Abstract
Cluster of differentation (CD)4+ T helper cells (Th)1s fail to produce interleukin (IL)-4. Even if restimulated in the presence of IL-4, a condition that induces IL-4–producing capacity in naive CD4+ T cells, Th1s fail to become IL-4 producers. We report that Th1 cells have a major impairment in IL-4 signaling. When compared to both Th2s and naive T cells, they display a striking diminution in phosphorylation of Stat6. They also show reduced phosphorylation of Janus kinase (JAK)-3 and insulin receptor substrate (IRS)-2 when compared to Th2s. Stat6 and JAK-3 are present in equivalent amounts in Th1s and Th2s, but IRS-2 protein levels are much lower in Th1s than in Th2s. Altered sensitivity to IL-4, the major inducer of the Th2 phenotype, may explain the stability of the Th1 state.
Naive CD4+ T cells develop into either of two major subsets of Ths that produce distinct sets of cytokines. Th1s generally produce IFN-γ, IL-2, and TNF-α, whereas Th2s produce IL-4, IL-5, IL-10, and IL-13 (1). The polarization of responses to Th1 or Th2 dominance often determines resistance or susceptibility of hosts to infections and the degree of tissue damage in many autoimmune diseases (2–4).
IL-4 is the major determinant of the differentiation of antigen-stimulated naive cluster of differentiation (CD)14+ T cells into Th2s (5–8). IL-4 mediates its functions through binding to and stimulation of the IL-4R. The IL-4R consists of the IL-4Rα chain, which binds IL-4 with high affinity (9, 10), and the common gamma chain (γc) (11, 12), shared by the receptors for IL-2, IL-7, IL-9, and IL-15 (11–15). Upon interaction with IL-4, the IL-4Rα and γc-associated kinases, Janus kinase (JAK)-1 and JAK-3, are activated (16–18). Both JAK-1 and JAK-3 play critical roles in IL-4 function. Cell lines expressing JAK-1 mutations fail to phosphorylate insulin receptor substrate (IRS)-1/2) and Stat6, two of the major substrates phosphorylated in normal cells in response to IL-4 (19, 20). T cells from JAK-3–deficient mice show similar defects (21–23).
Mice deficient in IL-4Rα chain (24) or in Stat6 (25–27), the major regulator of IL-4–mediated gene activation (28), fail to generate Th2s in vitro and show a marked diminution in the development of IL-4–producing CD4+ T cells in response to infection with Nippostrongylus brasiliensis (24, 26). Furthermore, Stat6 activation has been mainly implicated in the regulation of IL-4–mediated gene activation. Thus, Stat6-deficient mice fail to upregulate class II MHC molecules, CD23, and Thy1 in mouse B cells, and fail to support class switching to IgE. The major regulators of IL-4–mediated growth are the phosphotyrosine binding domain substrates IRS-1/IRS-2 and Shc, whose phosphorylation is determined by a domain in the IL-4 receptor distinct from that responsible for Stat6 activation (19, 29–34). However, thymocytes and B cells from Stat6-deficient mice also display diminished uptake of [3H] thymidine in response to IL-4–dependent stimuli (25–27). This may indicate a possible role for Stat6 in growth regulation; alternatively, a principal role of Stat6 may be the induction of factors that are critical in transmitting growth-inducing signals. Indeed, the IL-4Rα chain is itself upregulated by IL-4 (9, 35).
Th1s and Th2s differ from one another in several important respects. For example, Th1s not only fail to produce IL-4 when stimulated, but cannot support the transcription of transfected reporter genes under the control of the IL-4 promoter (36–39). It has recently been demonstrated that they fail to express c-maf, a transcription factor that binds to the IL-4 promoter (40). Transfection of Th1 lines with c-maf allows them to transcribe reporter genes under the control of the IL-4 promoter. This transcription is further enhanced by the cotransfection of the cDNA for NIP-45, a protein that interacts with nuclear factor of activated T cells (41). Furthermore, Th1s fail to express GATA-3, which is expressed in naive and in Th2s (42). GATA-3 transgenic mice primed under Th1 conditions (in the absence of IL-4) can develop into cells capable of inducing IL-4 messenger RNA (mRNA), indicating an important role for GATA-3 in the differentiation of naive cells into Th2s.
What is particularly striking is that Th1s not only fail to produce IL-4 upon challenge, but they fail to develop into IL-4–producing cells even if they are restimulated with antigen in the presence of IL-4 (43–45). These are conditions that cause the development of naive T cells into IL-4 producers. Thus, Th1s have lost the ability to induce IL-4– producing capacity. In addition, it has been reported that long-term lines of Th1s are incapable of growth in response to IL-4 despite the fact that Th1 and Th2 lines display essentially equal capacity to bind IL-4 with high affinity (46). It has been reported that these lines differ in their sensitivity to IL-1 and it has been suggested that this explains their differential sensitivity to IL-4 as a growth stimulus (47).
We wished to examine the possibility that the inability of Th1s to respond to IL-4 with the induction of IL-4–producing activity might reflect an insensitivity of these cells to IL-4. Here we show that while Th1s express amounts of IL-4R comparable to Th2s, they lose their capacity to phosphorylate Stat6 as they differentiate and that such loss is correlated with the inability of these cells to upregulate CD30 in response to IL-4 and to develop into IL-4 producers. Furthermore, we demonstrate that the level of expression of IRS-2, a principal regulator of IL-4–mediated growth, is enhanced upon development of naive cells into Th2s but not Th1s; this enhancement does not occur in cells from Stat6-deficient mice. In differentiated Th1s, IL-4 does not enhance expression of IRS-2. These results indicate that a defect in the activation of Stat6 in Th1s can explain many of the phenotypic properties of these cells.
Materials and Methods
Animals and Cell Cultures.
B10.A mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice transgenic for TCR-α and -β chains encoding a receptor specific for pigeon cytochrome C peptide 88–104 in association with the IEk class II MHC molecule were maintained in our animal quarters. Lymph node cells were depleted of CD8+ cells, B220+ cells, and IAk+ cells by negative selection using magnetic beads. The purified CD4+ cells were then centrifuged on a discontinuous 50, 60, and 70% Percoll gradient. Cells with a density of >70% were collected and used for priming (7). Primary stimulation of TCR transgenic cells was carried out by culturing 106 naive CD4+ T cells in the presence of 107 irradiated T cell–depleted spleen cells from B10.A, 1 μM pigeon cytochrome C peptide (prepared by National Institute of Allergy and Infectious Diseases Biologic Resources Branch) and IL-2 (10 U/ml) for 7 d. For differentiation of Th1s, monoclonal anti–IL-4 antibody (11B11; 10 μg/ml) and IL-12 (10 ng/ml) were also added to the culture; for the differentiation of Th2s, IL-4 (0.5 ng/ml) and anti–IL-12 antibody (R&D Systems, Inc., Minneapolis, MN) were added. In some instances, the priming culture was repeated one or two times. T and B cell–depleted APCs were prepared from splenocytes by depleting Thy 1.2 and B220 positive cells using the magnetic bead method. Stat6 knockout mice and littermate controls were provided by Dr. James Ihle (St. Jude Children's Research Hospital, Memphis, TN). These (129 × C57BL/6) mice were backcrossed to normal C57BL/6 mice.
