-4 and IL-5, as both messenger RNA and protein levels of these cytokines are elevated in bronchial biopsies (1), bronchoalveolar lavage (BAL)1 cells (2, 3), and blood (3) of allergic patients as compared to normal individuals. Since these cytokines promote the accumulation and activation of eosinophils (4, 5) as well as IgE synthesis by B cells (6), this cytokine pattern has been thought to be important in the pathogenesis of asthma. Although considerable circumstantial evidence exists for this hypothesis in human asthma, the use of animal models of allergic inflammation has allowed more in-depth examination of the importance of Th2 cytokines in the pathogenesis of allergic inflammation. We have previously demonstrated that in fact the development of airway hyperresponsiveness (AHR) and pulmonary eosinophilia after allergen provocation is CD4+ T cell dependent (7). Consistent with studies in allergic asthmatics, the allergic phenotype in murine models has been associated with elevations in lung messenger RNA and protein levels of the type 2 cytokines, IL-4 and IL-5 (8, 9). Of the Th2 cytokines, IL-4 in particular has been shown to be essential in the pathogenesis of allergen-induced AHR. Specifically, AHR, eosinophilic inflammation, and IgE production normally seen after local antigen challenge do not develop in mice in which the gene for IL-4 is disrupted (10) or in animals in which the cytokine (11) or its receptor (9) are blocked in vivo. In addition, recent studies demonstrate that IL-4 may also be important in the production of mucus by the airway epithelium (9). Despite substantial evidence for the importance of IL-4 in allergic asthma, it has been difficult to ascertain the specific mechanisms by which it mediates the pathogenesis of allergic disease because of its plethora of actions. IL-4 has been shown to be the primary determinant of differentiation of CD4+ T cells into Th2 cytokine–producing cells (12). In addition to its pivotal role in CD4+ T cell differentiation, IL-4 also exerts many biological actions that may be important in the pathogenesis of allergic asthma such as its role in B cell production of IgE (6), upregulation of vascular cell adhesion molecule 1 (VCAM-1) on vascular endothelial cells (13), and its role in mastocytosis (14). Studies in murine models have suggested that IL-4's primary role is in commitment of CD4+ T cells to the Th2 cytokine–producing phenotype and that subsequent IL-5 production may be the important factor (15); however, other studies demonstrate that IL-4 mediates antigen-induced AHR independently of IL-5 and eosinophils (16).
The pleiotropic actions of IL-4 are mediated via a cell surface receptor composed of a cytokine-specific α chain and the common γc chain used by several other cytokines (17). Recent studies also demonstrate that the α chain of the IL-4 receptor is a component of the high affinity IL-13 receptor (17). Ligation of the IL-4 receptor results in the activation of at least two distinct signaling pathways. One involves the activation of signal transducer and activator of transcription factor 6 (Stat6) through phosphorylation by Januse kinase (JAK)1 and JAK3 (18, 19). Once activated, Stat6 proteins form homodimers, translocate to the nucleus, and directly bind to specific promoter sequences to regulate gene transcription. In addition to Stat6 activation, stimulation of the IL-4 receptor has also been shown to induce the phosphorylation of an insulin receptor substrate (IRS) termed IRS-2 (20, 21). Activated IRS-2 associates with phosphatidylinositol 3-kinase and may be responsible for certain IL-4–induced responses such as proliferation in response to IL-4. Previous studies in Stat6-deficient (Stat−/−) mice have suggested that despite the multiple signaling pathways activated by IL-4, Stat6 signaling is essential for mediating most responses of lymphocytes to IL-4 (22–24). Specifically it has been shown that Stat6-deficient animals are unable to generate Th2s or undergo class switching to IgE production. In contrast to its role in mediating responses of lymphocytes to IL-4, the role of Stat6 signaling pathways in other IL-4– mediated processes is virtually unknown.
The goal of this study was to gain further insight into the mechanisms by which IL-4 mediates the development of allergen-induced AHR. To this end, we compared the effects of allergen exposure on the development of allergen-induced AHR, pulmonary eosinophilia, BAL cytokine levels, immunoglobulin production, and mucus cell numbers in Stat6-deficient mice and their wild-type littermates (Stat+/+). Our results demonstrate that Stat6 signaling is essential in the production of Th2 cytokines in response to antigen challenge and in subsequent development of allergen-induced AHR. However, Stat6 signaling only partially mediates antigen-induced eosinophilia with no role in VCAM-1 expression. These results suggest that agents that specifically inhibit this pathway may be useful therapeutically for the modulation of chronic inflammation in allergic disorders.
Materials and Methods
Mice.
Male and female Stat6-deficient and wild-type mice backcrossed six generations onto a BALB/c background were generated and bred at Harvard School of Public Health (Boston, MA) as previously described (22). Animals were housed under laminar flow hoods in an environmentally controlled pathogen-free animal facility for the duration of experiments. They were given free access to tap water and OVA-free rodent chow. The studies reported here conformed to the principles for laboratory animal research outlined by the animal Welfare Act and the Department of Health, Education and Welfare (National Institutes of Health) guidelines for the experimental use of animals (n = 4–9 mice/experimental group, 9–12 wk of age).
Antigen Sensitization and Challenge.
Stat6-deficient and wild-type mice were sensitized by intraperitoneal injection of 10 μg OVA (crude grade IV; Sigma Chemical Co., St. Louis, MO) in 0.2 M PBS/ALUM (Sigma Chemical Co.) or PBS/ALUM alone twice, 1 wk apart. 1 wk after the last sensitization, mice were anesthetized with a mixture of ketamine and xylazine (45 and 8 mg/kg, respectively) and challenged by aspiration with 50 μl of a 1.5% solution of OVA or an equivalent volume of PBS. The effect of Stat6 deficiency on airway responsiveness, pulmonary inflammation, cytokine expression, and immunoglobulin synthesis was compared in wild-type and Stat6-deficient mice either sensitized and challenged with PBS (i.e., PBS treated) or OVA (i.e., OVA treated). 3 d after aspiration challenge, airway responsiveness to intravenous acetylcholine chloride (Ach) administration was determined, the number of inflammatory cells and cytokine levels in BAL fluid was determined, lungs were fixed in 10% formalin (Lyne Laboratories, Stoughton, MA), and blood was collected for analysis of serum immunoglobulin levels.
Airway Responsiveness Measurements.
Airway responsiveness to intravenous Ach challenge was measured as previously described (8, 10). In brief, mice were anesthetized with sodium pentobarbital (90 mg/kg) and intubated with a 20-gauge tracheal cannula equipped with a port for airway pressure measurements. Mice were ventilated at a rate of 120 breaths/min with a constant tidal volume of air (0.2 ml). A laparotomy was performed to expose the abdominal vena cava for drug delivery. Muscle paralysis was provided by intravenous administration of decamethonium bromide (25 mg/kg). After establishment of a stable airway pressure, acetylcholine was injected intravenously (50 μg/kg) and dynamic airway pressure was followed for 5 min. Airway responsiveness was defined by the time-integrated rise in peak airway pressure termed the airway-pressure-time index (cm H2O-s).
