Abstract
p300 and cAMP response element–binding protein (CREB)–binding protein (CBP) are members of a family of coactivators involved in the regulation of transcription and chromatin. We show that transcription factors of the nuclear factor of activated T cells (NFAT) family bind p300/CBP and recruit histone acetyltransferase activity from T cell nuclear extracts. The NH2-terminal transactivation domain of NFAT1 and the phospho-CREB- and E1A-binding sites of p300/CBP are involved in the interaction. The viral oncoprotein E1A inhibits NFAT-dependent transactivation in a p300-dependent manner. Recruitment of the coactivators p300/CBP by the transactivation domains of NFAT proteins is likely to play a critical role in NFAT-dependent gene expression during the immune response.
Keywords: transcriptional regulation, histone acetyltransferases, T cells, nuclear factor of activated T cells, cytokine gene expression
Histone acetyltransferases (HATs)1 and histone deacetylases have been closely implicated in the mechanism of transcriptional activation and repression of multiple genes (for a review, see references 1 and 2). Acetylation of the NH2-terminal regions of histones is considered to be crucial for the accessibility of transcription factors to nucleosomal templates. All core histones can be variably acetylated by a number of proteins designated HAT (1, 3), which include p300/cAMP response element–binding protein (CREB)-binding protein (CBP) (4, 5), the p300/ CBP-associated factor P/CAF (6), and the nuclear receptor coactivator ACTR (7). A diverse and increasing number of transcription factors and some elements of the basal transcription machinery are able to form stable physical complexes with and respond to the coactivating properties of p300/CBP (for a review, see references 8 and 9). However, the precise mechanisms by which transcriptional activators stimulate the transcriptional machinery through p300/CBP remain unclear.
p300/CBP coactivators serve also as multifunctional adaptors that coordinate cell cycle progression with transcriptional regulation (10). These coactivators are recruited in signaling pathways dependent on p53, a tumor suppressor and a common target for genetic alteration in human cancers (11). Interestingly, there is accumulating evidence for the role of CBP and p300 in tumorigenesis (9). Specifically, certain cases of acute myeloid leukemia have been linked to recurrent chromosomal translocations that result in in frame fusions of CBP or p300 to the monocytic leukemia zinc finger protein (12) and myeloid/lymphoid leukemia (13, 14) gene products. Moreover, the viral oncoprotein E1A inhibits host gene transcription, by itself binding and presumably using the HAT activities of p300/ CBP (9).
The nuclear factor of activated T cells (NFAT) regulates the inducible transcription of cytokine genes and other genes critical for the immune response (for a review, see reference 15), thereby indirectly regulating immune cell proliferation. The most NH2-terminal region (∼100 amino acids [aa]) of NFAT proteins contains a strong acidic transactivation domain, whose function in resting cells is partially masked by the adjacent regulatory domain (16). We show here that the NH2-terminal transactivation domains of NFAT proteins are capable of recruiting HATs. Immunoprecipitates of NFAT1 from T cell nuclear extracts contain an associated HAT activity that is accounted for at least partially by p300/CBP. NFAT-dependent transactivation is inhibited by the viral oncoprotein E1A in a p300-dependent manner. The p300/CBP proteins, acting as chromatin-remodeling factors and/or interacting with the basal transcription machinery, may play an important role in the NFAT-dependent expression of inducible genes.
Materials and Methods
Antibodies.
Antihemagglutinin (anti-HA; 12CA5 murine mAb against the influenza HA peptide; Boehringer Mannheim Biochemicals, Indianapolis, IN) was used to immunoprecipitate HA-tagged p300. Rabbit polyclonal antibodies against an NH2-terminal peptide (67.1), the DNA-binding domain (DBD), and a COOH-terminal peptide (anti–NFAT1-C) of NFAT1 were used as described (17, 18). Tat antibody, used as a negative control for immunoprecipitation, was provided by Dr. B. Cullen (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD [19]).
Plasmids.