Immunoprecipitation and Western Blot Analysis.
Th1s or Th2s were washed with HBSS twice, recultured with 10 U/ml rIL-2 overnight, and then deprived of serum and cytokines for 2 h. Cells (5 × 106 ml) were stimulated with 5 ng/ml of IL-4 in complete RMPI at room temperature for 12 min. The reaction was stopped with cold PBS containing 100 μM Na3 VO4. Cells were then lysed with 0.5 ml lysis buffer (50 mM Hepes, 0.5% Nonidet P-40, 5 mM EDTA, 50 mM NaCl, 10 mM Na pyrophosphate, and 50 mM NaF) freshly supplemented with 1 mM Na3 VO4, 1 mM PMSF, and 10 μg/ml aprotinin, leupeptin, and pepstatin. Lysates were incubated with 5–10 μl antibody for 2 h on ice. Immune complexes were precipitated with protein G agarose beads (Pierce Chemical Co., Rockford, IL), eluted with SDS-PAGE loading buffer, separated in a 7.5% acrylamide gel, and transferred onto Immobilon-P membranes (Millipore, Bedford, MA), which were probed with specific antibodies (17) and visualized with an enhanced chemiluminescent detection system (Pierce Chemical Co.). Anti-Stat6 antibody was provided by Dr. James Ihle (St. Jude Children's Research Hospital, Memphis, TN), anti–JAK-3 by Dr. John O'Shea (National Institutes of Health, Bethesda, MD), and anti–IRS-2 antibody and anti–phosphotyrosine (4G10) were purchased from Upstate Biotechnology Inc. (Lake Placid, NY)
Reverse Transcriptase PCR.
TCR transgenic Th2s were washed extensively at the end of the priming culture and recultured in IL-2 supplemented complete medium for 14 d until these cells had become small and round shaped. These well-“rested” cells (106) were then either unstimulated or stimulated with 2 × 105 irradiated T and B cell–depleted APCs, IL-2, and cytochrome C peptide with or without IL-4 (0.5 ng/ml) for 6 or 22 h. Total RNA were prepared by the guanidinium method and reverse transcribed into cDNA. PCRs were performed using IRS-2 primers (forward: TGGTGAGGCAGGTACCCGTCT; reverse: TCTGCACGGATGACCTTAGCA; reference 48) and actin primers (forward: GATGACGATATCGCTGCGCTG; reverse: GTACGACCAGAGGCATACAGG). Amplification was performed (geneAmp PCR system 9600; Perkin-Elmer Corp., Foster City, CA). The cycling conditions were 94°C for 15 s, 59°C for 15 s, and 72°C for 30 s for 35 cycles, followed by extension at 72°C for 5 min. PCR products were analyzed by electrophoresis in a 2% agarose gel.
Flow Cytometry Analysis and ELISA.
For IL-4R staining, cells were incubated with 10% goat serum for 5 min to block nonspecific binding. M1 anti–IL-4R monoclonal antibody or a rat isotype control (Genzyme, Cambridge, MA) were added to the cells and incubated for 20 min in FACS® buffer (Becton Dickinson, Mountain View, CA; PBS/3% fetal calf serum/0.1% sodium azide). Cells then were washed with FACS® buffer. FITC-labeled goat Fab′ fragment against rat IgG (Southern Biotechnology Associates, Birmingham, AL) were added to stained cells for 20 min. For CD30 staining, cells were first blocked with 10 μl of 2.4G2 rat anti–mouse FcγRII/III ascitic fluid for 5 min before staining with PE-labeled monoclonal antibody against mouse CD30 (PharMingen, San Diego, CA) for 20 min in FACS® buffer. The stained cells were washed twice with FACS® buffer and analyzed in a FACScan®. IL-4 and IFN-α production were measured using commercial ELISA detection kits (Endogen, Woburn, MA).
Results
Th1 Cells Display a Marked Diminution in IL-4–induced Stat6 and JAK-3 Phosphorylation.
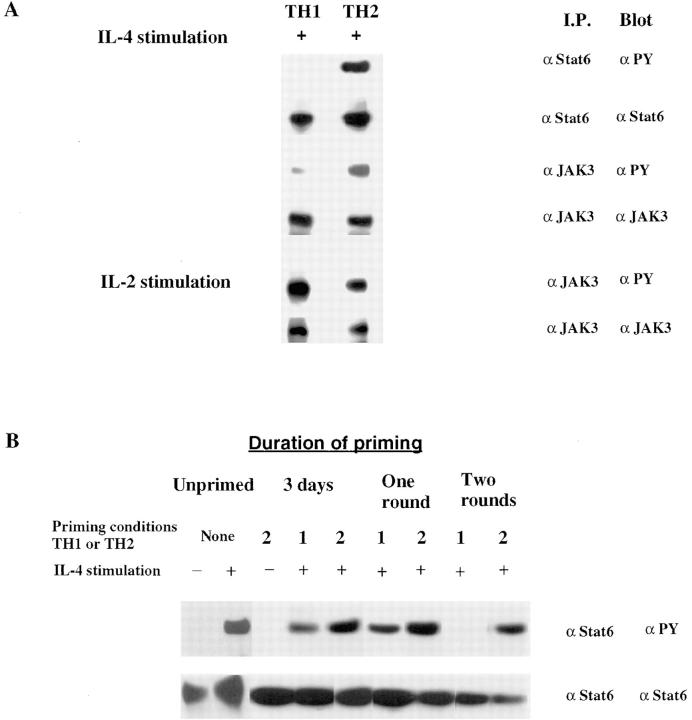
We prepared dense CD4+ T cells from mice transgenic for TCR-α and -β chains specific for a cytochrome C peptide in association with IEk (7). These cells were primed in vitro by culturing for two rounds of 7 d each with T cell–depleted spleen cells from B10.A mice together with 1 μM cytochrome C peptide and IL-2. To generate Th1s, we added monoclonal anti– IL-4 antibody and IL-12; to generate Th2s, we added IL-4 and anti–IL-12 antibody. Extracts prepared from Th2s that had been stimulated with IL-4 for 12 min were immunoprecipitated with anti-Stat6. When analyzed by SDS-PAGE and Western blotting with antiphosphotyrosine, we observed striking tyrosine phosphorylation of Stat6. By contrast, immunoprecipitates of extracts from IL-4–stimulated Th1s showed no detectable Stat6 phosphorylation. Western blots with anti-Stat6 antibody revealed comparable amounts of Stat6 in both Th1s and Th2s (Fig. 1 A).