Assessment of Airway Inflammation.
After airway responsiveness measurements, lungs were lavaged three times with one 1.0-ml aliquot of HBSS (Biofluids, Gaithersburg, MD). The lavage fluid was centrifuged (1,500 rpm × 8 min), the supernatant was removed for cytokine analysis, and the cell pellet was resuspended in normal saline after a brief hypotonic exposure to lyse red blood cells. Total cells were counted with a hemacytometer. Cytospin preparations were prepared and stained with Diff-Quik (Baxter, McGraw Park, IL), and BAL cell differential percentages were determined based on light microscopic evaluation of >500 cells/ slide.
BAL Cytokine Analysis.
Cytokine protein levels were measured in duplicate samples of unconcentrated BAL fluid (100 μl) from each animal by ELISA as previously described (8). Assays for IL-4, IL-5, and IFN-γ were conducted using matched antibody pairs obtained from PharMingen (San Diego, CA) according to the manufacturer's instructions. Optical density readings of samples were converted to picograms per milliliter using values obtained from standard curves generated with varying concentrations of mouse recombinant IL-4, IL-5, and IFN-γ (5–2,000 pg/ ml). The limit of detection of each assay was 15 pg/ml.
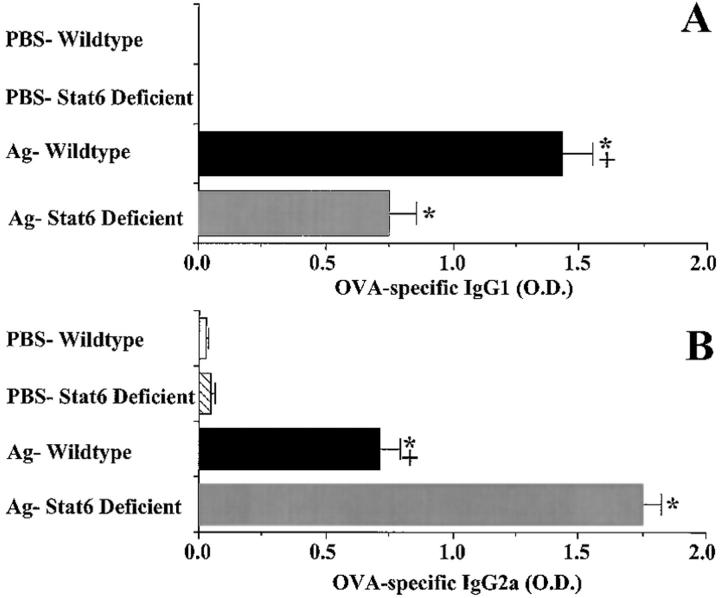
OVA-specific Serum IgG1 and IgG2a Analysis.
After airway responsiveness measurements, a kidney was excised and pooled blood was collected for antibody analysis. Serum was separated by centrifugation (1,200 g × 10 min), and stored at −80°C until analyzed. OVA-specific IgG1 and IgG2a serum antibody levels were determined by sandwich ELISA in 96-well ELISA plates. Sample wells were coated with a 0.01% OVA solution in PBS (50 μl/well) and incubated at room temperature overnight. Wells were then blocked with 10% FBS in PBS (200 μl/well) at room temperature for 2 h. After a wash procedure with 0.05% Tween-20 in PBS, serum samples were thawed and diluted 1:100, 1:250, and 1:500 with 10% FBS in PBS. Samples (100 μl/well) were incubated overnight at 4°C. Plates were washed with 0.05% Tween-20/PBS and incubated with biotin-conjugated anti– mouse IgG1 (1/2,000; γl chain specific) or IgG2a (1/2,000; γ2a chain specific; PharMingen, San Diego, CA; 100 μl/well) at room temperature for 1 h. After another wash procedure, plates were incubated for 1 h at room temperature with 100 μl/well of 0.0025 mg/ml avidin peroxidase (Sigma Chemical Co.) in 10% FBS/PBS. Plates were developed with ABTS (2.2′-azino-did[3-ethyl-benzthiazone sulfonate]; Kirkegaard & Perry Labs., Inc., Gaithersburg, MD; 100 μl/well). Plates were read at 405 nm within 30 min. Reported OD values are of serum samples diluted 1:250 with 10% FBS/PBS since these values were proven to be below the saturation point of the assay by comparison of OD values obtained from serum samples diluted 1:100. There were no detectable OVA-specific IgG1 antibodies in samples from PBS control animals.
Total Serum IgE Analysis.
Sera were obtained from blood taken during exsanguination of the animals after airway responsiveness measurements. An IgE-specific ELISA was used to quantitate total IgE Ab levels in serum using complementary antibody pairs for mouse IgE (R35-72 and R35-92) obtained from PharMingen according to the manufacturer's instructions. Duplicate samples (100 μl of a 1/50 dilution in 10% FBS in PBS) were examined from each animal. OD readings of samples were converted to picograms per milliliter using values obtained from standard curves generated with varying known concentrations of recombinant mouse IgE (5–2,000 pg/ml), and the final concentration was obtained by multiplying by the dilution factor.
Lung Histology.
To examine mucus cell content in the airway wall, lungs were excised after airway measurements and placed directly in 10% formalin. They were then washed in 70% ethanol, dehydrated, embedded in glycol methacrylate, cut into 10-μm sections, mounted on slides, and stained with hematoxylin and eosin and periodic acid Schiff.
Immunohistochemical Detection of VCAM-1.
3 d after aspiration challenge, lungs were excised and placed in periodate-lysine-paraformaldehyde fixative overnight at 4°C. They were then soaked in 30% sucrose for 2 d at 4°C and then snap frozen in a liquid nitrogen cooled isopentane mixture and stored at −80°C. Cryostat sections (10 μm) were cut and melted onto coated slides. Endogenous peroxidase was blocked with 0.1% sodium azide in 1.0% hydrogen peroxide diluted in PBS. Nonspecific reactions were minimized by blocking with CAS-Block (Zymed Laboratories, South San Francisco, CA) and 2% normal goat serum. Sections were incubated with either rat anti–mouse VCAM-1 antibodies (M/K-2, rat IgG; Southern Biotechnology Associates, Inc., Birmingham, AL; 5 mg/ml, 1:1,500) or an isotype-matched control antibody (rat IgG; Southern Biotechnology Associates, Inc.) at the same dilution for 1 h at room temperature. After a wash procedure, sections were incubated with mouse-absorbed goat anti–rat IgG antibodies (Southern Biotechnology Associates, Inc.; 0.5 mg/ml, diluted 1:1,500) for 30 min at room temperature. Positive VCAM-1 staining was detected using the avidin-biotin-peroxidase complex method (ABC kit; Biogenex, San Ramon, CA) using 3.3′ diaminobenzidine (DAB substrate kit; Biogenex, San Ramon, CA).