pLGP3-mNFAT1ΔReg (Δ146–398) was made by subcloning a SacI-HindIII fragment from pBSTΔSX into the expression plasmid pLGP3, between SacI and HindIII in the multicloning site. pBSTΔSX was made by removing a fragment SfiI-XhoI from pBS-mNFAT1-C and religating blunt ends. 6xHis-hNFAT1(1–415), was made by subcloning pGEX-2T-NFAT1(1– 415) into the BamHI/HindIII sites of pQE32 (QIAGEN Inc., Chatsworth, CA) that had been filled in with Klenow. The construction of pEFBOS-mNFAT1-C, pLGP3-mNFAT1-C, GAL4-hNFAT1(1–415), GAL4-ΔSP2, and GAL4-hNFAT2(1–418) has been described previously (16, 20). The NFAT luciferase reporter plasmid (NFAT3x-Luc) containing three copies of the distal NFAT site of the murine IL-2 promoter (21), was provided by Dr. D. McKean (Mayo Clinic, Rochester, MN). The GAL4- luciferase reporter plasmid (GAL4-Luc) (22), the cDNA plasmids pCMVβ-HA-p300, pCMVβ-E1A (23), the E1A mutant defective in binding to p300 (pE1A.15-35 [24]), and plasmids encoding the glutathione S-transferase (GST)-CBP fusion proteins, CBP1 (aa 1–117), CBP2 (aa 117–737), CBP3 (aa 737–1626), and CBP4 (aa 1680–1891) (25), were provided by Dr. M. Montminy (Joslin Diabetes Center, Boston, MA). pRSV–human growth hormone (hGH) was used to normalize transfection levels.
Transient Transfection.
Plasmid DNAs were introduced into Jurkat human T cells by electroporation (0.25 V, 960 μF) using a gene pulser (Bio-Rad Laboratories, Hercules, CA) and 0.4-cm gap cuvettes (26). Transfection of 293T cells (human embryonic kidney fibroblasts), originally referred to as 293tsA1609neo (27), was performed using the calcium phosphate coprecipitation technique. For each lane of the immunoprecipitation experiments, five 10-cm dishes with 0.5 × 106 cells each were transfected with 4 μg plasmid DNA. 36 h after transfection, cells were stimulated for 25 min at 37°C with 1 μM ionomycin and 10 nM PMA or left untreated, before lysis and immunoprecipitation. Transfection experiments for transactivation studies were performed in Jurkat cells.
Cell Extracts and Immunoprecipitation.
Whole-cell extracts were prepared from transfected 293T cells using buffer A (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% Triton, 10% glycerol, 20 mM sodium pyrophosphate, 1 mM dithiothreitol, 0.4 mM PMSF, 100 μg/ml aprotinin, 25 μM leupeptin). Cell lysates (5 × 106 cells/ml) were incubated for 10 min at 4°C and cleared by centrifugation at 2,500 g for 10 min at 4°C. High salt nuclear extracts from the murine T cell line Cl.7W2 (28) were prepared as described previously (29). Cell extracts were precleared with protein A and B–Sepharose (Pharmacia Biotech, Piscataway, NJ) for 1 h at 4°C and immunoprecipitated for 3 h at 4°C. Samples were analyzed by Western blotting (ECL kit; New England Nuclear, Boston, MA) or used for HAT assays.
HAT Assay.
HAT assays were performed using a modification of the assay described previously (30). Nuclear extracts from Cl.7W2 cells were precleared and immunoprecipitated as described above. Beads were washed in buffer B (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 5 mM EDTA, 0.5% NP-40, 0.4 mM PMSF, 100 μg/ml aprotinin, 25 μM leupeptin). The HAT assay was performed in 100 μl buffer B containing 8 μg crude histones (type IIA; Sigma Chemical Co., St. Louis, MO) and 600 nM [3H]acetyl-CoA (2.5 Ci/mmol; New England Nuclear). After incubation at 30°C for 1 h, the reaction mixture was spotted onto P81 phosphocellulose filter paper (Whatman Inc., Clifton, NJ) and washed with 0.2 M sodium carbonate buffer (pH 9.2). [3H]Acetyl incorporation into histones was determined by liquid scintillation counting of dried filters.
In Vitro Binding Analysis with GST Fusion Proteins.
GST fusion proteins containing CBP regions 1–4 were expressed in and purified from Escherichia coli as reported previously (25). 6xHis-hNFAT1(1–415) (0.5 μg), expressed and purified using Ni-NTA–agarose beads (QIAGEN Inc.), was incubated for 2 h at 4°C with 5–10 μg of CBP-GST fusion proteins immobilized on glutathione-Sepharose beads. The beads were washed five times with buffer B and once with the same buffer containing no detergent. Bound NFAT1(1–415) was analyzed by Western blotting with anti-67.1.
Transactivation Experiments.
Jurkat cells (12 × 106) were transfected with 2–5 μg of reporter plasmid DNA, 0.5 μg of pSRV-hGH plasmid, and 2–5 μg of different NFAT plasmid DNAs. 24 h after transfection, cells were stimulated overnight with 1 μM ionomycin and 10 nM PMA. Cell extracts were assayed for luciferase activity (Luciferase Assay System; Promega Corp., Madison, WI) as described previously (26). Transfection efficiencies were determined by measuring the hGH concentration in the culture media with a radioimmunometric assay kit (Nichols Institute, San Juan Capistrano, CA).
Results and Discussion
NFAT Associates with HATs in T Cell Nuclear Extracts.