Figure 1.
Impaired IL-4–induced phosphorylation of Stat6 and JAK-3 in Th1s. (A) Two-round–primed TCR transgenic Th1s and Th2s were recultured in IL-2 for 1 d and then deprived of serum and cytokines for 2 h. Cells (2.5 × 107) were stimulated with IL-4 (5 ng/ml) or with IL-2 (100 U/ml) in separate experiments for 12 min. Cell lysates were incubated with anti-Stat6 or anti–JAK-3 antibody and precipitated with protein G/ agarose beads. Phosphorylated Stat6 or JAK-3 was detected by blotting with antiphosphotyrosine antibody (αPY). The same filter was stripped and reprobed with anti-Stat6 or anti–JAK-3 antibody. (B) Lysates were prepared from TCR transgenic cells that had been stimulated under Th1 or Th2 conditions for 3 d, for one round of 7 d, or for two rounds of 7 d each. B10.A CD4+ lymph node cells (Unprimed) were treated with or without IL-4, lysed, and analyzed for Stat6 phosphorylation and content.
Naive CD4+ T cells respond to IL-4 with Stat6 phosphorylation comparable to that observed for Th2s (Fig. 1 B). Unstimulated cells expressed no detectable phosphorylation. The degree of such IL-4–induced phosphorylation remains stable in the course of priming CD4+ T cells with cytochrome C peptide, APCs, IL-2, IL-4, and anti–IL-12 (i.e. to the Th2 phenotype). When cells are primed with anti–IL-4 and –IL-12 (i.e., to the Th1 phenotype), IL-4– induced Stat6 phosphorylation remains detectable during the initial round of priming (i.e., at 3 d). Stat6 phosphorylation is sometimes completely lost by the end of the first round of priming, but in most experiments (as illustrated in Fig. 1 B), IL-4–induced Stat6 phosphorylation is not lost in Th1s until two rounds of priming.
IL-4–induced phosphorylation of JAK-3 was also diminished in “two-round” primed Th1s (Fig. 1 A). The relative degree of phosphorylation of JAK-3 in Th1s was reduced by ∼3.7-fold compared to Th2s. The amount of JAK-3 protein in Th1s was no different than the amount in comparably primed Th2s. The diminution in IL-4–induced JAK-3 phosphorylation in Th1s did not reflect a generalized inactivation of this kinase or of the γc chain in Th1s since IL-2 caused striking phosphorylation of JAK-3 in these cells (Fig. 1 A). This is particularly important since IL-2 signaling, like IL-4 signaling, uses both JAK-1 and JAK-3.
Th1s and Th2s Have Comparable Numbers of IL-4Rs.
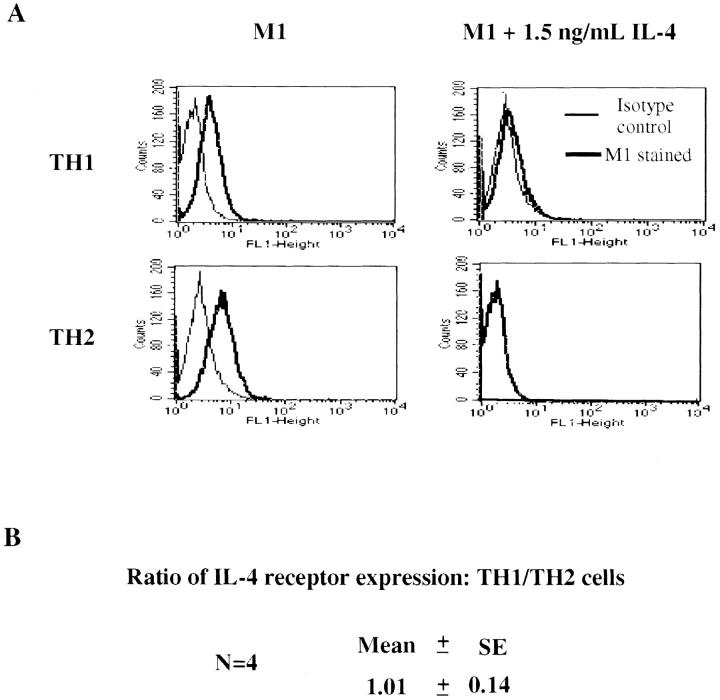
The defect that Th1s show in IL-4–induced signaling could not be explained by the failure of these cells to express IL-4Rs. Two-round–primed Th1s and Th2s from transgenic donors displayed comparable numbers of IL-4Rs as demonstrated by flow cytometric analysis with the anti–mouse IL-4R antibody M1; the specificity and high affinity binding of IL-4 was established by the capacity of IL-4 (1.1 × 10−10M) to inhibit binding by both cell types (Fig. 2 A). Indeed, in four independent experiments, the relative degree of expression of IL-4Rs by Th1s was 1.01 ± 0.14 that of Th2s (Fig. 2 B). In contrast to naive T cells, IL-4 does not further upregulate IL-4R expression in recently primed Th1s or Th2s (data not shown).
Figure 2.
IL-4R expression in Th1s and Th2s. (A) 105 two-round– primed TCR transgenic Th1s and Th2s were incubated with the M1 anti–IL-4R monoclonal antibody or a rat isotype control in the presence or absence of IL-4 (1.5 ng/ml). The cells were washed and stained with FITC-labeled Fab goat anti–rat IgG. Samples were analyzed with a FACSCAN®. (B) To calculate the ratio of IL-4R expression in Th1s and Th2s, the mean fluorescence intensity (MFI) of M1-stained Th1s minus the MFI of isotype control Th1s was divided by the MFI of M1-stained Th2s minus the MFI of isotype control Th2s.
Th1s Have Diminished Expression of IRS-2.