Data Analysis.
Data was reported as mean ± SE. Differences between means were determined by analysis of variance using Fisher's least significant difference test for multiple comparisons. Significance was assumed at P values of <0.05.
Results
Antigen-induced AHR Is Mediated by Stat6.
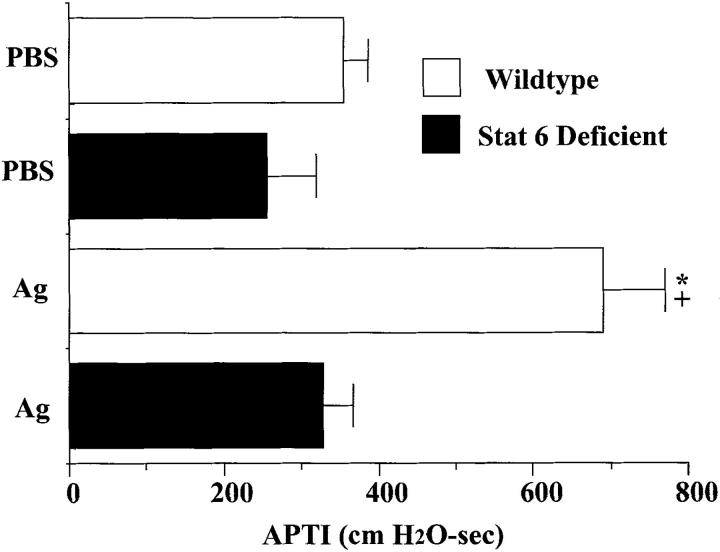
To determine the importance of Stat6 signaling in the development of allergic responses, measurements of airway reactivity to intravenous Ach were performed on PBS- and OVA-treated wild-type and Stat6-deficient mice (Fig. 1). Wild-type animals sensitized and challenged with OVA developed significant increases in airway reactivity to Ach compared to wild-type animals challenged with PBS. In marked contrast, OVA-sensitized and challenged Stat6-deficient mice did not develop a significant increase in airway reactivity compared to their PBS-treated controls. These results indicate that the development of antigen-induced AHR is completely dependent upon Stat6 signaling.
Figure 1.
Effects of Stat6 gene disruption on airway reactivity to intravenous acetylcholine (50 μg/kg) in mice 3 d after a single aspiration challenge with antigen (1.5% OVA) or PBS. Values shown are mean and SE (n = 9). * P value <0.05 versus all other groups; + P value <0.05 versus Ag-treated Stat6-deficient group. APTI, airway-pressure-time index, cm H2O-sec.
Stat6 Partially Mediates the Development of Pulmonary Eosinophilia.
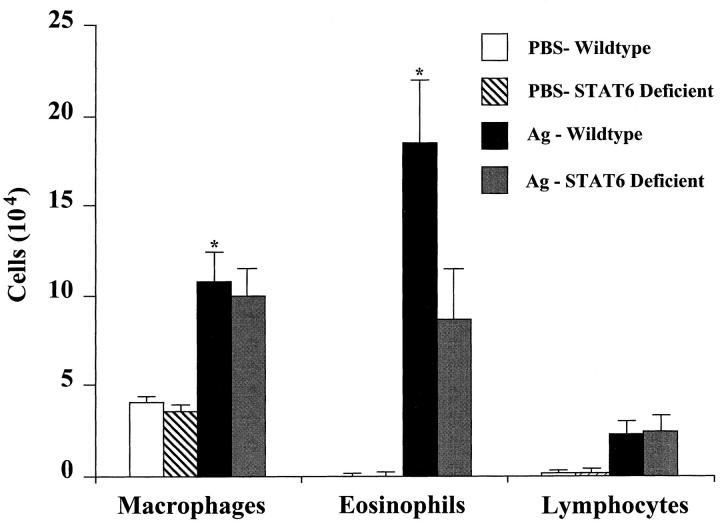
To examine the role of Stat6 signaling in antigen-induced pulmonary inflammation, we examined the cell profile of bronchoalveolar lavage fluids obtained from Stat6-deficient and wild-type BALB/c mice. OVA sensitization and challenge induced equivalent increases in BAL macrophages and lymphocytes in OVA-treated wild-type and Stat6-deficient animals (Fig. 2). Striking increases were observed in the number of eosinophils recovered in the lavage fluid of OVA-treated wild-type mice as compared to their PBS controls (P <0.05). Although increases in BAL eosinophils were also observed in OVA-treated Stat6-deficient mice, they were ∼50% of that found in OVA-treated wild-type animals. These results suggest that antigen-induced pulmonary eosinophilia is only partially dependent on Stat6 signaling pathways.
Figure 2.
Effects of Stat6 gene disruption on the numbers of BAL inflammatory cells recovered from mice 3 d after a single antigen or PBS aspiration challenge. Mice were treated as described in Fig. 1. Values shown are mean and SE (n = 9). * P value <0.05 versus PBS-treated groups.
Allergen-induced Increases in BAL IL-4 and IL-5 Cytokine Levels Are Mediated by Stat6.
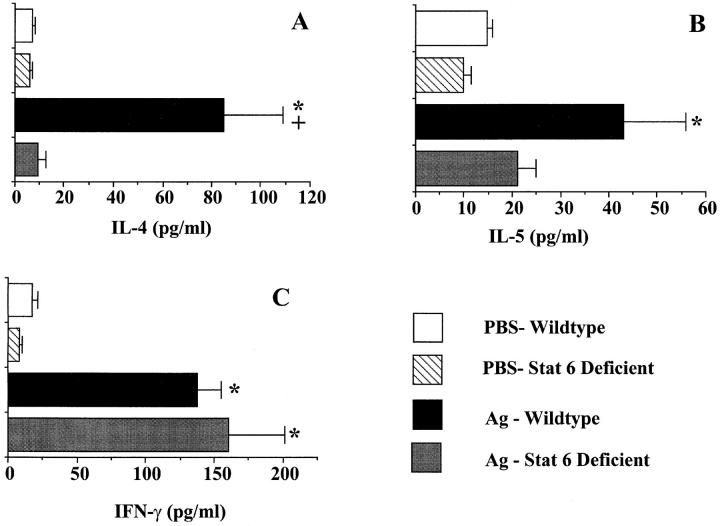
Since previous studies have shown that Stat6 signaling is important in T helper cell development (22–24), we examined the role of Stat6 in cytokine production in the lung after antigen provocation. IL-4, IL-5, and IFN-γ proteins were detected at low levels in the BAL fluids of both PBS-exposed wild-type and Stat6-deficient animals. Antigen challenge of OVA-treated wild-type animals resulted in significant increases in BAL protein levels of the Th2 cytokines, IL-4 and IL-5, compared to their PBS-treated controls. In contrast, antigen challenge of Stat6-deficient mice did not result in elevations in either IL-4 or IL-5 levels as compared to their PBS-treated controls (Fig. 3, A and B). Antigen exposure did result in significant increases in IFN-γ levels in both wild-type and Stat6-deficient mice; however, no differences were noted between these groups (Fig. 3 C). These results indicate that Stat6 signaling is essential for the development of a type 2 cytokine pattern in the lung after pulmonary antigen exposure.