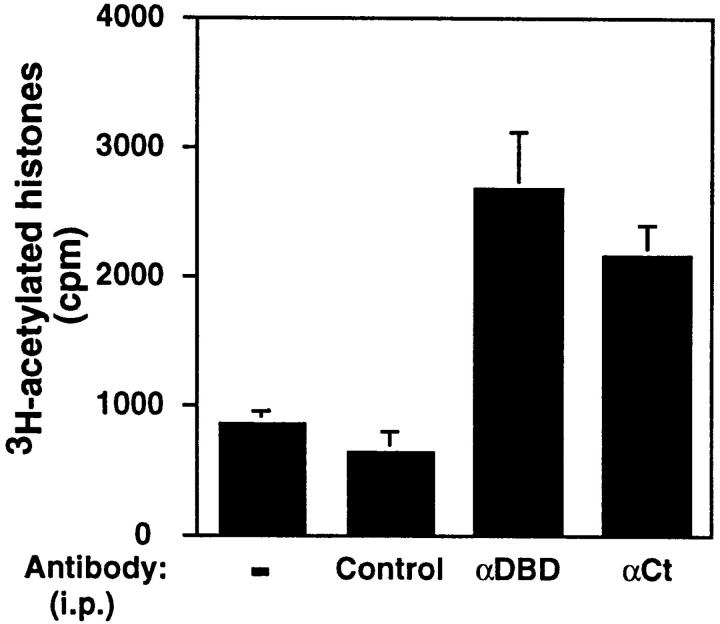
Cl.7W2 T cells were stimulated with ionomycin and phorbol esters to localize all endogenous NFAT proteins to the nucleus and activate their transcriptional activity. Nuclear extracts were prepared from the activated cells, and NFAT1 was immunoprecipitated using several antibodies. After extensive washes, the immune complexes were tested for HAT activity. As shown in Fig. 1, antibodies directed against the DBD and COOH-terminal regions of NFAT1 immunoprecipitated significant levels of HAT activity from stimulated T cell nuclear extracts compared with the background levels immunoprecipitated with an irrelevant antibody. In contrast, an antibody against an NH2-terminal epitope of NFAT1 immunoprecipitated background levels of HAT activity (data not shown). These data suggested that the HAT interaction might involve the NH2-terminal region of NFAT1.
Figure 1.
NFAT1 associates with HATs in nuclear extracts. Cl.7W2 T cells were stimulated with ionomycin and PMA for 25 min at 37°C, and nuclear extracts were immunoprecipitated (i.p.) with antibodies against the DBD (αDBD) or a COOH-terminal peptide (αCt) of NFAT1. An irrelevant antibody was used as a control. The immune complexes were tested for HAT activity as described in Materials and Methods. The results are representative of three independent experiments. Bars, The mean and range of duplicates in one such experiment.
NFAT Interacts with p300 in Cells.
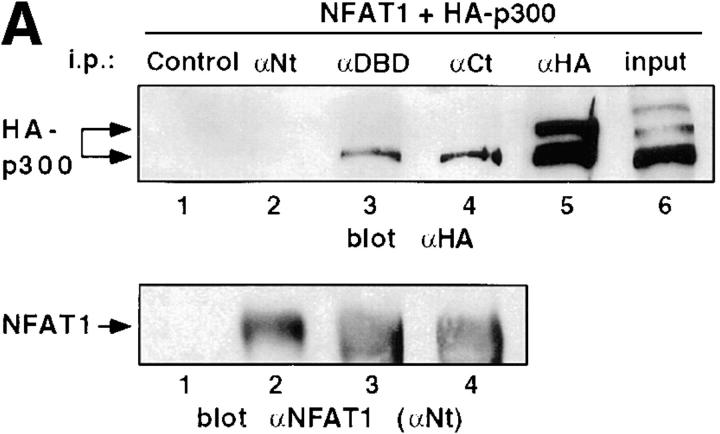
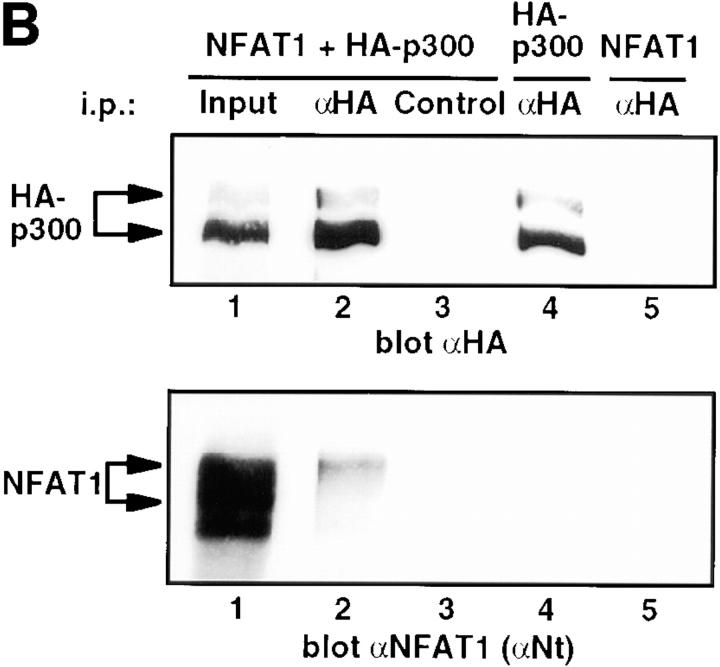
Since p300/CBP possess intrinsic HAT activity (4, 5) and also associate with other HATs (6, 7), we asked whether the HAT activity associated with NFAT1 was accounted for at least partly by p300/CBP. HA epitope–tagged p300 and NFAT1 were coexpressed in 293T cells, and the cells were stimulated with PMA and ionomycin to localize NFAT1 to the nucleus and activate it transcriptionally. NFAT1 was then immunoprecipitated from whole-cell lysates, and the immunoprecipitates were tested for the presence of coprecipitating HA-p300. Antibodies against the DBD and COOH-terminal regions of NFAT1 coimmunoprecipitated HA-p300, as judged by Western blotting of the