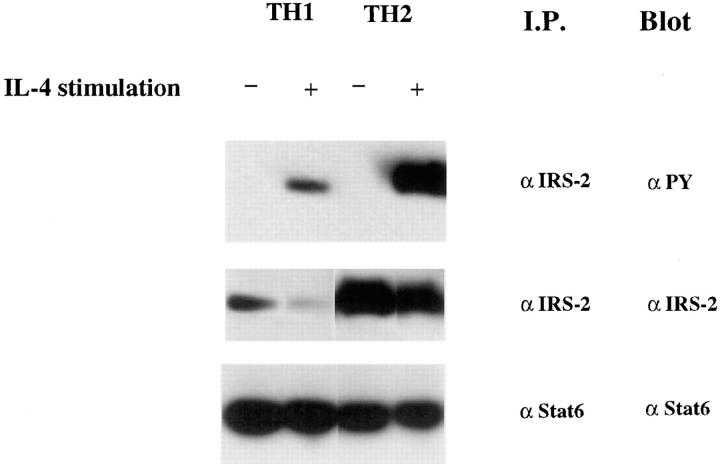
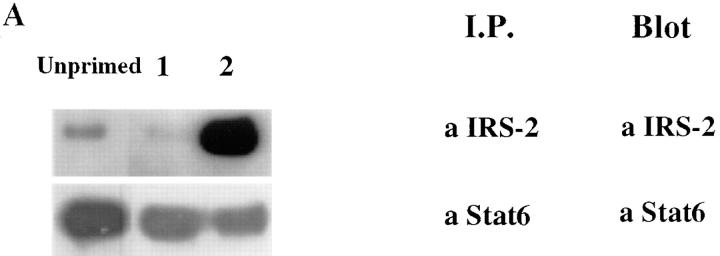
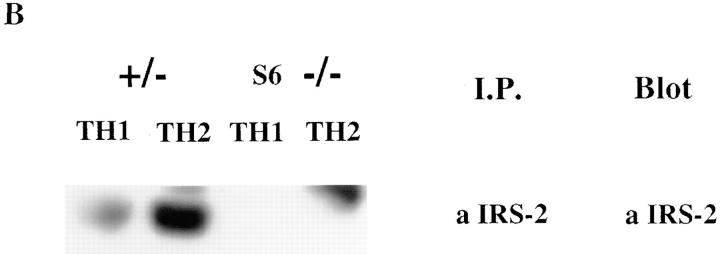
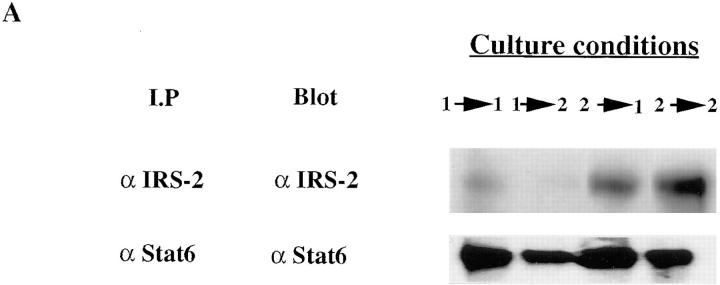
To get a fuller picture of the signaling abnormality of IL-4Rs in Th1s, we examined the phosphorylation of a molecule that has been implicated in IL-4–mediated growth, IRS-2. Fig. 3 demonstrates that IL-4–induced phosphorylation of IRS-2 is strikingly diminished in Th1s compared to Th2s. However, in contrast to Stat6 and JAK-3, which are present in similar amounts in Th1s and Th2s, the level of expression of IRS-2 protein, as detected by immunoblotting of anti– IRS-2 immunoprecipitates, is markedly diminished in Th1s compared to Th2s. Furthermore, although Stat6 is present in relatively similar amounts in unprimed CD4+ T cells, in Th1s and Th2s, IRS-2 is present in very limited amounts in unprimed cells (Fig. 4 A). The sustained induction of IRS-2 expression in Th2s appears to be Stat6 dependent. Thus, when CD4+ T cells from Stat6-deficient mice are primed with soluble anti-CD3, anti-CD28, APCs, IL-2, IL-4, and anti–IL-12 for one round, they fail to express detectable IRS-2, whereas littermate heterozygotes show induction of IRS-2 under Th2- but not Th1-priming conditions (Fig. 4 B).
Figure 3.
Th1s possess less IRS-2 and display less IRS-2 phosphorylation in response to IL-4. Two-round–primed TCR transgenic Th1s or Th2s (2.5 × 107) were stimulated with or without IL-4 for 12 min. Lysates were prepared and immunoprecipitated with anti–IRS-2 or anti-Stat6 antibody and analyzed for phosphorylation and content.
Figure 4.
Induction of IRS-2 protein is Stat6 dependent. (A) Lysates prepared from B10.A CD4+ lymph node T cells or from two-round– primed TCR transgenic Th1s or Th2s were immunoprecipitated with anti–IRS-2 or anti-Stat6 antibody. The amount of IRS-2 and Stat6 protein was determined by Western blot analysis using anti–IRS-2 or anti-Stat6 antibody. (B) Naive CD4+ T cells, prepared from lymph nodes of Stat6-deficient mice or their heterozygous littermates, were primed (one round) with 3 μg/ml soluble anti-CD3 and anti-CD28 under Th1 or Th2 conditions. The amount of IRS-2 protein was determined by immunoprecipitation and Western blot analysis using anti–IRS-2 antibody.
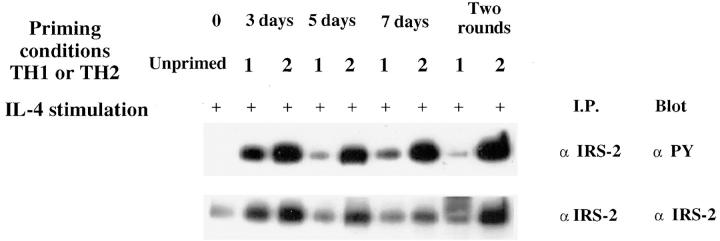
An analysis of the induction of IRS-2 protein using CD4+ T cells from TCR transgenic donors in the course of priming with cytochrome C peptide reveals that cells primed in the Th1 and Th2 “directions” both initially induce IRS-2 (i.e., at 3 d), but IRS-2 is only sustained in the cells primed in the presence of IL-4, so that by 5 d of priming, cells primed under Th1 conditions have less IRS-2 than do cells primed under Th2 conditions (Fig. 5). Thereafter, IRS-2 levels in Th1s are lower than in Th2s, particularly by 2 wk of priming. The levels of phosphorylation of IRS-2 in Th1s are also strikingly diminished compared to Th2s from 5 d of priming and after (Fig. 5).
Figure 5.
Upregulation of IRS-2 expression and IL-4–induced phosphorylation of IRS-2 during the course of Th2 development. Lysates were prepared from purified TCR transgenic lymph node CD4+ (Unprimed) or TCR transgenic lymph node CD4+ cells that were stimulated with T and B cell–depleted APCs, cytochrome C, and IL-2 under either Th1 or Th2 conditions for 3, 5, or 7 d, or for two rounds of 7 d each. IRS-2 phosphorylation and content was analyzed by immunoprecipitation with anti– IRS-2 antibody and Western blot analysis using antiphosphotyrosine or anti–IRS-2 antibody. Lysates were prepared from equal numbers of cells.