Figure 3.
Effect of Stat6 deficiency on IL-4 (A), IL-5 (B), and IFN-γ (C) protein levels in BAL fluids of mice 3 d after a single antigen or PBS aspiration challenge. Mice were treated as described in Fig. 1. Protein levels were analyzed by ELISA. OD readings were converted to picograms per milliliter by comparison with standard curves. Values shown are mean and SE (n = 9). * P value <0.05 versus PBS-treated groups; + P value <0.05 versus Ag-treated Stat6-deficient group.
Stat6 Signaling Is Essential for IgE Production.
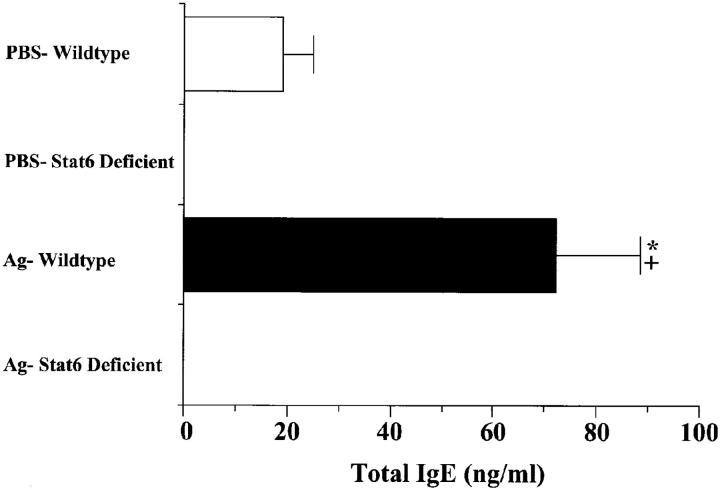
Since IgE is thought to be important in the pathogenesis of allergic responses (25), we measured the total IgE levels in OVA-treated wild-type and Stat6-deficient animals. OVA-treated wild-type animals produced significantly more IgE than that produced by their PBS-treated littermates. Strikingly, neither PBS- nor OVA-treated Stat6-deficient animals produced detectable levels of serum IgE (Fig. 4). This finding indicates that both basal and antigen-induced IgE production is completely dependent on Stat6 signaling.
Figure 4.
Effect of Stat6 gene disruption on serum total IgE levels of mice 3 d after a single antigen or PBS aspiration challenge. Mice were treated as described in Fig. 1. Serum was diluted 1:50 for total IgE and analyzed by ELISA. OD readings were converted to nanograms per milliliter by comparison with a standard curve. Values are reported as mean and SE (n = 9). * P value <0.05 versus PBS-treated groups; + P value <0.05 versus Ag-treated Stat6-deficient animals.
Differential Expression of Specific IgG1 and IgG2a Ab in OVA-treated Wild-type and Stat6-deficient Animals.
We examined serum levels of OVA-specific IgG1 and IgG2a Abs after OVA challenge. This method has been used previously to assess the relative influence of Th1 versus Th2 cytokines in vivo, since the production of the mouse γl subclass is augmented by IL-4 and inhibited by IFN-γ, whereas that of γ2 is augmented by IFN-γ and inhibited by IL-4 (26). In wild-type mice exposed to OVA, significant levels of both IgG1 and IgG2a are observed; however, IgG1 levels were predominant (Fig. 5, A and B). In contrast, the levels of OVA-specific IgG1 were significantly lower in Stat6-deficient animals than in wild type, whereas their OVA-specific IgG2a levels were significantly higher. As expected, the levels of OVA-specific Ab isotypes in PBS-sensitized and challenged mice of both wild type and Stat6 deficiency were below the level of detection. These results are consistent with a lack of Th2 cytokine production in antigen-treated Stat6-deficient animals.
Figure 5.
Effect of Stat6 deficiency on serum OVA-specific IgG1 (A) and OVA-specific IgG2a (B) antibody levels of mice 3 d after a single antigen or PBS aspiration challenge. Mice were treated as described in Fig. 1. Serum was diluted 1:100, 1: 250, and 1:500 with FBS for analysis of OVA-specific IgG1 and IgG2a antibody levels by ELISA. Since recombinant OVA-specific antibodies were not available to generate a standard curve, relative antibody levels are reported using OD readings obtained after background absorbance was subtracted. Reported OD values are from samples diluted 1:250, all of which were below the saturation point of the assay as demonstrated by comparison to OD values obtained from samples diluted 1:100. Values are reported as mean and SE (n = 9). * P value <0.05 versus PBS-treated groups; + P value <0.05 versus OVA-treated Stat6-deficient animals.
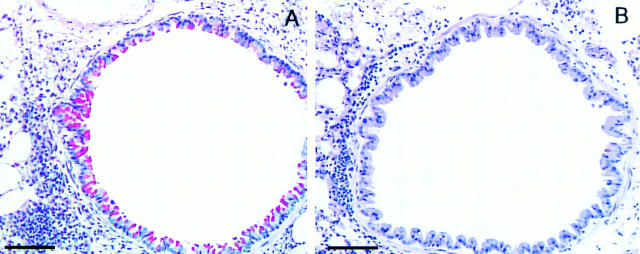
Stat6 Signaling Is Essential for Airway Goblet Cell Differentiation.
As we (9) and others (27, 28) have previously demonstrated that IL-4 is important in regulating mucus production in the airways, we examined the potential role of Stat6 signaling in this process. We find that many cells in the epithelial layer of the airway wall stain positive for mucus after antigen challenge of wild-type mice. In marked contrast, Stat6 deficiency completely prevented the increase in mucus containing cells in the airway epithelium of antigen-exposed mice (Fig. 6, A and B). This finding is significant in that airway mucus hypersecretion is a common feature of allergic asthma and has been found to be particularly profound in autopsy examination of lungs from patients who have died in status asthmaticus.
Figure 6.