immunoprecipitations with anti-HA (Fig. 2 A, lanes 3 and 4). Antibody against the NH2-terminal region of NFAT1 gave very weak coimmunoprecipitation of HA-p300 (Fig. 2 A, lane 2), which was only detected by overexposure (data not shown), again suggesting that the NH2-terminal region of NFAT1 was involved in the interaction with HA-p300. All antibodies immunoprecipitated equivalent amounts of NFAT1 (Fig. 2 A, bottom). The antibody control showed no immunoreactive band corresponding to HA-p300 (Fig. 2 A, lane 1), indicating the specificity of the interaction. Conversely, anti-HA coimmunoprecipitated a fraction of nuclear NFAT1 as shown by Western blotting with anti-NFAT1 (Fig. 2 B, lane 2). The coimmunoprecipitated fraction of NFAT1 migrated with higher apparent molecular weight than the bulk of the NFAT1 protein (compare lanes 1 and 2), suggesting that some posttranslational modification of NFAT1 (e.g., phosphorylation) might be required for that optimal interaction with p300. These results are the first demonstration of an interaction of NFAT transcription factors with the coactivators and HATs p300 and CBP.
Figure 2.
Interaction of p300 and NFAT1 in cells. (A) 293T cells expressing NFAT1 and HA-p300 were stimulated with ionomycin and PMA for 25 min at 37°C. Whole-cell extracts were immunoprecipitated (i.p.) with antibodies against an NH2-terminal peptide (αNt), the DBD (αDBD), and a COOH-terminal peptide (αCt) of NFAT1. An irrelevant antibody was used as a control. The immune complexes were analyzed by Western blotting for HA-p300 (top) and NFAT1 (bottom, blot with αNt). The expressed HA-p300, detected with anti-HA (αHA), appeared as several bands (lanes 5 and 6), and the bottom band seemed to be preferentially coimmunoprecipitated with NFAT1 (lanes 3 and 4). (B) Lysates from 293T cells expressing NFAT1 alone, HA-p300 alone, or both were immunoprecipitated (i.p.) with anti-HA (αHA), and immune complexes were analyzed by Western blotting for HA-p300 (top) and NFAT1 (bottom). Input, 1% of the total amount of cell extract used for immunoprecipitations. Arrows, The different forms detected by immunoblotting.
The NH2-terminal Region of NFAT1 Interacts with CBP In Vitro.