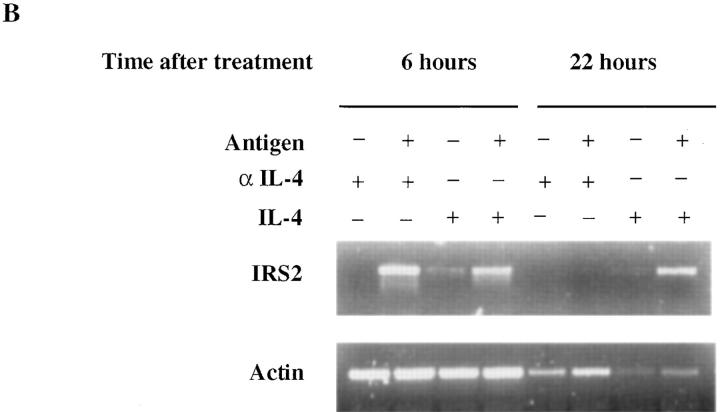
Th1s not only fail to express substantial amounts of IRS-2, they fail to show stable induction of IRS-2 when restimulated for 7 d in the presence of cytochrome C peptide, APC, IL-2, IL-4, and anti–IL-12 (Th2-inducing conditions; Fig. 6 A). Thus, in fully primed Th1s, IL-4 does not appear to be able to induce stable expression of IRS-2, in keeping with the “desensitization” of the IL-4R in these cells. Interestingly, Th2s restimulated for 7 d in Th1-inducing conditions continue to express IRS-2, although possibly at somewhat lower levels than Th2s that have been restimulated in Th2 conditions. Indeed, IRS-2 expression in Th2s is not entirely stable. If Th2s are rested by culture in IL-2 for 14 d, their expression of IRS-2 falls markedly. Upon stimulation with cytochrome C peptide and APC without IL-4, they show a transient induction of IRS-2 mRNA (Fig. 6 B). By contrast, if IL-4 is included in the culture, IRS-2 mRNA expression is more sustained, indicating the importance of IL-4 in regulating IRS-2 expression in Th2s.
Figure 6.
Failure of culture under Th2 conditions to induce IRS-2 expression in well-differentiated Th1s. (A) Two-round–primed TCR transgenic Th1s or Th2s were restimulated with cytochrome C plus irradiated APCs under Th1 (1 > 1; 2 > 1) or Th2 (1 > 2; 2 > 2 ) conditions for 7 d. IRS-2 and Stat6 protein content were determined by immunoprecipitation and Western blot analysis using anti–IRS-2 and anti-Stat6 antibody. (B) Well-rested two-round–primed TCR transgenic Th2s left unstimulated (i.e., treated with anti–IL-4), stimulated with IL-4 alone, or stimulated with irradiated T and B cell–depleted spleen cells, cytochrome C peptide, IL-2, and anti–IL-4 or IL-4 for 6 h and 22 h. Total RNAs were prepared with RNAzol and reverse transcribed into cDNA. PCR was performed with IRS-2 or actin primers. Products were analyzed in 2% agarose gel.
Failure of Th1s to Respond to IL-4 Correlates with the Lack of IL-4–dependent Upregulation of CD30.
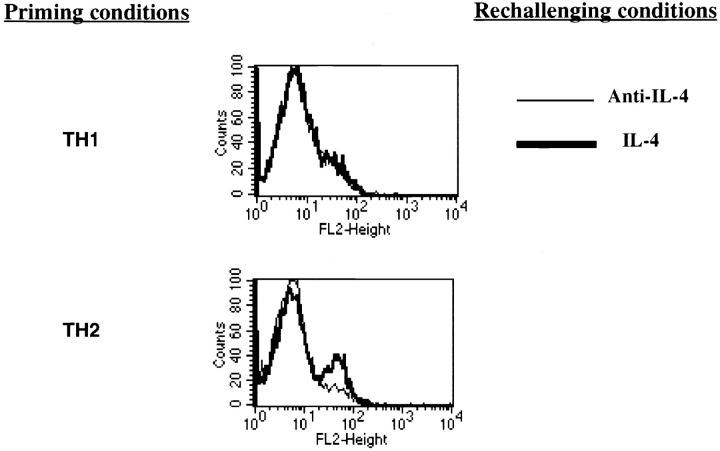
To examine further whether the diminished Stat6 phosphorylation induced by IL-4 in Th1s correlated with a failure to upregulate IL-4–dependent molecules, we examined the capacity of IL-4 to enhance expression of CD30 (49) in Th1s and Th2s. Naive C57BL/6 CD4+ T cells were primed with soluble anti-CD3 and anti-CD28 with irradiated APCs under Th1 or Th2 conditions for two rounds of 7 d each. They were then stimulated for 3 d with anti-CD3, anti-CD28, and irradiated APCs with IL-4 or anti-IL–4. A subset of Th2 cells from C57BL/6 donors stimulated in the presence of IL-4 show induction of CD30 at 3 d (Fig. 7), whereas stimulation of these cells in the presence of anti– IL-4 failed to induce CD30. Th1s from C57BL/6 mice failed to induce CD30 when challenged in the presence of IL-4 or of anti–IL-4.
Figure 7.
Failure of Th1s to respond to Th2 conditions with elevated expression of CD30 or production of IL-4. CD4+ lymph node T cells from C57BL/6 cells were primed for two rounds with anti-CD3, anti-CD28, T-depleted irradiated APCs, and IL-2 under Th1 or Th2 conditions. They were then restimulated with anti-CD3, anti-CD28, T-depleted irradiated APCs, and IL-2 in the presence of IL-4 or anti–IL-4 for 3 d. CD30 expression was determined by FACS® analysis using PE-labeled anti-CD30 antibody after the 3-d culture. The brackets delimit the area of fluorescence of unstained cells.
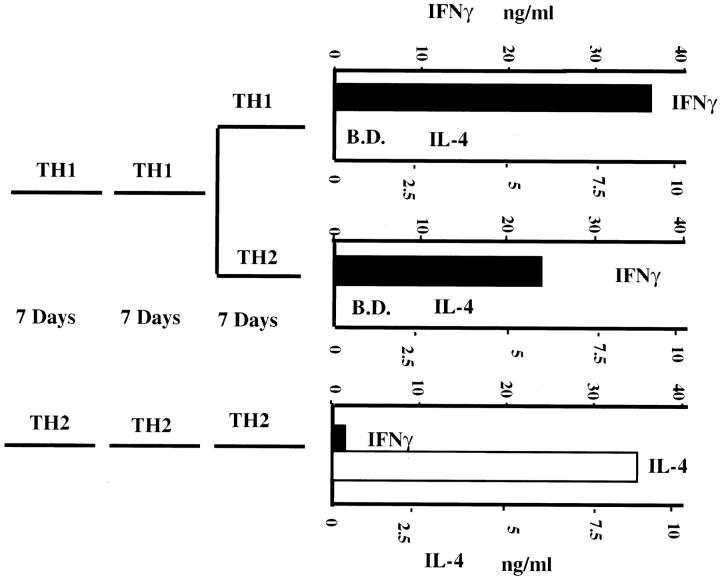
Similarly, as we and others have previously reported (43– 45), “twice-primed” TCR transgenic Th1s fail to develop the capacity to produce IL-4 even if restimulated with cytochrome C peptide, APC, IL-2, and IL-4 for 7 d (Fig. 8).