Effects of Stat6 gene disruption on antigen-induced increases in the number of mucus-containing cells in the airway epithelium. Lung sections (n = 4/experimental group, four sections per animal) from OVA-treated wild-type (A) and Stat6-deficient (B) mice were stained with periodic acid Schiff. (A) Lung section of antigen-treated wild-type mouse demonstrating interstitial inflammatory cells and a large degree of goblet cell hyperplasia. (B) Lung section of antigen-treated Stat6-deficient mouse demonstrating interstitial inflammatory cells and an absence of mucus-containing cells. Bars, 100 μm.
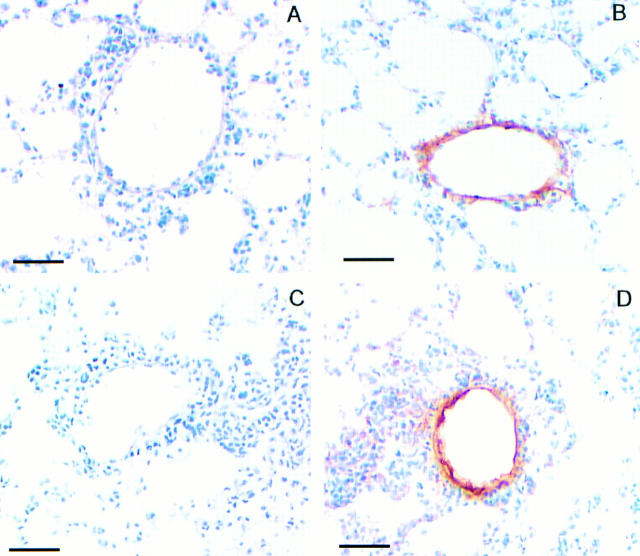
Stat6 Signaling Does Not Mediate Lung Endothelial Cell VCAM-1 Expression.
As Stat6 appears to only partially mediate antigen-induced pulmonary eosinophilia, we sought to determine whether the Stat6-dependent eosinophilia was due to induction of VCAM-1 expression. We examined VCAM-1 immunoreactivity in lung sections from OVA-treated Stat6-deficient and wild-type animals. Our results suggest that lung endothelial cells stain positive for VCAM-1 in sections taken from both OVA-treated wild-type and Stat6-deficient animals (Fig. 7, B and D). Lung sections treated with the secondary antibody only, showed no staining, indicating that the protocol used resulted only in specific staining for VCAM-1. These results indicate that Stat6 does not mediate VCAM-1 expression on lung endothelial cells after allergen challenge.
Figure 7.
Immunohistochemical detection of VCAM-1 expression in lung sections from antigen-treated wild-type and Stat6-deficient mice treated as described in Fig. 1 legend. Lung sections (n = 2, four sections per animal) from either antigen-treated wild-type (A and B) or Stat6-deficient animals (C and D) were incubated with either isotype-matched control IgG mAb (A and C) or anti–VCAM-1 mAb (B and D). Notice comparable VCAM-1–positive staining in sections from antigen-treated wild-type and Stat6-deficient mice (B and D). Notice lack of positive staining in sections incubated with the isotype-matched control antibody (A and C). Bars, 50 μm.
Discussion
In this study, we demonstrate that Stat6 signaling is essential for the development of allergen-induced AHR since Stat6-deficient animals do not develop hyperresponsiveness in response to an antigen exposure regime that induces AHR in their wild-type littermates. In addition, antigen-induced mucus cell hypertrophy was completely dependent on Stat6 signaling. Interestingly, allergen-induced pulmonary eosinophilia was only partially dependent on Stat6 signaling. The Stat6-dependent eosinophilia did not appear to be mediated via VCAM-1 upregulation since positive staining of VCAM-1 was observed in lungs from both OVA-treated wild-type and Stat6-deficient animals. These results provide the first in vivo evidence that Stat6 signaling is required for the development of Th2-mediated allergic airway responses to inhaled antigens.
As Th2 cytokines have been hypothesized to mediate allergen-induced allergic airway responses (1–3, 8) and Stat6 proteins have been shown to be essential in Th2 differentiation in vitro (22–24), we examined the role of this transcription factor in cytokine production in the lung after antigen provocation. Our results demonstrate that Stat6 signaling is essential for antigen-induced increases in Th2 cytokine production in vivo. Antigen challenge of wild-type animals resulted in significant elevations in the type 2 cytokines (i.e., IL-4 and IL-5) in the bronchoalveolar lavage fluids of wild-type animals. In marked contrast, Stat6-deficient animals were unable to produce IL-4 or IL-5 in response to local antigen provocation. The lack of Th2 cytokine production after antigen challenge in Stat6-deficient animals was also reflected in the balance of Th1- and Th2-dependent antibody isotype production in the serum of wild-type and Stat6-deficient animals. OVA-treated wild-type animals developed a predominant IgG1 and IgE antibody profile with lower IgG2a levels. In contrast, Stat6-deficient animals have predominant IgG2a antibody production and virtually no detectable IgE. These results are consistent with previous reports of the inability of lymphocytes from Stat6-deficient mice to differentiate into Th2s when stimulated in vitro under conditions that favor the generation of Th2s. Despite the lack of antigen-induced increases in IL-4 production in Stat6-deficient animals, low levels of IL-4 and IL-5 were observed in PBS-treated Stat6-deficient animals, perhaps suggesting that these cytokines may be produced by non–T cells independent of Stat6 pathways. Interestingly, Th1 cytokine production was not impaired in Stat6-deficient animals as antigen-induced increases in IFN-γ were equivalent in wild-type and Stat6-deficient animals. Thus, this study provides in vivo evidence for the essential role of Stat6 in mediating the differentiation signals induced by either IL-4 or IL-13 after allergen provocation.
This study reveals that Stat6 is essential for the development of allergen-induced AHR as Stat6-deficient animals did not become hyperresponsive after antigen sensitization and challenge, as did their wild-type littermates. The lack of antigen-induced AHR in Stat6-deficient animals was associated with the inability of these animals to produce Th2 cytokines in response to antigen challenge. The association of antigen-induced AHR with increased Th2 type cytokine expression is consistent with our previous demonstration that these functional responses are dependent on CD4+ T cells (7) and associated with the production of type 2 cytokines (8).
One of the characteristics of allergic asthma is the presence of elevated serum IgE levels. IL-4 has clearly been shown to be a key regulator of the synthesis of IgE (6). In addition, IL-4 in conjunction with IL-3 acts as a mast cell growth factor (14). Together, these actions of IL-4 may promote IgE-Fc receptor–mediated mast cell degranulation. Upon degranulation, mast cells release a variety of bronchoconstrictor mediators, including histamine and leukotrienes, that may contribute to the development of AHR. In addition, activated mast cells can secrete IL-4, IL-5, and platelet activating factor (PAF) that may further amplify inflammatory processes (29, 30). The results of our study support a role for IgE-dependent processes in AHR in that after allergen sensitization and challenge Stat6-deficient animals do not have increased IgE levels or AHR. However, several recent studies in mice deficient in either B cells (31) or IgE (32) have shown that allergen-induced AHR develops in the absence of IgE-mediated mast cell activation. Although our study clearly demonstrates that Stat6 signaling is essential for IgE production after allergen exposure, further studies are necessary to determine the exact role of IgE-dependent processes in the pathogenesis of AHR.