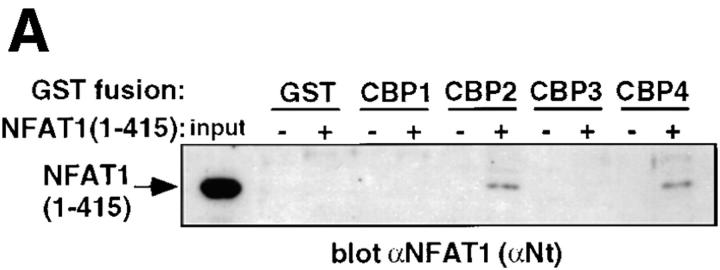
To identify the region of NFAT1 and p300/CBP involved in the NFAT1–p300/CBP interaction, we performed binding experiments using bacterially expressed recombinant proteins. GST fusion proteins containing different regions of CBP were incubated with hexahistidine-tagged NFAT1(1–415), and the level of NFAT1 binding was assessed by Western analysis. These experiments showed that NFAT1 interacted directly with regions CBP2 (aa 117– 737) and CBP4 (aa 1680–1891), where CBP2 includes the phospho-CREB–binding site and CBP4 includes the E1A-binding site (Fig. 3 A). Equivalent amounts of each GST fusion protein were used in the binding assay as seen by Ponceau red staining (data not shown). Binding between CBP and NFAT was specific because no immunoreactive band was seen with GST alone or with other portions of CBP. The interaction seen in these in vitro experiments was weak, and may reflect either the lack of some posttranslational modification of NFAT1 required for optimal interaction or the lack of an accessory protein present in nuclear extracts (see Fig. 2 B). The interaction of p300/ CBP with signal transducer and activator of transcription Stat1 also involves the participation of two widely separated regions of p300/CBP (31), although most of the transcription factors described to date interact only with a single region (9).
Figure 3.
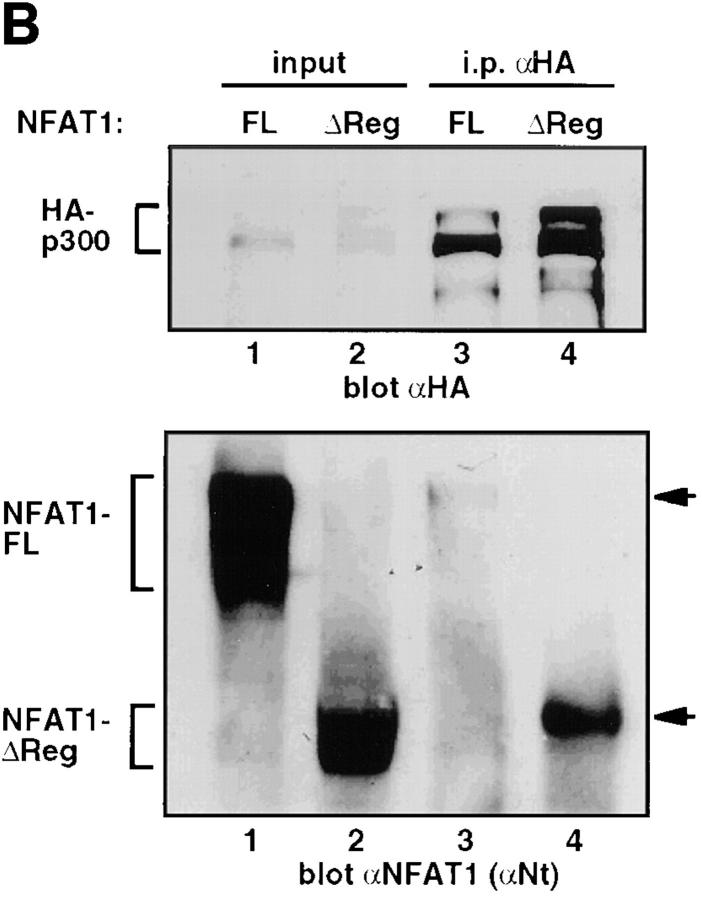
Regions of NFAT1 and CBP involved in the interaction. (A) In vitro binding experiments were performed using 6xHisNFAT1(1–415) and GST-CBP fusion proteins immobilized on glutathione-Sepharose beads. After six washes, the samples were analyzed by Western blotting analysis for NFAT1. (B) 293T cells coexpressing HA-p300 and either full-length NFAT1 (NFAT1-FL) or NFAT1ΔReg were stimulated with ionomycin and PMA for 25 min at 37°C. Whole-cell extracts were immunoprecipitated with anti-HA (αHA). Immune complexes were analyzed by Western blotting for HA-p300 (top) and NFAT1 (bottom). Input, 1% of the total amount of cell extract used for immunoprecipitations. Arrows, The different forms detected by immunoblotting.
An Intact Regulatory Domain Is Not Required for Interaction with p300.
The NH2-terminal region of NFAT1 comprises a strong acidic transactivation domain and an adjacent regulatory domain, the NFAT homology region (15). To test the importance of the regulatory domain, we cotransfected 293T cells with HA-p300 and either full-length NFAT1 or NFAT1ΔReg, which lacks aa 146–398 encoding the bulk of the regulatory domain. Immunoprecipitation of stimulated cell lysates with anti-HA followed by Western analysis revealed that NFAT1ΔReg was still capable of interaction with p300 (Fig. 3 B, lane 4); indeed, the interaction was reproducibly stronger than observed with the full-length protein (compare lanes 3 and 4). A control antibody, unable to immunoprecipitate HA-tagged epitope p300, was also unable to precipitate NFAT1ΔReg (data not shown). Taken together with the in vitro binding experiments using recombinant proteins, these data suggested that an intact regulatory domain was not required for NFAT1–p300 interaction, and that the NH2-terminal transactivation domain of NFAT1 constituted at least one region of interaction with p300.