Figure 8.
Th1s fail to acquire IL-4–producing capacity even if cultured under Th2 conditions. Two-round–primed TCR transgenic Th1s were restimulated under Th1 or Th2 conditions for 7 d; similarly primed Th2 cells were restimulated under Th2 conditions for 7 d. At the end of culture, cells were washed extensively. 106 washed cells were restimulated with cytochrome C peptide plus 106 irradiated spleen cells for 24 h. Supernatants were assayed for IFN-γ and IL-4 by ELISA. B.D. below detection.
Discussion
Our results show that as naive CD4+ T cells differentiate to the Th1 phenotype, a major impairment of signaling through the IL-4R occurs. This is most apparent in the degree of induced phosphorylation of Stat6, which is the principal regulator of IL-4–mediated gene activation. The level of activation of JAK-3 is also impaired, whereas differences in degree of expression of the IL-4R are minimal. This result could explain the stability of the Th1 phenotype. The molecular basis of this impaired sensitivity of the IL-4R in Th1 cells is not known. The decreased phosphorylation of JAK-3 and the presence of normal amounts of Stat6 and JAK-3 protein suggest that it may reflect an overall effect in the receptor signaling activity rather than a specific effect on Stat6. The capacity of IL-2 to induce JAK-3 phosphorylation in Th1s indicates that the defect is not specific for JAK-3 and the γc chain. In light of the recent discovery of a new family of cytokine-induced JAK/STAT inhibitors (50–53), it is conceivable that during the development of Th1s one or more such inhibitors are induced and prevent JAK-3/Stat6–dependent IL-4–mediated signaling in these cells. However, we have not observed any difference in levels of expression of mRNA for SOCS 2 (suppressor of cytokine signaling 2) in Th1s and Th2s; we could not detect expression of SOCS 1 or 3 in Th1s or Th2s, although we could detect these mRNAs in bone marrow cells treated with IFN-α and IL-3 for 1 h (Huang, H., unpublished data).
It is also striking that IL-4 regulates the level of expression of IRS-2 in recently differentiated Ths. Naive T cells display very low levels of IRS-2 protein and mRNA. Initial stimulation with antigen and APC causes a transient induction of IRS-2, which is only sustained in the presence of IL-4. Thus, Th1s that have been stimulated through two rounds of priming display little or no IRS-2, whereas Th2s that have been primed for two rounds show substantial amounts of IRS-2 and vigorous phosphorylation in response to IL-4 stimulation.
The diminished sensitivity of Th1s to IL-4–mediated signaling may explain the failure of upregulation of IRS-2 in these cells. The regulation of IRS-2 expression by IL-4 and particularly the failure of Stat6 knockout cells to show such a response might also explain the diminished IL-4– stimulated growth of thymocytes from Stat6-deficient mice (25–27). These cells should fail to respond to IL-4 with enhanced expression of IRS-2 and IL-4Rs, since both are controlled by the IL-4/Stat6 signaling pathway; low levels of both IL-4R and IRS-2 might then limit IL-4 induced growth.
Kubo et al. (54) have recently reported that a Th1 T cell clone and a hybridoma with a Th1 phenotype failed to phosphorylate Stat6 in response to IL-4. Thus, the lack of IL-4–mediated signaling by Th1 cells is likely to be a very stable property of these cells. Kubo et al. (53) argue that Th1s fail to secrete IL-4 because of a silencer element located 3′ of exon 4 of the IL-4 gene that contains a Stat6-binding site. They further argue that Stat6 binding to this site inactivates the silencer element and thus allows IL-4 to be made in response to phorbol ester and ionomycin stimulation. Thus, the failure of Th1s to produce IL-4 is explained by the lack of activated Stat6 in these cells, whereas the capacity of Th2s to secrete IL-4 depends upon the activation of Stat6 and the consequent blocking of silencer function. Although this concept has attractive features, our previous results (55) have shown that once naive T cells have been differentiated into Th2s, IL-4 is no longer required for the production of IL-4 or other Th2 cytokines by these cells. Thus, Th2s stimulated with antigen and APC produce IL-4 mRNA in the presence of neutralizing concentrations of anti–IL-4 or anti–IL-4R antibody. Furthermore, IL-4 does not enhance IL-4 production by these cells even when limiting concentrations of antigen are used for stimulation. Finally, Th2s prepared from IL-4 knockout mice produce normal amounts of IL-13 mRNA in response to stimulation with anti-CD3; IL-4 does not enhance production of IL-13 by these differentiated Th2s.
Our results, indicating a failure of recently differentiated Th1s to phosphorylate Stat6, and those of Kubo et al. (53) describing absent Stat6 phosphorylation in a Th1 clone and a Th1 hybridoma, contrast with results that have been described showing that extracts from IL-4–stimulated Th1s can form complexes with a gamma-activated sequence (GAS) element as detected by electrophoretic mobility shift analysis (EMSA). Szabo et al. (56) and Lederer et al. (57) observed an IL-4–inducible EMSA in cultures that had been differentiated in the Th1 direction for one round of stimulation; cells from such cultures retained the capacity to develop IL-4–producing activity if cultured for an additional 4–7 d with antigen and IL-4. Indeed, it was subsequently demonstrated that cells that had been primed in the Th1 direction for several rounds lost the capacity to develop into IL-4–producing cells (43), consistent with our observation that IL-4–induced phosphorylation of Stat6 is often not extinguished until cells have undergone two rounds of Th1 priming. Pernis et al. (58) reported that in a Th1 clone (AE.7), IL-4 induced a factor (then designated STF–IL-4) that formed a complex with the GAS element from FcγR1. The significance of this finding remains to be evaluated further. This could be explained by differences among individual long-term Th1-like clones and recently differentiated Th1s. Alternatively, the IL-4R may retain some signaling properties in Th1s that nonetheless do not allow full activation of Stat6. Indeed, EMSA often reveals that IL-4 induces multiple bands containing the GAS element, only one of which is supershifted by anti–Stat6 antibody.
Understanding the molecular basis of the diminished sensitivity of IL-4Rs in well-differentiated Th1s might provide interesting targets for the development of agents that could reverse the polarity of these cells. Such agents could be important in the development of strategies for treatment of tissue damaging autoimmunity or to reverse inappropriate responses to certain pathogenic agents.