Eosinophilic inflammation is clearly a hallmark of allergic asthma and considerable evidence suggests an association between pulmonary eosinophil infiltration and AHR in human asthma (33). Eosinophils are postulated to induce AHR through release of several cationic proteins, eosinophilic cationic protein (ECP), and major basic protein (MBP), which are thought to damage the airway epithelium leaving smooth muscle more susceptible to contractile mechanisms (34). Although IL-5 has clearly been shown to be important in promoting the activation (35) and survival (36) of eosinophils in tissues, IL-4 may also contribute to eosinophilia by promoting IL-5 production and the upregulation of endothelial VCAM-1 expression that controls the attachment and migration of eosinophils. In previous studies, it has been difficult to dissociate direct effects of IL-4 on eosinophil recruitment from its role in expansion of Th2s and subsequent increases in IL-5 production. This study suggests that multiple mechanisms are likely to contribute to antigen-induced tissue eosinophilia since Stat6 gene disruption only partially suppresses antigen-induced increases in BAL eosinophils. Thus, we conclude that there are both Stat6-dependent and -independent mechanisms of antigen-induced eosinophilia. The Stat6-dependent mechanism of eosinophilia is likely due to Stat6's role in induction of Th2 differentiation as the increases in BAL eosinophils in wild-type animals were correlated with increases in IL-5 levels. The reduction in eosinophils in the BAL of Stat6-deficient mice, as compared to their littermates, was likely due to the lack of Th2 differentiation and IL-5 production in these animals. The Stat6-independent mechanism(s) are not likely to be IL-5 mediated as antigen challenge of Stat6-deficient animals resulted in significant increases in eosinophils in the BAL without concomitant increases in IL-5 protein levels. These results suggest that the Stat6-independent eosinophilia is due to either IL-4–controlled processes mediated by intracellular signaling pathways besides Stat6 or to IL-4–independent processes such as the production of eotaxin and monocyte chemoattractant protein 5, which have recently been shown to play a role in lung eosinophilia.
Based on previous reports that IL-4 contributes to tissue eosinophilia via upregulation of VCAM-1 (13) and that IL-4 induction of VCAM-1 expression on endothelial cells may be Stat6 mediated (37), we sought to determine whether the Stat6-dependent eosinophilia observed in our study was mediated via induction of VCAM-1 expression on vascular endothelial cells. We examined VCAM-1 expression in lung sections from OVA-treated Stat6-deficient and wild-type animals. Surprisingly, we found that VCAM-1 staining is evident in both wild-type and Stat6-deficient animals, suggesting that Stat6 is not essential for VCAM-1 expression. However, as we did not quantitate the level of VCAM-1 staining, we cannot rule out some degree of Stat6 involvement. These findings suggest that antigen-induced VCAM-1 expression is not mediated via IL-4–induced activation of Stat6, but may be due to either other IL-4–dependent signaling pathways besides Stat6 or entirely IL-4 independent. Consistent with the hypothesis that VCAM-1 expression may not be entirely IL-4 dependent, Najakima et al. (38) has previously demonstrated that anti–IL-4 mAb administration to antigen-challenged mice only partially blocked VCAM-1 expression.
Stat6 deficiency resulted in the complete prevention of antigen-induced AHR, even in the presence of eosinophilic inflammation, albeit attenuated, indicating that the development of antigen-induced AHR in this model is not mediated directly by eosinophils. As we did not evaluate the activation state of eosinophils in this study, one might argue that the eosinophils in Stat6-deficient animals may not have been activated. However, several other studies in murine models support our conclusion that airway eosinophilia and AHR are separable events (16, 39). For instance, Corry et al. (16) demonstrated that anti–IL-5 antibody administration to OVA-sensitized mice at levels that suppressed eosinophils had no effect on subsequent antigen-induced airway reactivity. Furthermore, Van Oosterhout et al. (39) demonstrated that administration of IL-5–producing cells into the peritoneal cavity of guinea pigs produced AHR several days before any evidence of eosinophil airway recruitment. Lastly, naive IL-4 transgenic mice that have a profound pulmonary eosinophilia are not hyperresponsive to cholinergic stimulation (27). On the other hand, studies of IL-5–deficient mice support a role for IL-5 and eosinophils in the development of allergen-induced AHR (40). A potential explanation for these discrepancies may be provided by Kraneveld et al. (41) who demonstrate that IL-5 is indeed important in AHR, but perhaps not via recruitment of eosinophilia alone but through release of neuropeptides capable of inducing bronchoconstriction and AHR to contractile agents. Our results would support this conclusion in that when levels of IL-5 were low in Stat6-deficient mice, no AHR occurred despite the presence of eosinophils. Further studies are clearly necessary to define the exact role of eosinophils in allergen-induced AHR.
Our results indicate that Stat6 signaling is essential in antigen-induced mucus production as mucus containing cells were not evident in the lungs of antigen-challenged Stat6-deficient mice. This finding is consistent with our previous demonstration that blockade of the IL-4 receptor prevented antigen-induced increases in mucus-containing cells (9) and with the findings of Rankin et al. (27) who demonstrated that naive IL-4 transgenic mice had profound increases in mucus-containing cells in the airway epithelium. As the mucus cell hyperplasia in both of these studies was associated with the presence of eosinophils in the airways, the precise effects of IL-4 could not be separated from those of other inflammatory cells found in the lung. However, in this study, no mucus-containing cells were present in the lungs of allergen-exposed Stat6-deficient animals, despite the presence of eosinophils, suggesting that the differentiation of airway epithelial cells to mucus-containing cells is likely a result of direct IL-4 stimulation. This hypothesis is consistent with a recent study demonstrating that IL-4 induces expression of a specific mucin gene, MUC5AC, in the airways of IL-4 transgenic mice (28). Taken together, these studies strongly support a pivotal role for IL-4 or IL-13 and Stat6 activation in mucus hypersecretion and plugging in the airways, which is characteristic of patients who die from asthma (42), cystic fibrosis (43), and chronic bronchitis (43).
Although many of the processes examined in this study are clearly IL-4 dependent, Stat6 has also been shown to be activated in response to IL-13 and platelet-derived growth factor (PDGF) receptor stimulation (44). IL-13 is another pleiotropic regulatory cytokine that shares a number of biological properties with IL-4, such as the differentiation of Th2s (22), the induction of VCAM-1 in cultured human endothelial cells (37), and stimulation of B cell class switching to IgE production. Although IL-13 levels have been shown to be elevated in the BAL cells of asthmatics (45), no direct determination of its role in the pathogenesis of allergic asthma has been conducted. PDGF is thought to be important in tissue repair processes and is a potent activating factor for mesenchymal cells including fibroblasts and smooth muscle cells. A thickened basement membrane due to increased collagen deposition and hypertrophy of airway smooth muscle cells is a common feature of asthmatic airways upon biopsy. However, two separate studies have shown no correlation between either PDGF levels in BAL or PDGF receptor expression in lung biopsies and asthma (46, 47).