E1A Inhibits NFAT1- and NFAT2-dependent Transactivation.
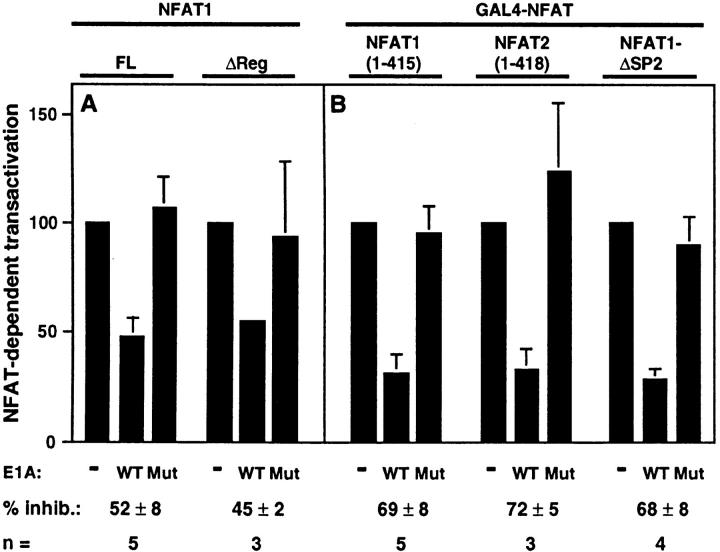
The functional consequence of the interaction between p300 and NFAT was investigated by E1A, a viral protein that interferes with p300/CBP functions (9). Jurkat T cells were cotransfected with expression vectors encoding these two proteins and a reporter construct containing the distal NFAT site of the IL-2 promoter (NFAT3x-Luc). 24 h after transfection, cells were stimulated overnight with 1 μM ionomycin and 10 nM PMA to localize NFAT to the nucleus and activate NFAT-dependent transcription. Cells were harvested and lysed, and the luciferase assay was performed. In five experiments, E1A inhibited significantly NFAT-dependent transactivation (Fig. 4 A). An E1A mutant lacking the binding site for p300 was impaired in its ability to inhibit NFAT-dependent transactivation, indicating that the effect was indeed mediated by interference with p300. E1A was also able to inhibit transactivation mediated by NFAT1ΔReg (Δ146–398), the protein lacking the regulatory domain.
Figure 4.
E1A inhibits NFAT-dependent transactivation in a p300-dependent manner. (A) The NFAT3x-Luc reporter plasmid and expression plasmids encoding full-length NFAT1 (NFAT1-FL) or NFAT1ΔReg were cotransfected into Jurkat cells along with expression plasmids encoding wild-type E1A (WT) or the E1A mutant defective for p300 binding (Mut). (B) The GAL4-Luc reporter and expression plasmid encoding GAL4-NFAT1(1–415), GAL4-NFAT2(1–418), or GAL4-NFAT1ΔSP2 were cotransfected into Jurkat cells along with expression plasmids encoding wild-type E1A (WT) or the E1A mutant defective in p300 binding (Mut). After 36 h of transfection, cells were left unstimulated or stimulated overnight with ionomycin and PMA and assayed for luciferase assay. Results are representative of several experiments. Transfection efficiencies were normalized by measuring hGH expression from a cotransfected RSV-hGH plasmid.
Most NFAT-dependent transactivation requires the interaction of NFAT with activating protein-1 (AP-1), a heterodimer of Fos and Jun proteins (for a review, see reference 15). Fos and Jun both interact with p300, and E1A can modulate AP-1 activity by competing directly for p300/CBP (32, 33). To determine whether at least part of the effect of E1A on NFAT-dependent transactivation was due to interference with the p300–NFAT rather than the p300–AP-1 interaction, we used GAL4 fusion proteins containing the NH2-terminal transactivation domains of NFAT1 and NFAT2 (16). As seen in Fig. 4 B, E1A also inhibited transactivation mediated by GAL4-NFAT1(1–145) and GAL4-NFAT2(1–418), whereas the p300 binding site–defective mutant of E1A did not. Similar results were obtained using GAL4-ΔSP2 (Δ145-387) (16), a deleted version containing the NH2-terminal transactivation domain but lacking an intact regulatory domain (Fig. 4 B). Taken together, these data indicated that E1A inhibits NFAT-dependent transactivation in a p300-dependent manner, and that an intact regulatory domain of NFAT1 was not required for effective inhibition.
Physiological Implications of the NFAT and p300/CBP Interaction.