Acknowledgments
We thank Dr. Achasah D. Keegan for helpful discussion, Drs. James Ihle and John O'Shea for the gift of anti-Stat antibodies, and Dr. James Ihle for providing breeding pairs of Stat6 knockout mice. We acknowledge the assistance of Mrs. Jane Hu-Li, Mrs. Cynthia Watson, and Jeff Nyswaner, and thank Shirley Starnes for editorial assistance.
Footnotes
H. Huang is a fellow of the Leukemia Society of America.
Abbreviations used in this paper: γc, common gamma chain; CD, cluster of differentiation; EMSA, electrophoretic mobility shift assay; GAS, gamma-activated sequence; IRS, insulin receptor substrate; JAK, Janus kinase; mRNA, messenger RNA.
References
- 1.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Romagnani S. Th1 and Th2 in human diseases. Clin Immunol Immunopathol. 1996;80:225–235. doi: 10.1006/clin.1996.0118. [DOI] [PubMed] [Google Scholar]
- 4.Reiner SL, Locksley RM. The regulation of immunity to Leishmania major. . Annu Rev Immunol. 1995;13:151–177. doi: 10.1146/annurev.iy.13.040195.001055. [DOI] [PubMed] [Google Scholar]
- 5.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)–producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4–producing cells. J Exp Med. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swain SL, Weinberg AD, English M, Huston G. IL-4 directs the development of Th2-like helper effectors. J Immunol. 1990;145:3796–3806. [PubMed] [Google Scholar]
- 7.Seder RA, Paul WE, Davis MM, Fazekas de St B, Groth, Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+T cells from T cell receptor transgenic mice. J Exp Med. 1992;176:1091–1098. doi: 10.1084/jem.176.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh CS, Heimberger AB, Gold JS, O'Garra A, Murphy KM. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an αβ T-cell-receptor transgenic system. Proc Natl Acad Sci USA. 1992;89:6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohara J, Paul WE. Receptors for B-cell stimulatory factor–1 expressed on cells of haematopoietic lineage. Nature. 1987;325:537–540. doi: 10.1038/325537a0. [DOI] [PubMed] [Google Scholar]
- 10.Park LS, Friend D, Sassenfeld HM, Urdal DL. Characterization of the human B cell stimulatory factor 1 receptor. J Exp Med. 1987;166:476–488. doi: 10.1084/jem.166.2.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russell SM, Keegan AD, Harada N, Nakamura Y, Noguchi M, Leland P, Friedmann MC, Miyajima A, Puri RK, Paul WE, Leonard WJ. Interleukin-2 receptor γ chain: a functional component of the interleukin-4 receptor. Science. 1993;262:1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- 12.Kondo M, Takeshita T, Ishii N, Nakamura M, Watanabe S, Arai K, Sugamura K. Sharing of the interleukin-2 (IL-2) receptor γ chain between receptors for IL-2 and IL-4. Science. 1993;262:1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- 13.Noguchi M, Nakamura Y, Russell SM, Ziegler SF, Tsang M, Cao X, Leonard WJ. Interleukin-2 receptor γ chain: a functional component of the interleukin-7 receptor. Science. 1993;262:1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- 14.Kimura Y, Takeshita T, Kondo M, Ishii N, Nakamura M, Van Snick J, Sugamura K. Sharing of the IL-2 receptor γ chain with the functional IL-9 receptor complex. Int Immunol. 1995;7:115–120. doi: 10.1093/intimm/7.1.115. [DOI] [PubMed] [Google Scholar]
- 15.Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, Namen A, Park LS, Cosman D, Anderson D. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO (Eur Mol Biol Organ) J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ihle JN. The Janus protein tyrosine kinase family and its role in cytokine signaling. Adv Immunol. 1995;60:1–35. doi: 10.1016/s0065-2776(08)60582-9. [DOI] [PubMed] [Google Scholar]
- 17.Johnston JA, Kawamura M, Kirken RA, Chen YQ, Blake TB, Shibuya K, Ortaldo JR, McVicar DW, O'Shea JJ. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 18.Witthuhn BA, Silvennoinen O, Miura O, Lai KS, Cwik C, Liu ET, Ihle JN. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 19.Wang LM, Keegan AD, Paul WE, Heidaran MA, Gutkind JS, Pierce JH. IL-4 activates a distinct signal transduction cascade from IL-3 in factor-dependent myeloid cells. EMBO (Eur Mol Biol Organ) J. 1992;11:4899–4908. doi: 10.1002/j.1460-2075.1992.tb05596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen XH, Patel BK, Wang LM, Frankel M, Ellmore N, Flavell RA, LaRochelle WJ, Pierce JH. Jak1 expression is required for mediating interleukin-4–induced tyrosine phosphorylation of insulin receptor substrate and Stat6 signaling molecules. J Biol Chem. 1997;272:6556–6560. doi: 10.1074/jbc.272.10.6556. [DOI] [PubMed] [Google Scholar]
- 21.Nosaka T, Van Deursen JM, Tripp RA, Thierfelder WE, Witthuhn BA, McMickle AP, Doherty PC, Grosveld GC, Ihle JN. Defective lymphoid development in mice lacking Jak3. Science. 1995;270:800–802. doi: 10.1126/science.270.5237.800. [DOI] [PubMed] [Google Scholar]
- 22.Park SY, Saijo K, Takahashi T, Osawa M, Arase H, Hirayama N, Miyake K, Nakauchi H, Shirasawa T, Saito T. Developmental defects of lymphoid cells in Jak3 kinase–deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 23.Thomis DC, Gurniak CB, Tivol E, Sharpe AH, Berg LJ. Defects in B lymphocyte maturation and T lymphocyte activation in mice lacking Jak3. Science. 1995;270:794–797. doi: 10.1126/science.270.5237.794. [DOI] [PubMed] [Google Scholar]
- 24.Noben-Trauth N, Shultz L, Brombacher F, Urban J, Gu H, Paul WE. An IL-4–independent pathway for CD4+T cell IL-4 production is revealed in interleukin receptor deficient mice. Proc Natl Acad Sci USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 26.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 27.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DA, et al. Lack of IL-4–induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 28.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4–induced transcription factor: IL-4 Stat. Science. 1994;265:1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 29.Keegan AD, Nelms K, White M, Wang LM, Pierce JH, Paul WE. An IL-4 receptor region containing an insulin receptor motif is important for IL-4–mediated IRS-1 phosphorylation and cell growth. Cell. 1994;76:811–820. doi: 10.1016/0092-8674(94)90356-5. [DOI] [PubMed] [Google Scholar]