In summary, our results demonstrate that Stat6 is essential in the development of allergen-induced AHR. Stat6 likely mediates AHR via its pivotal role in Th2 differentiation resulting in subsequent stimulation of IgE synthesis and mucus production in the airways. Conversely, our studies indicate that Stat6 only partially mediates antigen-induced eosinophilia, and that eosinophils are not required for the development of allergen-induced AHR in this model. Furthermore, although VCAM-1 expression on the pulmonary vasculature may contribute to eosinophil recruitment, it is not mediated via IL-4– or IL-13–activated Stat6 pathways as previously hypothesized. We conclude that interrupting Stat6 signaling provides a very effective means of preventing many of the symptoms that are common to allergic asthma. Drugs designed to block Stat6 activation may provide a specific and effective means of combating the multiple symptoms of allergic asthma without the toxic side effects of steroid treatments that are now commonly in use.
Acknowledgments
This work was supported by grants from the Center for Indoor Air Research, National Institutes of Health grants RO1-HL58527 to M.Wills-Karp, and a gift from the Mathers Foundation and RO1AI40171 to M.J. Grusby. M.J. Grusby is a scholar of the Leukemia Society of America. Douglas Kuperman was supported by multidisciplinary training grant HL07534 from the National Heart, Lung and Blood Institute.
Footnotes
Abbreviations used in this paper: Ach, acetylcholine chloride; AHR, airway hyperresponsiveness; BAL, bronchoalveolar lavage; IRS, insulin receptor substrate; PDGF, platelet-derived growth factor; Stat6, signal transducer and activator of transcription factor 6; VCAM-1, vascular cell adhesion molecule 1.
References
- 1.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley A, Corrigan C, Durham S, Kay B. Predominant Th2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 2.Walker C, Bode E, Boer L, Hansel T, Blaser K, Johann-Christian J, Virchow Allergic and nonallergic asthmatics have distinct patterns of T-cell activation and cytokine production in peripheral blood and bronchoalveolar lavage. Am Rev Respir Dis. 1992;146:109–115. doi: 10.1164/ajrccm/146.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Del Prete GF, De Carli M, D'Elios MM, Maestrelli P, Ricci M, Fabbri L, Romagnini S. Allergen exposure induces the activation of allergen-specific Th2 cells in the airway mucosa of patients with allergic respiratory disorders. Eur J Immunol. 1993;23:1445–1449. doi: 10.1002/eji.1830230707. [DOI] [PubMed] [Google Scholar]
- 4.Lopez AF, Sanderson CJ, Gamble JR, Campbell HR, Young IG, Vadas MA. Recombinant human interleukin-5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang JM, Rambaldi A, Biondi A, Chen ZG, Sanderson CJ, Montovani A. Recombinant human interleukin-5 is a selective eosinophil chemoattractant. Eur J Immunol. 1989;19:701–705. doi: 10.1002/eji.1830190420. [DOI] [PubMed] [Google Scholar]
- 6.Finkelman FD, Katona IM, Urban JF, Jr, Holmes J, Ohara J, Tung AS, Sample JG, Paul WE. Interleukin-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 7.Gavett SH, Chen X, Finkelman FD, Wills-Karp M. Depletion of murine CD4+T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am J Respir Cell Mol Biol. 1994;10:587–593. doi: 10.1165/ajrcmb.10.6.8003337. [DOI] [PubMed] [Google Scholar]
- 8.Gavett SH, O'Hearn DJ, Li X, Huang S, Finkelman FD, Wills-Karp M. Interleukin-12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med. 1995;182:1527–1536. doi: 10.1084/jem.182.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brusselle G, Kips J, Joos G, Bluethmann H, Pauwels R. Allergen-induced airway inflammation and bronchial responsiveness in wild-type and interleukin-4–deficient mice. Am J Respir Cell Mol Biol. 1995;12:254–259. doi: 10.1165/ajrcmb.12.3.7873190. [DOI] [PubMed] [Google Scholar]
- 10.Gavett SH, O'Hearn DJ, Karp CL, Patel EA, Schofield BH, Finkelman FD, Wills-Karp M. Interleukin-4 receptor blockade prevents airway responses induced by antigen challenge in mice. Am J Physiol. 1997;272:L253–L261. doi: 10.1152/ajplung.1997.272.2.L253. [DOI] [PubMed] [Google Scholar]
- 11.Lukacs NW, Strieter RM, Chensue SW, Kunkel SL. Interleukin-4 dependent pulmonary eosinophil infiltration in a murine model of asthma. Am J Respir Cell Mol Biol. 1994;10:526–532. doi: 10.1165/ajrcmb.10.5.8179915. [DOI] [PubMed] [Google Scholar]
- 12.Swain SL, Weinberg AD, Inglish M, Hutson G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 13.Schleimer RP, Sterbinsky S, Kaiswer S. IL-4 induced adherence of human eosinophils and basophils but not neutrophils to endothelium. Association with expression of VCAM-1. J Immunol. 1992;148:1086–1092. [PubMed] [Google Scholar]
- 14.Madden KB, Urban JF, Ziltener HJ, Schrader JW, Finkelman FD, Katona IM. Antibodies to IL-3 and IL-4 suppress helminth-induced intestinal mastocytosis. J Immunol. 1991;147:1387–1391. [PubMed] [Google Scholar]
- 15.Coyle AJ, Le Gros G, Bertrand C, Tsuyuki S, Heusser CH, Kopf M, Anderson GP. Interleukin-4 is required for the induction of lung Th2 mucosal immunity. Am J Respir Cell Mol Biol. 1995;13:54–59. doi: 10.1165/ajrcmb.13.1.7598937. [DOI] [PubMed] [Google Scholar]
- 16.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MD, Wiener-Kronish JP, Locksley RM. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–117. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin J-X, Migone T-S, Friedman M, Weatherbee JA, Zhou L, Yamauchi A, Bloom ET, Mietz J, John S, Leonard WJ. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotrophy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–339. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 18.Johnston JA, Kawamura M, Kirken RA, Chen Y-Q, Blake TB, Shibuya K, Ortaldo JR, McVicar DW, O'Shea JJ. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 19.Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu E, Ihle JN. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 20.Keegan AD, Nelms K, Wang L, Pierce JH, Paul WE. Interleukin-4 receptor: signaling mechanisms. Immunol Today. 1994;15:423–431. doi: 10.1016/0167-5699(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 21.Sun XL, Wang L-M, Zhang Y, Yenush L, Myers MG, Jr, Glasheen E, Lane WS, Pierce JH, White MF. Role of IRS-2 in insulin and cytokine signalling. Nature. 1995;377:173–177. doi: 10.1038/377173a0. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for the development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 23.Shimoda K, Van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4–induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 25.Sears MR, Burrows B, Flannery EM, Herbison G, Hewitt CJ, Hodaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 26.Golding B, Zaitseva M, Golding H. The potential for recruiting immune responses toward type-1 or type-2 help. Am J Trop Med Hyg. 1994;50:33–40. doi: 10.4269/ajtmh.1994.50.33. [DOI] [PubMed] [Google Scholar]