HATs p300 and CBP play a role in cell transcription regulation as well as cell cycle control (9). Transcription of eukaryotic genes is a multistep process with many potential levels of control, including the assembly of sequence-specific DNA-binding proteins on gene-regulatory elements, and the recruitment of coactivators that facilitate the activity of the basal transcription complex (8, 34). By interacting with the basal transcription machinery, p300/CBP may facilitate the recruitment of the RNA polymerase as part of the holoenzyme complex to NFAT-dependent promoters (35, 36). Since acetylation of core histones has been associated with chromatin assembly and the regulation of gene expression (1, 2), the recruitment of HAT activity by NFAT might be an important step for the regulation of NFAT-dependent gene expression in the context of chromatin during the immune response. Specific interactions with coactivators, in conjunction with the distinct site preferences of NFAT family proteins, may determine the cell-specific expression of inducible genes.
Acknowledgments
We are grateful to Dr. Marc Montminy, Dr. Heidi Okamura, Dr. David McKean, and Dr. Chun Luo for generously providing reagents. Antiserum to HIV-1 Tat was obtained from Dr. Bryan Cullen through the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
This work was supported by National Institutes of Health grant CA42471 (to A. Rao). A. Rao is a Scholar of the Leukemia Society of America. C. Garcia-Rodriguez is the recipient of a Lady Tata Memorial Trust Fellowship.
Abbreviations used in this paper
- aa
amino acid(s)
- AP-1
activating protein-1
- CBP
CREB-binding protein
- CREB
cAMP response element–binding protein
- DBD
DNA-binding domain
- GST
glutathione S-transferase
- HA
hemagglutinin
- HAT
histone acetyltransferase
- hGH
human growth hormone
- Luc
luciferase
- NFAT
nuclear factor of activated T cells
References
- 1.Brownell JE, Allis D. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev. 1996;6:176–184. doi: 10.1016/s0959-437x(96)80048-7. [DOI] [PubMed] [Google Scholar]
- 2.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 3.Wu C. Chromatin remodeling and the control of gene expression. J Biol Chem. 1997;272:28171–28174. doi: 10.1074/jbc.272.45.28171. [DOI] [PubMed] [Google Scholar]
- 4.Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 5.Bannister AJ, Kourazides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 6.Yang XJ, Ogryzko VV, Nishikawa J, Howard BH, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 7.Chen H, Lin RJ, Schiltz RL, Chakravarti D, Nash A, Nagy L, Privalsky ML, Nakatani Y, Evans RM. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 8.Janknecht R, Hunter T. Transcriptional control: versatile molecular glue. Curr Biol. 1996;6:951–954. doi: 10.1016/s0960-9822(02)00636-x. [DOI] [PubMed] [Google Scholar]
- 9.Shikama N, Lyon J, La NB, Thangue The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 10.Perkins ND, Felzien LK, Betts JC, Leung K, Beach DH, Nabel GJ. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science. 1997;275:523–527. doi: 10.1126/science.275.5299.523. [DOI] [PubMed] [Google Scholar]
- 11.Avantaggiatti ML, Ogryzko VV, Gardner K, Giordano A, Levine AS, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. doi: 10.1016/s0092-8674(00)80304-9. [DOI] [PubMed] [Google Scholar]
- 12.Borrow J, Stanton JVP, Andresen M, Becher R, Behm FG, Chaganti RSK, Civin CI, Disteche C, Dube I, Frischauf AM, et al. The translocation t(8;16)(p11; p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 13.Sobulo OM, Borrow J, Tomeck R, Reshmi S, Harden A, Schlegelberger B, Housman D, Doggett NA, Rowley JD, Zeleznik-Le NJ. MLL is fused to CBP, a histone acetyltransferase, in therapy-related acute myeloid leukemia with a t(11;16)(q23;p13.3) Proc Natl Acad Sci USA. 1997;94:8732–8737. doi: 10.1073/pnas.94.16.8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ida K, Kitabayshi I, Taki T, Taniwaki M, Noro K, Yamamoto M, Ohki M, Hayashi Y. Adenoviral 1A-associated protein p300 is involved in acute myeloid leukemia with t(11;22)(q23;q13) Blood. 1997;90:4699–4704. [PubMed] [Google Scholar]
- 15.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 16.Luo C, Burgeon E, Rao A. Mechanisms of transactivation by nuclear factor of activated T cells-1. J Exp Med. 1996;184:141–147. doi: 10.1084/jem.184.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho AM, Jain J, Rao A, Hogan PG. Expression of the transcription factor NFATp in a neuronal cell line and in the murine nervous system. J Biol Chem. 1994;269:28181–28186. [PubMed] [Google Scholar]