- 30.Keegan AD, Paul WE. Multichain immune recognition receptors: similarities in structure and signaling pathways. Immunol Today. 1992;13:63–68. doi: 10.1016/0167-5699(92)90136-U. [DOI] [PubMed] [Google Scholar]
- 31.Pernis A, Witthuhn B, Keegan AD, Nelms K, Garfein E, Ihle JN, Paul WE, Pierce JH, Rothman P. Interleukin 4 signals through two related pathways. Proc Natl Acad Sci USA. 1995;92:7971–7975. doi: 10.1073/pnas.92.17.7971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quelle FW, Shimoda K, Thierfelder W, Fischer C, Kim A, Ruben SM, Cleveland JL, Pierce JH, Keegan AD, Nelms K, Ihle JN. Cloning of murine Stat6 and human Stat6, Stat proteins that are tyrosine phosphorylated in responses to IL-4 and IL-3 but are not required for mitogenesis. Mol Cell Biol. 1995;15:3336–3343. doi: 10.1128/mcb.15.6.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan JJ, McReynolds LJ, Keegan A, Wang LH, Garfein E, Rothman P, Nelms K, Paul WE. Growth and gene expression are predominantly controlled by distinct regions of the human IL-4 receptor. Immunity. 1996;4:123–132. doi: 10.1016/s1074-7613(00)80677-9. [DOI] [PubMed] [Google Scholar]
- 34.Wang HY, Paul WE, Keegan AD. IL-4 function can be transferred to the IL-2 receptor by tyrosine containing sequences found in the IL-4 receptor α chain. Immunity. 1996;4:113–121. doi: 10.1016/s1074-7613(00)80676-7. [DOI] [PubMed] [Google Scholar]
- 35.Kotanides H, Reich NC. Interleukin-4–induced STAT6 recognizes and activates a target site in the promoter of the interleukin-4 receptor gene. J Biol Chem. 1996;271:25555–25561. doi: 10.1074/jbc.271.41.25555. [DOI] [PubMed] [Google Scholar]
- 36.Todd MD, Grusby MJ, Lederer JA, Lacy E, Lichtman AH, Glimcher LH. Transcription of the interleukin 4 gene is regulated by multiple promoter elements. J Exp Med. 1993;177:1663–1674. doi: 10.1084/jem.177.6.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruhn KW, Nelms K, Boulay JL, Paul WE, Lenardo MJ. Molecular dissection of the mouse interleukin-4 promoter. Proc Natl Acad Sci USA. 1993;90:9707–9711. doi: 10.1073/pnas.90.20.9707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szabo SJ, Gold JS, Murphy TL, Murphy KM. Identification of cis-acting regulatory elements controlling interleukin-4 gene expression in T cells: roles for NF-Y and NF-ATc. Mol Cell Biol. 1993;13:4793–4805. doi: 10.1128/mcb.13.8.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tara D, Weiss DL, Brown MA. Characterization of the constitutive and inducible components of a T cell IL-4 activation responsive element. J Immunol. 1995;154:4592–4602. [PubMed] [Google Scholar]
- 40.Ho IC, Hodge MR, Rooney JW, Glimcher LH. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell. 1996;85:973–983. doi: 10.1016/s0092-8674(00)81299-4. [DOI] [PubMed] [Google Scholar]
- 41.Hodge MR, Chun HJ, Rengarajan J, Alt A, Lieberson R, Glimcher LH. NF-AT-driven interleukin-4 transcription potentiated by NIP45. Science. 1996;274:1903–1905. doi: 10.1126/science.274.5294.1903. [DOI] [PubMed] [Google Scholar]
- 42.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 43.Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, Murphy K, O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sornasse T, Larenas PV, Davis KA, deVries J, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473–483. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu LJ, Huang H, Ryan J, Paul WE. In differentiated CD4+ T cells, interleukin 4 production is cytokine-autonomous, whereas interferon gamma production is cytokine-dependent. Proc Natl Acad Sci USA. 1997;94:3189–3194. doi: 10.1073/pnas.94.7.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kurt JE, Hamberg S, Ohara J, Paul WE, Abbas AK. Heterogeneity of helper/inducer T lymphocytes. I. Lymphokine production and lymphokine responsiveness. J Exp Med. 1987;166:1774–1787. doi: 10.1084/jem.166.6.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lichtman AH, Chin J, Schmidt JA, Abbas AK. Role of interleukin 1 in the activation of T lymphocytes. Proc Natl Acad Sci USA. 1988;85:9699–9703. doi: 10.1073/pnas.85.24.9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zamorano J, Wang HY, Wang LM, Pierce JH, Keegan AD. IL-4 protects cells from apoptosis via the insulin receptor substrate pathway and a second independent signaling pathway. J Immunol. 1996;157:4926–4934. [PubMed] [Google Scholar]
- 49.Nakamura T, Lee RK, Nam SY, Al-Ramadi BK, Koni PA, Bottomly K, Podack ER, Flavell RA. Reciprocal regulation of CD30 expression on CD4+T cells by IL-4 and IFN-γ. J Immunol. 1997;158:2090–2098. [PubMed] [Google Scholar]
- 50.Yoshimura A, Ohkubo T, Kiguchi T, Jenkins NA, Gilbert DJ, Copeland NG, Hara T, Miyajima A. A novel cytokine-inducible gene CIS encodes an SH2-containing protein that binds to tyrosine-phosphorylated interleukin 3 and erythropoietin receptors. EMBO (Eur Mol Biol Organ) J. 1995;14:2816–2826. doi: 10.1002/j.1460-2075.1995.tb07281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Endo TA, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, et al. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 52.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 53.Starr R, Willson TA, Viney EM, Murray LJ, Rayner JR, Jenkins BJ, Gonda TJ, Alexander WS, Metcalf D, Nicola NA, Hilton DJ. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 54.Kubo M, Ransom J, Webb D, Hashimoto Y, Tada T, Nakayama T. T-cell subset-specific expression of the IL-4 gene is regulated by a silencer element and STAT6. EMBO (Eur Mol Biol Organ) J. 1997;16:4007–4020. doi: 10.1093/emboj/16.13.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang H, Hu-Li J, Chen H, Ben-Sasson SZ, Paul WE. IL-4 and IL-13 production in differentiated Th2 cells is not IL-4 dependent. J Immunol. 1997;159:3731–3738. [PubMed] [Google Scholar]
- 56.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 57.Lederer J A, Perez VL, DesRoches L, Kim SM, Abbas AK, Lichtman AH. Cytokine transcriptional events during helper T cell subset differentiation. J Exp Med. 1996;184:397–406. doi: 10.1084/jem.184.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pernis A, Gupta S, Gollob KJ, Garfein E, Coffman R, Schindler C, Rothman P. Lack of interferon γ receptor β chain and the prevention of interferon γ signaling in TH1 cells. Science. 1995;269:245–247. doi: 10.1126/science.7618088. [DOI] [PubMed] [Google Scholar]