- 27.Rankin JA, Picarella DE, Geba GP, Temann U, Prasad B, DiCosmo B, Tarallo A, Stripp B, Whitsett J, Flavell RA. Phenotypic and physiologic characterization of transgenic mice expressing interleukin 4 in the lung: lymphocytic and eosinophilic inflammation without airway hyperreactivity. Proc Natl Acad Sci USA. 1996;93:7821–7825. doi: 10.1073/pnas.93.15.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Temann U-A, Prasad B, Gallup MW, Basbaum C, Ho SB, Flavell RA, Rankin JA. A novel role for murine IL-4 in vivo: induction of MUC5AC gene expression and mucin hypersecretion. Am J Respir Cell Mol Biol. 1997;16:471–478. doi: 10.1165/ajrcmb.16.4.9115759. [DOI] [PubMed] [Google Scholar]
- 29.Bradding P, Okayama Y, Howartth PH, Church MK, Holgate ST. Heterogeneity of human mast cells based on cytokine content. J Immunol. 1995;155:297–307. [PubMed] [Google Scholar]
- 30.Brown MA, Pierce JH, Watson CJ, Falco J, Ihle JN, Paul WE. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Clin Sci. 1987;68:427–432. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- 31.Korsgren M, Erjefalt JS, Korsgren O, Sundler F, Persson CGA. Allergic eosinophil-rich inflammation develops in lungs and airways of B cell–deficient mice. J Exp Med. 1997;185:885–892. doi: 10.1084/jem.185.5.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehlhop, P.D., M. Van de Rijn, A.B. Goldberg, J.P. Brewer, V.P. Kurup, T.R. Martin, and H.C. Oettgen. 1997. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc. Natl. Acad. Sci. USA. 1344–1349. [DOI] [PMC free article] [PubMed]
- 33.Wardlaw AJ, Dunnette S, Gleich GJ, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Relationship to bronchial hyperreactivity. Am Rev Respir Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- 34.Frigas E, Loegering DA, Gleich GJ. Cytotoxic effects of the guinea pig derived major basic protein on tracheal epithelium. Lab Invest. 1980;42:35–43. [PubMed] [Google Scholar]
- 35.Lopez AF, Sanderson CJ, Gamble JR, Campbell HR, Young IG, Vadas MA. Recombinant human interleukin-5 is a selective activator of human eosinophil function. J Exp Med. 1988;167:219–224. doi: 10.1084/jem.167.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi Y, Hayashi Y, Sugama Y, Miura Y, Kasatsu T, Suda T. Highly purified murine interleukin-5 (IL-5) stimulates eosinophil function and prolongs in vitro survival: IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988;167:1737–1742. doi: 10.1084/jem.167.5.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer-Crocker RL, Hughes CCW, Pober JS. IL-4 and IL-13 activate the JAK2 tyrosine and Stat6 in cultured human vascular endothelial cells through a common pathway that does not involve the γc chain. J Clin Invest. 1996;98:604–609. doi: 10.1172/JCI118829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakajima H, Sano H, Nishimura T, Yoshida S, Iwamoto I. Role of vascular cell adhesion molecule 1/ very late activation antigen 4 and intercellular adhesion molecule 1/lymphocyte function–associated antigen 1 interactions in antigen-induced eosinophil and T cell recruitment into the tissue. J Exp Med. 1994;179:1145–1154. doi: 10.1084/jem.179.4.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Oosterhout AJ, Ladenius AR, Savelkoul HF, Van Ark I, Delsman KC, Nijkamp FP. Effect of anti-IL-5 and IL-5 on airway hyperreactivity and eosinophils in guinea pigs. Am Rev Respir Dis. 1993;147:548–552. doi: 10.1164/ajrccm/147.3.548. [DOI] [PubMed] [Google Scholar]
- 40.Foster PS, Hogan SP, Ramsay AJ, Matthaei KI, Young IG. Interleukin 5 deficiency abolishes eosinophilia, airways hyperreactivity, and lung damage in a mouse asthma model. J Exp Med. 1996;183:195–201. doi: 10.1084/jem.183.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kraneveld AD, Nijkamp FP, Van Oosterhout AJM. Role for neurokinin-2 receptor in interleukin-5–induced airway hyperresponsiveness but not eosinophilia in guinea pigs. Am J Respir Crit Care Med. 1997;156:367–374. doi: 10.1164/ajrccm.156.2.9608101. [DOI] [PubMed] [Google Scholar]
- 42.Aikawa T, Shimura S, Hidetada S, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 43.Larivee, P., S.J. Levine, R.D. Rieves, and J.H. Shelhamer. 1994. Airway inflammation and mucus hypersecretion. In Airway Secretion: Physiological Bases for the Control of Mucus Hypersecretion. S. Shimura and T. Takishima, editors. Marcel Dekker, New York. 469–511.
- 44.Huang SK, Xiao HQ, Kleince-Tebbe J, Paciotti G, Marsh DG, Lichtenstein LM, Liu MC. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688–2694. [PubMed] [Google Scholar]
- 45.Patel BKR, Wang L, Lee C, Taylore WG, Pierce JH, LaRochelle WJ. Stat6 and Stat1 are common elements in platelet-derived growth factor and interleukin-4 signal transduction pathways in NIH 3T3 fibroblasts. J Biol Chem. 1996;271:22175–22182. doi: 10.1074/jbc.271.36.22175. [DOI] [PubMed] [Google Scholar]
- 46.Chanez P, Vignola M, Stenger R, Vic P, Michel FB, Bousquet J. Platelet-derived growth factor in asthma. Allergy. 1995;50:878–883. doi: 10.1111/j.1398-9995.1995.tb02493.x. [DOI] [PubMed] [Google Scholar]
- 47.Aubert JD, Hayashi S, Hards J, Bai TR, Pare PD, Hogg JC. Platelet-derived growth factor and its receptor in lungs from patients with asthma and chronic airflow obstruction. Am J Physiol. 1994;266:L655–L663. doi: 10.1152/ajplung.1994.266.6.L655. [DOI] [PubMed] [Google Scholar]