- 18.Wang DZ, McCaffrey PG, Rao A. The cyclosporin-sensitive transcription factor NFATp is expressed in several classes of cells in the immune system. Ann NY Acad Sci. 1995;766:182–194. doi: 10.1111/j.1749-6632.1995.tb26661.x. [DOI] [PubMed] [Google Scholar]
- 19.Hauber J, Perkins A, Heimer E, Cullen B. Trans-activation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc Natl Acad Sci USA. 1987;84:6364–6368. doi: 10.1073/pnas.84.18.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo C, Shaw KTY, Raghavan A, Aramburu J, Garcia-Cozar F, Perrino BA, Hogan PG, Rao A. Interaction of calcineurin with a domain of the transcription factor NFAT1 that controls nuclear import. Proc Natl Acad Sci USA. 1996;93:8907–8912. doi: 10.1073/pnas.93.17.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedin KE, Bell MP, Kalli KR, Huntoon CJ, Sharp BM, McKean DJ. Delta-opioid receptors expressed by Jurkat T cells enhance IL-2 secretion by increasing AP-1 complexes and activity of the NF-AT/AP-1-binding promoter element. J Immunol. 1997;159:5431–5440. [PubMed] [Google Scholar]
- 22.Chakravarti D, LaMorte VJ, Nelson MC, Nakajima T, Schulman IG, Juguilon H, Montminy M, Evans RM. Role of CBP/p300 in nuclear receptor signalling. Nature. 1996;383:99–102. doi: 10.1038/383099a0. [DOI] [PubMed] [Google Scholar]
- 23.Eckner R, Ewen ME, Newsome D, Gerdes M, DeCaprio JA, Bentley J, Lawrence, Livingston DM. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-KD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 24.Stein RW, Corrigan M, Yaciuk P, Whelan J, Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990;64:4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakajima T, Fukamizu A, Takahashi J, Gage FH, Fisher T, Blenis J, Montminy M. The signal-dependent coactivator CBP is a nuclear target for pp90rsk. Cell. 1996;86:465–474. doi: 10.1016/s0092-8674(00)80119-1. [DOI] [PubMed] [Google Scholar]
- 26.Luo C, Burgeon E, Carew JA, McCaffrey PG, Badalian TM, Lane WS, Hogan PG, Rao A. Recombinant NFAT1 (NFATp) is regulated by calcineurin in T cells and mediates transcription of several cytokine genes. Mol Cell Biol. 1996;16:3955–3966. doi: 10.1128/mcb.16.7.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DuBridge RB, Tang P, Hsia HC, Phaik-Mooi L, Miller JH, Calos MP. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987;7:379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valgue-Archer VE, De Villiers J, Sinskey AJ, Rao A. Transformation of T lymphocytes by the v-fos oncogene. J Immunol. 1990;145:4355–4364. [PubMed] [Google Scholar]
- 29.McCaffrey PG, Jain J, Jamieson C, Sen R, Rao A. A T cell nuclear factor resembling NF-AT binds to an NF-κB site and to the conserved lymphokine promoter sequence “Cytokine-1.” . J Biol Chem. 1992;267:1864–1871. [PubMed] [Google Scholar]
- 30.Brownell KE, Allis D. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in Tetrahymenamacronuclei. Proc Natl Acad Sci USA. 1995;92:6364–6368. doi: 10.1073/pnas.92.14.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang JJ, Vinkemeier U, Gu W, Chakravarti D, Horvath CM, Darnell JEJ. Two contact regions between Stat1 and CBP/p300 in interferon γ signaling. Proc Natl Acad Sci USA. 1996;93:15092–15096. doi: 10.1073/pnas.93.26.15092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JS, See RH, Deng T, Shi Y. Adenovirus E1A downregulates cJun- and JunB-mediated transcription by targeting their coactivator p300. Mol Cell Biol. 1996;16:4312–4326. doi: 10.1128/mcb.16.8.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin SC, Heyman RA, Rose DW, Glass CK, Rosenfeld MG. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 34.Tjian R, Maniatis T. Transcriptional activation: a complex puzzle with few easy pieces. Cell. 1994;77:5–8. doi: 10.1016/0092-8674(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 35.Kee B, Arias J, Montminy M. Adaptor-mediated recruitment of RNA polymerase II to a signal-dependent activator. J Biol Chem. 1996;271:2373–2375. doi: 10.1074/jbc.271.5.2373. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima T, Uchida C, Anderson SF, Parvin JD, Montminy M. Analysis of a cAMP-responsive activator reveals a two-component mechanism for transcriptional induction via signal-dependent factors. Genes Dev. 1997;11:738–747. doi: 10.1101/gad.11.6.738. [DOI] [PubMed] [Google Scholar]